Surgical and Clinical Outcomes of Microvascular Decompression: A Comparative Study between Young and Elderly Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients′ Selection
2.2. Outcome Data
- -
- Clinical pre-operative data: age, sex, affected side, TN type, pain duration (years), Barrow Neurological Institute Pain Intensity (BNI) score [15], previous surgery;
- -
- Intraoperative data: craniotomy size; use of neuronavigation during surgery [16]; surgical duration and mastoid opening. Intraoperative conflict was classified as: “contact only” when the offending artery was in contact with the nerve root but without any visible indentation and “distortion and/or indentation” when there was a distortion and/or an indentation of the nerve root caused by the offending artery [17,18];
- -
- Post-operative/follow-up data: acute pain relief (APR) (pain-free at hospital discharge); cerebrospinal fluid (CSF) leak after surgery and complication other than CSF leak; length of stay; pain-free survival determined at the most recent follow-up visit, need for re-operation and BNI at follow-up.
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
3.1. Patients Characteristics
3.2. Outcome Data
3.3. Risk Factor Analysis for Pain Recurrence
4. Discussion
4.1. Comparative Analysis between Elderly and Non-Elderly Group
4.2. Risk Factor Analysis for Pain Recurrence
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Lambru, G.; Zakrzewska, J.; Matharu, M. Trigeminal neuralgia: A practical guide. Pract. Neurol. 2021, 21, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Maarbjerg, S.; Gozalov, A.; Olesen, J.; Bendtsen, L. Concomitant persistent pain in classical trigeminal neuralgia--evidence for different subtypes. Headache 2014, 54, 1173–1183. [Google Scholar] [CrossRef]
- Obermann, M.; Yoon, M.S.; Ese, D.; Maschke, M.; Kaube, H.; Diener, H.-C.; Katsarava, Z. Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 2007, 69, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, G.; De Stefano, G.; Leone, C.; Cruccu, G.; Tardioli, S.; Cartocci, G.; Fiorelli, M.; Truini, A.; Caramia, F. Concomitant continuous pain in patients with trigeminal neuralgia is associated with trigeminal nerve root atrophy. Cephalalgia 2020, 40, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Di Stefano, G.; Truini, A. Trigeminal Neuralgia. N. Engl. J. Med. 2020, 383, 754–762. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, L.; Floden, L.; Vinikoor-Imler, L.; Symonds, T.; Giblin, K.; Hartford, C.; Zakrzewska, J.M. Burden of illness of trigeminal neuralgia among patients managed in a specialist center in England. J. Headache Pain 2020, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Rapisarda, A.; Ioannoni, E.; Olivi, A. Microvascular decompression in patients with trigeminal neuralgia and multiple sclerosis: Results and analysis of possible prognostic factors. Acta Neurol. Belg. 2020, 120, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhao, W. Microvascular decompression for recurrent trigeminal neuralgia. J. Clin. Neurosci. 2014, 21, 1549–1553. [Google Scholar] [CrossRef]
