Approaching or Decentering? Differential Neural Networks Underlying Experiential Emotion Regulation and Cognitive Defusion
Abstract
1. Introduction
1.1. Approaching versus Decentering?
Experiential Emotion Regulation versus Cognitive Defusion
1.2. Experiential Emotion Regulation: An Experiential View of Emotion Regulation
1.3. Cognitive Defusion as a “Decentering” Strategy of Emotion Regulation
1.4. The Current Study
2. Materials and Methods
2.1. Participants
2.2. Emotion Regulation Training
2.3. Stimuli
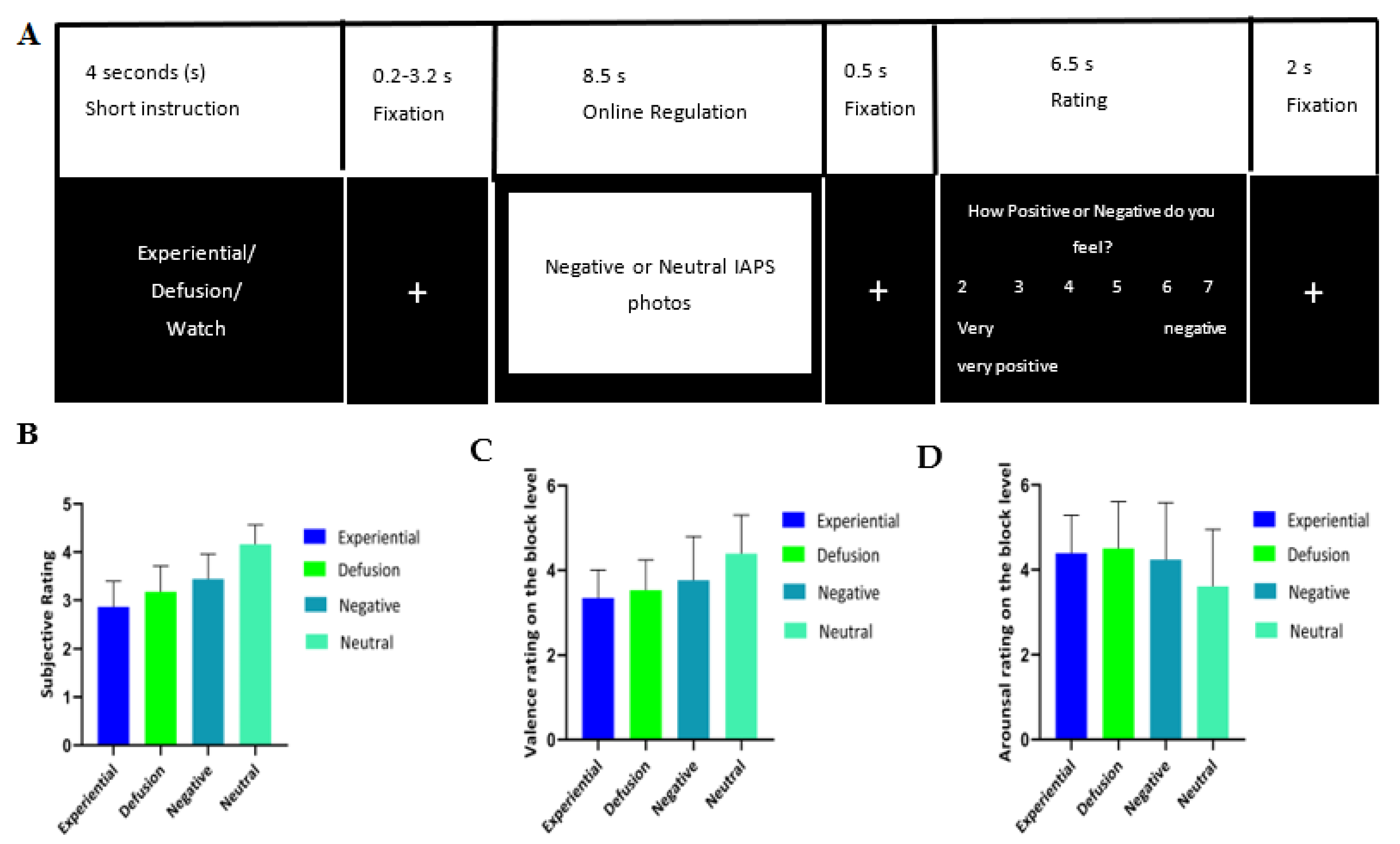
2.4. Task Paradigm
2.5. Experimental Procedure
2.6. Data Acquisition
2.6.1. Task fMRI
2.6.2. T1 Scanning
2.7. Behavioral Data Analysis
2.8. fMRI Data Analysis
2.8.1. Pre-Processing
2.8.2. Analysis of Brain Activations
2.8.3. Analysis of Functional Connectivity Using gPPI
3. Results
3.1. Behavioral Results
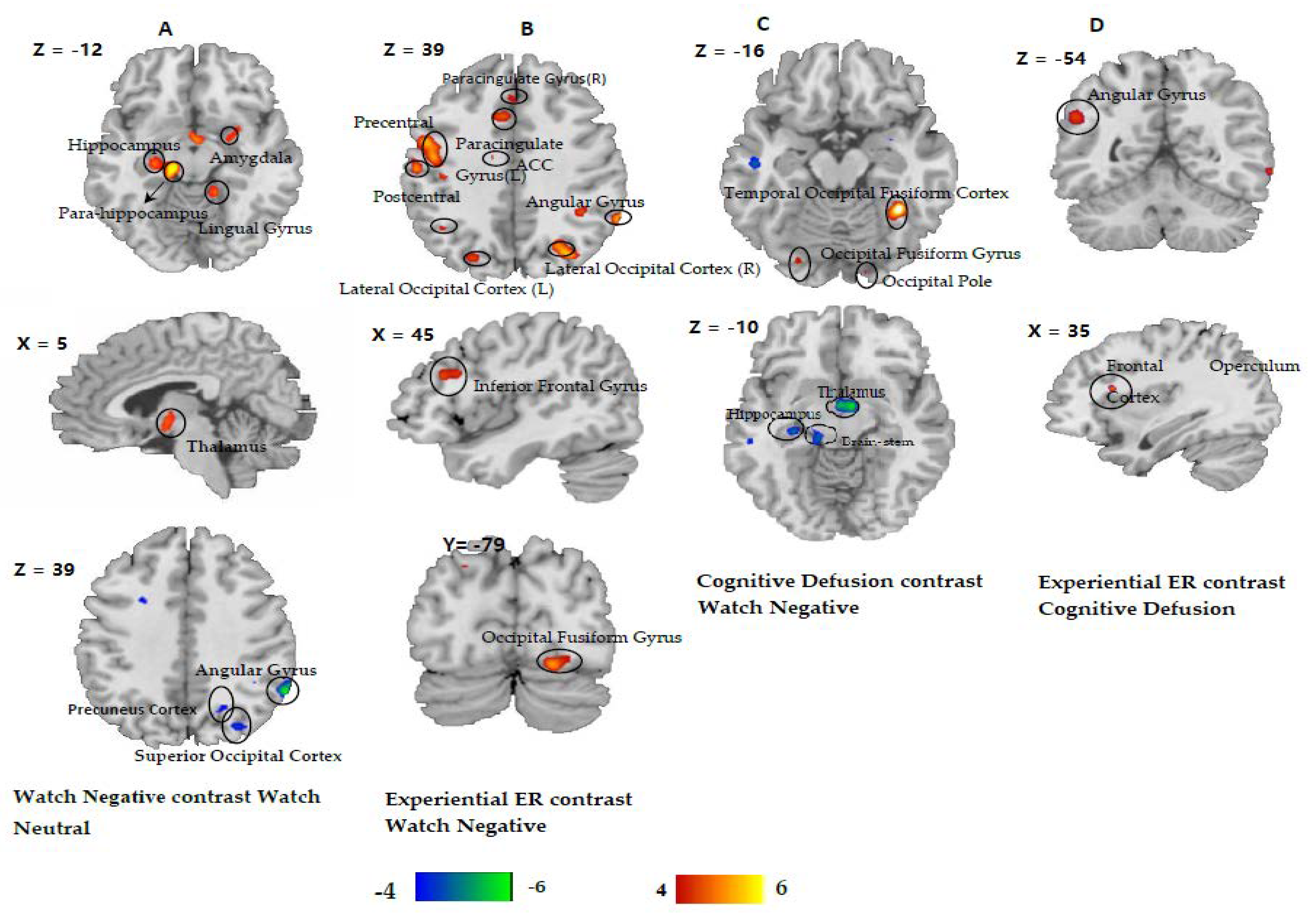
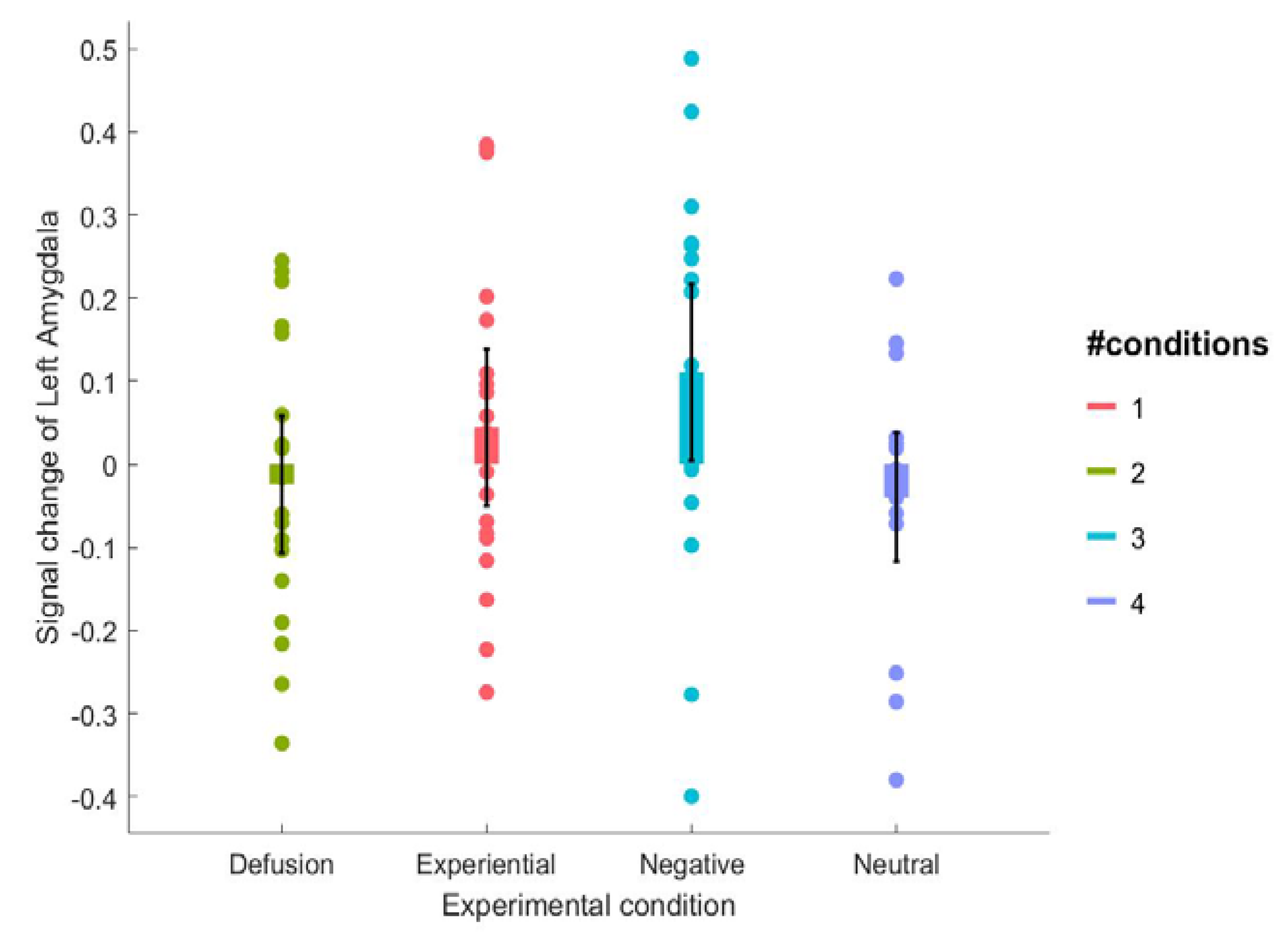
3.2. fMRI Results
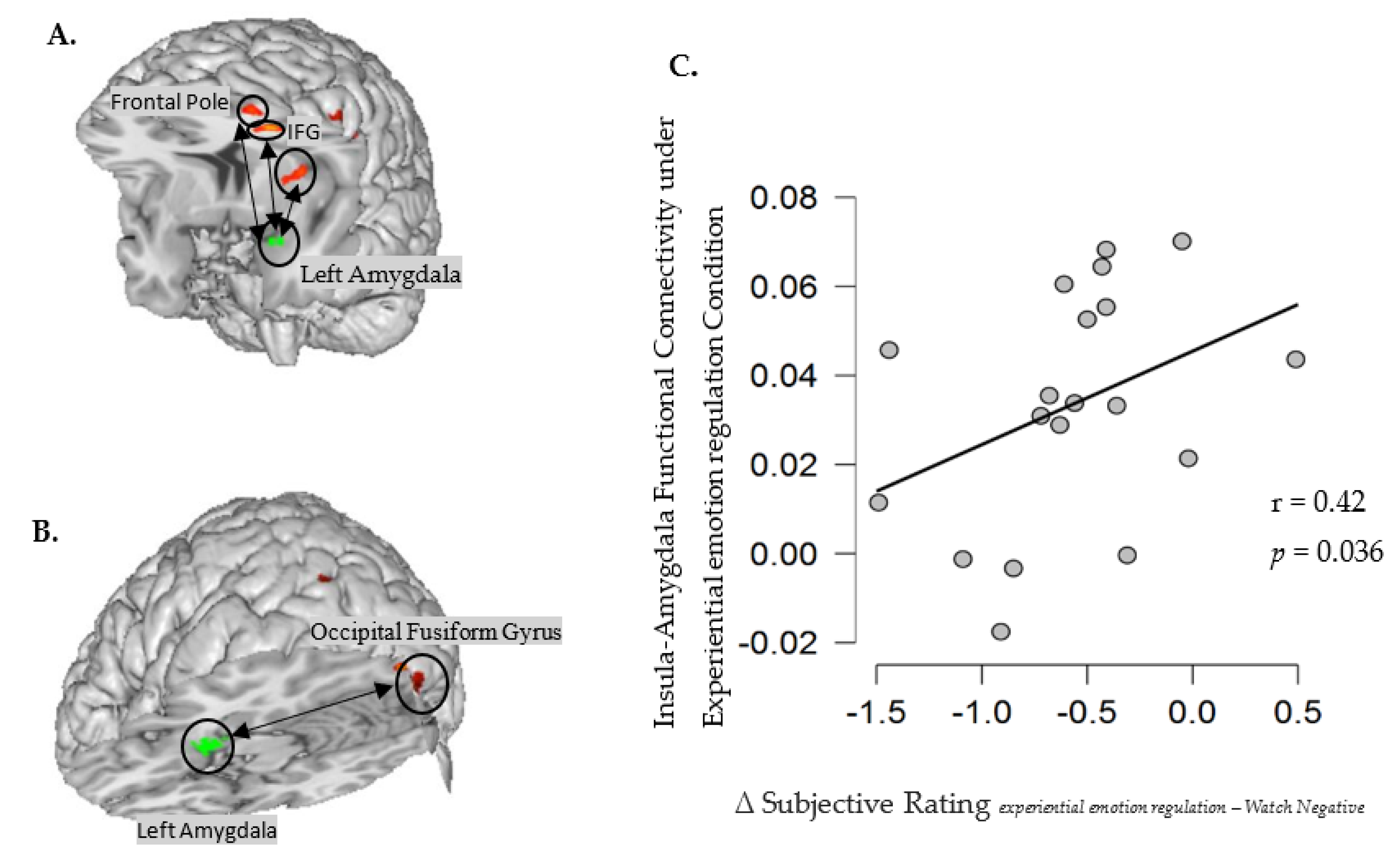
Functional Connectivity Analysis
4. Discussion
4.1. Emotion Experience in Experiential Emotion Regulation
4.2. Emotion Experience in Cognitive Defusion
4.3. Neural Network of Experiential Emotion Regulation
4.3.1. Anterior Cingulate Cortex
4.3.2. Angular and Postcentral Gyrus
4.3.3. Insular Cortex
4.3.4. Prefrontal Cortex
4.4. Neural Network for Cognitive Defusion
4.5. Experiential Emotion Regulation versus Cognitive Defusion
4.6. Limitations and Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvers, J.A.; Weber, J.; Wager, T.D.; Ochsner, K.N. Bad and Worse: Neural Systems Underlying Reappraisal of High-and Low-Intensity Negative Emotions. Soc. Cogn. Affect. Neurosci. 2015, 10, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tamir, M.; Schwartz, S.H.; Oishi, S.; Kim, M.Y. The Secret to Happiness: Feeling Good or Feeling Right? J. Exp. Psychol. Gen. 2017, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J. The Emerging Field of Emotion Regulation: An Integrated Review. Rev. Gen. Psychol. 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Gross, J.J.; Thompson, R.A. Emotion Regulation: Conceptual Foundations. In Handbook of Emotion Regulation; Gross, J., Ed.; The Guilford Press: New York, NY, USA, 2007; pp. 3–24. ISBN 1593851480. [Google Scholar]
- Kanske, P.; Heissler, J.; Schonfelder, S.; Bongers, A.; Wessa, M. How to Regulate Emotion? Neural Networks for Reappraisal and Distraction. Cereb. Cortex 2010, 21, 1379–1388. [Google Scholar] [CrossRef]
- Morawetz, C.; Oganian, Y.; Schlickeiser, U.; Jacobs, A.M.; Heekeren, H.R. Second Language Use Facilitates Implicit Emotion Regulation via Content Labeling. Front. Psychol. 2017, 8, 366. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional Imaging Studies of Emotion Regulation: A Synthetic Review and Evolving Model of the Cognitive Control of Emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef]
- Gross, J.J.; Uusberg, H.; Uusberg, A. Mental Illness and Well-being: An Affect Regulation Perspective. World Psychiatry 2019, 18, 130–139. [Google Scholar] [CrossRef]
- McCracken, L.M.; Gutieŕrez-Martínez, O.; Smyth, C. ‘Decentering’ Reflects Psychological Flexibility in People with Chronic Pain and Correlates with Their Quality of Functioning. Health Psychol. 2013, 32, 820–823. [Google Scholar] [CrossRef]
- Bernstein, A.; Hadash, Y.; Lichtash, Y.; Tanay, G.; Shepherd, K.; Fresco, D.M. Decentering and Related Constructs: A Critical Review and Metacognitive Processes Model. Perspect. Psychol. Sci. 2015, 10, 599–617. [Google Scholar] [CrossRef]
- Vandekerckhove, M.; Kestemont, J.; Weiss, R.; Schotte, C.; Exadaktylos, V.; Haex, B.; Verbraecken, J.; Gross, J.J. Experiential versus Analytical Emotion Regulation and Sleep: Breaking the Link between Negative Events and Sleep Disturbance. Emotion 2012, 12, 1415–1421. [Google Scholar] [CrossRef]