- Jannetta, P.J. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia, 1967. J. Neurosurg. 2007, 107, 216–219. [Google Scholar] [CrossRef]
- Bendtsen, L.; Zakrzewska, J.M.; Heinskou, T.B.; Hodaie, M.; Lacerda Leal, P.R.; Nurmikko, T.; Obermann, M.; Cruccu, G.; Maarbjerg, S. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020, 19, 784–796. [Google Scholar] [CrossRef]
- Phan, K.; Rao, P.J.; Dexter, M. Microvascular decompression for elderly patients with trigeminal neuralgia. J. Clin. Neurosci. 2016, 29, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Greve, T.; Tonn, J.C.; Mehrkens, J.H. Microvascular decompression for trigeminal neuralgia in the elderly: Efficacy and safety. J. Neurol. 2021, 268, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Amagasaki, K.; Watanabe, S.; Naemura, K.; Shono, N.; Nakaguchi, H. Safety of microvascular decompression for elderly patients with trigeminal neuralgia. Clin. Neurol. Neurosurg. 2016, 141, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.L.; Shetter, A.G.; Fiedler, J.A.; Smith, K.A.; Han, P.P.; Speiser, B.L. Gamma knife radiosurgery for trigeminal neuralgia: The initial experience of The Barrow Neurological Institute. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1013–1019. [Google Scholar] [CrossRef]
- Legninda Sop, F.Y.; D’Ercole, M.; Izzo, A.; Rapisarda, A.; Ioannoni, E.; Caricato, A.; Olivi, A.; Montano, N. The Impact of Neuronavigation on the Surgical Outcome of Microvascular Decompression for Trigeminal Neuralgia. World Neurosurg. 2021, 149, 80–85. [Google Scholar] [CrossRef]
- Maurya, V.; Sreedhar, C.M.; Khera, A.; Bhatia, M.; Sharma, V. Trigeminal neuralgia: When does neurovascular contact turn into a conflict? Med. J. Armed Forces India 2019, 75, 134–139. [Google Scholar] [CrossRef]
- Wang, W.; Yu, F.; Kwok, S.C.; Wang, Y.; Yin, J. Microvascular Decompression for Trigeminal Neuralgia Caused by Venous Offending on the Ventral Side of the Root Entrance/Exit Zone: Classification and Management Strategy. Front Neurol. 2022, 13, 864061. [Google Scholar] [CrossRef]
- Montano, N.; Conforti, G.; Di Bonaventura, R.; Meglio, M.; Fernandez, E.; Papacci, F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther. Clin. Risk Manag. 2015, 11, 289–299. [Google Scholar] [CrossRef]
- Montano, N.; Gaudino, S.; Giordano, C.; Pignotti, F.; Ioannoni, E.; Rapisarda, A.; Olivi, A. Possible Prognostic Role of Magnetic Resonance Imaging Findings in Patients with Trigeminal Neuralgia and Multiple Sclerosis Who Underwent Percutaneous Balloon Compression: Report of Our Series and Literature Review. World Neurosurg. 2019, 125, e575–e581. [Google Scholar] [CrossRef]
- Ferroli, P.; Acerbi, F.; Tomei, M.; Tringali, G.; Franzini, A.; Broggi, G. Advanced age as a contraindication to microvascular decompression for drug-resistant trigeminal neuralgia: Evidence of prejudice? Neurol Sci. 2010, 31, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, M.; Eldridge, P.R.; Varma, T.R.; Miles, J.B.; Nurmikko, T.J. Microvascular decompression for trigeminal neuralgia in patients over 70 years of age. Neurology 2003, 60, 520. [Google Scholar] [CrossRef] [PubMed]