- Vandekerckhove, M. Neural Networks in Bottom up ‘Experiential Emotion Regulation’. Behav. Brain Res. 2020, 383, 111242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vlemincx, E.; Vleminck Vantieghem, I.; Dhar, M.; Dong, D.; Vandekerckhove, M. Bottom-Up and Cognitive Top-Down Emotion Regulation: Experiential Emotion Regulation and Cognitive Reappraisal on Stress Relief and Follow-Up Sleep Physiology. Int. J. Environ. Res. Public Health 2022, 19, 7621, in press. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.R. The Necessary and Sufficient Conditions of Therapeutic Personality Change. J. Consult. Psychol. 1957, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Gendlin, E.T. Experiential Psychotherapy. In Current Psychotherapies; Peacock: Itasca, IL, USA, 1973; pp. 317–352. [Google Scholar]
- Greenberg, L.S.; Vandekerckhove, M. Emotional Experience, Expression, and Regulation in the Psychotherapeutic Processes. In Regulating Emotion; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 240–268. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Review of Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. Cogn. Behav. Pract. 2002, 9, 164–166. [Google Scholar]
- Hayes, S.C. Acceptance and Commitment Therapy, Relational Frame Theory, and the Third Wave of Behavioral and Cognitive Therapies. Behav. Ther. 2004, 35, 639–665. [Google Scholar] [CrossRef]
- Grégoire, S.; Lachance, L.; Bouffard, T.; Dionne, F. The Use of Acceptance and Commitment Therapy to Promote Mental Health and School Engagement in University Students: A Multisite Randomized Controlled Trial. Behav. Ther. 2018, 49, 360–372. [Google Scholar] [CrossRef]
- Assaz, D.A.; Roche, B.; Kanter, J.W.; Oshiro, C.K.B. Cognitive Defusion in Acceptance and Commitment Therapy: What Are the Basic Processes of Change? Psychol. Rec. 2018, 68, 405–418. [Google Scholar] [CrossRef]
- Harries, R. ACT with Love: Stop Struggling, Reconcile Differences, and Strengthen Your Relationship with Acceptance and Commitment Therapy; New Harbinger Publications: Oakland, CA, USA, 2009. [Google Scholar]
- Gendlin, E.T. The Town and Human Attention. 2007. Available online: http://previous.focusing.org/gendlin/docs/gol_2180.html (accessed on 20 April 2022).
- Vandekerckhove, M.; Bulnes, L.C.; Panksepp, J. The Emergence of Primary Anoetic Consciousness in Episodic Memory. Front. Behav. Neurosci. 2014, 7, 210. [Google Scholar] [CrossRef][Green Version]
- Vandekerckhove, M.; Panksepp, J. The Flow of Anoetic to Noetic and Autonoetic Consciousness: A Vision of Unknowing (Anoetic) and Knowing (Noetic) Consciousness in the Remembrance of Things Past and Imagined Futures. Conscious. Cogn. 2009, 18, 1018–1028. [Google Scholar] [CrossRef]
- Vandekerckhove, M.; Panksepp, J. A Neurocognitive Theory of Higher Mental Emergence: From Anoetic Affective Experiences to Noetic Knowledge and Autonoetic Awareness. Neurosci. Biobehav. Rev. 2011, 35, 2017–2025. [Google Scholar] [CrossRef]