- Ashkan, K.; Marsh, H. Microvascular decompression for trigeminal neuralgia in the elderly: A review of the safety and efficacy. Neurosurgery 2004, 55, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Sekula, R.F.; Marchan, E.M.; Fletcher, L.H.; Casey, K.F.; Jannetta, P.J. Microvascular decompression for trigeminal neuralgia in elderly patients. J. Neurosurg. 2008, 108, 689–691. [Google Scholar] [CrossRef]
- Rughani, A.I.; Dumont, T.M.; Lin, C.T.; Tranmer, B.I.; Horgan, M.A. Safety of microvascular decompression for trigeminal neuralgia in the elderly. J. Neurosurg. 2011, 115, 202–209. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, D.; You, C.; Mao, B.Y.; Wu, B.; Fang, Y. The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg. 2013, 79, 756–762. [Google Scholar] [CrossRef]
- Kumar, S.; Rastogi, S.; Kumar, S.; Mahendra, P.; Bansal, M.; Chandra, L. Pain in trigeminal neuralgia: Neurophysiology and measurement: A comprehensive review. J. Med. Life 2013, 6, 383–388. [Google Scholar]
- Revuelta-Gutiérrez, R.; López-González, M.A.; Soto-Hernández, J.L. Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surg. Neurol. 2006, 66, 32–36. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.T.; Zhu, J.; Guan, H.X.; Zhou, Q.M.; Jiao, W.; Ying, T.T.; Yang, X.S.; Zhan, W.C.; Hua, X.M. A clinical analysis on microvascular decompression surgery in a series of 3000 cases. Clin. Neurol. Neurosurg. 2012, 114, 846–851. [Google Scholar] [CrossRef]
- Rapisarda, A.; Baroni, S.; Gentili, V.; Moretti, G.; Burattini, B.; Sarlo, F.; Olivi, A.; Urbani, A.; Montano, N. The role of biomarkers in drug-resistant trigeminal neuralgia: A prospective study in patients submitted to surgical treatment. Neurol. Sci. 2022, 43, 4425–4430. [Google Scholar] [CrossRef] [PubMed]
- Holste, K.; Chan, A.Y.; Rolston, J.D.; Englot, D.J. Pain Outcomes Following Microvascular Decompression for Drug-Resistant Trigeminal Neuralgia: A Systematic Review and Meta-Analysis. Neurosurgery 2020, 86, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schär, R.T.; Tashi, S.; Branca, M.; Soll, N.; Cipriani, D.; Schwartz, C.; Pollo, C.; Schucht, P.; Ulrich, C.T.; Beck, J.; et al. How safe are elective craniotomies in elderly patients in neurosurgery today? A prospective cohort study of 1452 consecutive cases. J. Neurosurg. 2020, 134, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Soleman, J.; Ullmann, M.; Greuter, L.; Ebel, F.; Guzman, R. Mortality and Outcome in Elderly Patients Undergoing Emergent or Elective Cranial Surgery. World Neurosurg. 2021, 146, e575–e589. [Google Scholar] [CrossRef] [PubMed]
- Johans, S.J.; Garst, J.R.; Burkett, D.J.; Grahnke, K.; Martin, B.; Ibrahim, T.F.; Anderson, D.E.; Prabhu, V.C. Identification of Preoperative and Intraoperative Risk Factors for Complications in the Elderly Undergoing Elective Craniotomy. World Neurosurg. 2017, 107, 216–225. [Google Scholar] [CrossRef]

| ALL CASES N = 76 | AGE < 65 N = 51 | AGE ≥ 65 N = 25 | p Value | |
|---|---|---|---|---|
| Sex (M) | 29 (38.1%) | 22 (43.1%) | 7 (28%) | 0.201 |
| Side (L) | 29 (38.1%) | 17 (33.3%) | 12 (48%) | 0.216 |
| Type (Classical TN) | 75 (98.6%) | 51 (100%) | 24 (96%) | 0.151 |
| Pain duration (years) | 6.37 ± 4.63 | 6.36 ± 5.13 | 6.40 ± 3.55 | 0.977 |
| BNI pre-op | 4.13 ± 0.71 | 4.11 ± 0.65 | 4.16 ± 0.85 | 0.811 |
| Previous surgery | 6 (7.9%) | 5 (9.8%) | 1 (4%) | 0.657 |
| ALL CASES N = 76 | AGE < 65 N = 51 | AGE ≥ 65 N = 25 | p Value | |
|---|---|---|---|---|
| Craniotomy size (cm2) | 3.60 ± 1.44 | 3.65 ± 1.53 | 3.51 ± 1.26 | 0.696 |
| Neuronavigation (Yes) | 40 (52.6%) | 30 (58.8%) | 10 (40%) | 0.164 |
| Surgical duration (min) | 116.34 ± 24.98 | 116.86 ± 27.42 | 115.28 ± 19.53 | 0.797 |
| Mastoid opening (Yes) | 13 (17.1%) | 9 (17.6%) | 4 (16%) | 0.857 |
| Double Conflict | 6 (7.8%) | 5 (9.8%) | 1 (4%) | 0.657 |
| Nerve root distortion/indentation | 63 (82.8%) | 43 (84.3%) | 20 (80%) | 0.748 |
| ALL CASES N = 76 | AGE < 65 N = 51 | AGE ≥ 65 N = 25 | p Value | |
|---|---|---|---|---|
| APR (Yes) | 75 (98.6%) | 50 (98%) | 25 (100%) | 0.481 |
| CSF leak | 4 (5.2%) | 2 (3.9%) | 2 (8%) | 0.594 |
| Complications other than CSF leak | 5 (6.5%) | 3 (5.8%) | 2 (8%) | 0.534 |
| Length of stay (days) | 5.09 ± 1.8 | 5 ± 1.68 | 5.28 ± 2.05 | 0.528 |
| Median follow-up (range 3–48 months) | 13.5 ± 11.99 | 12.47 ± 11.93 | 15.60 ± 12.06 | 0.288 |
| BNI at follow-up | 1.40 ± 0.96 | 1.35 ± 0.86 | 1.52 ± 1.15 | 0.483 |
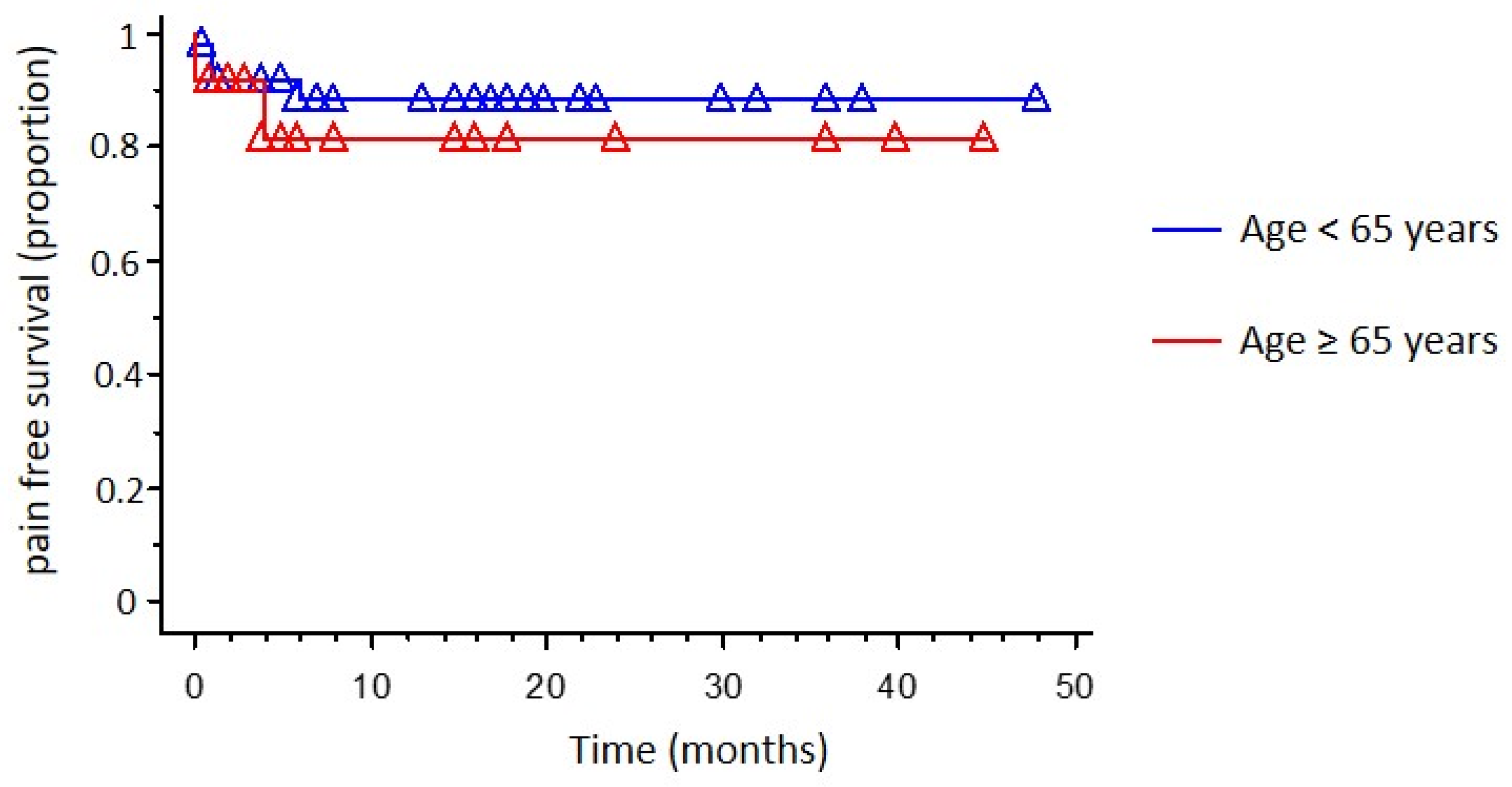
| Recurrence | 9 (11.84%) | 5 (9.8%) | 4 (16%) | 0.432 |
| Coef | Coef/SE | Chi-Square | p Value | HR | 95% Lower | 95% Upper | |
|---|---|---|---|---|---|---|---|
| Age | −0.592 | −0.795 | 0.632 | 0.426 | 0.55 | 0.129 | 2.380 |
| Sex | −1.438 | −1.642 | 2.69 | 0.1 | 0.23 | 0.043 | 1.322 |
| Previous surgery | −0.45 | −0.388 | 0.15 | 0.69 | 0.63 | 0.064 | 6.305 |
| Post-op complications | −1.02 | −0.953 | 0.909 | 0.34 | 0.33 | 0.034 | 3.202 |
| Intraoperative conflict type | 3.781 | 3.89 | 15.132 | 0.0001 | 43.86 | 6.52 | 294.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menna, G.; Rapisarda, A.; Izzo, A.; D’Ercole, M.; D’Alessandris, Q.G.; Olivi, A.; Montano, N. Surgical and Clinical Outcomes of Microvascular Decompression: A Comparative Study between Young and Elderly Patients. Brain Sci. 2022, 12, 1216. https://doi.org/10.3390/brainsci12091216
Menna G, Rapisarda A, Izzo A, D’Ercole M, D’Alessandris QG, Olivi A, Montano N. Surgical and Clinical Outcomes of Microvascular Decompression: A Comparative Study between Young and Elderly Patients. Brain Sciences. 2022; 12(9):1216. https://doi.org/10.3390/brainsci12091216
Chicago/Turabian StyleMenna, Grazia, Alessandro Rapisarda, Alessandro Izzo, Manuela D’Ercole, Quintino Giorgio D’Alessandris, Alessandro Olivi, and Nicola Montano. 2022. "Surgical and Clinical Outcomes of Microvascular Decompression: A Comparative Study between Young and Elderly Patients" Brain Sciences 12, no. 9: 1216. https://doi.org/10.3390/brainsci12091216
APA StyleMenna, G., Rapisarda, A., Izzo, A., D’Ercole, M., D’Alessandris, Q. G., Olivi, A., & Montano, N. (2022). Surgical and Clinical Outcomes of Microvascular Decompression: A Comparative Study between Young and Elderly Patients. Brain Sciences, 12(9), 1216. https://doi.org/10.3390/brainsci12091216