- Panksepp, J.; Clarici, A.; Vandekerckhove, M.; Yovell, Y. Neuro evolutionary foundations of infant minds: Visions of how primal emotions guide construction of human affective states and their disorders. Psychoanal. Inq. 2019, 39, 36–51. [Google Scholar] [CrossRef]
- Stanton, A.L.; Danoff-Burg, S. Emotional Expression, Expressive Writing, and Cancer; American Psychological Association: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Horn, A.B.; Pössel, P.; Hautzinger, M. Promoting Adaptive Emotion Regulation and Coping in Adolescence: A School-Based Programme. J. Health Psychol. 2011, 16, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J. Antecedent- and Response-Focused Emotion Regulation: Divergent Consequences for Experience, Expression, and Physiology. J. Pers. Soc. Psychol. 1998, 74, 224–237. [Google Scholar] [CrossRef]
- Sheppes, G.; Gross, J.J. Is Timing Everything? Temporal Considerations in Emotion Regulation. Personal. Soc. Psychol. Rev. 2011, 15, 319–331. [Google Scholar] [CrossRef]
- Herwig, U.; Kaffenberger, T.; Jancke, L.; Bruhl, A.B. Self-Related Awareness and Emotion Regulation. Neuroimage 2010, 50, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; McFarlin, D.R.; Perlman, D.M.; Salomons, T.V.; Davidson, R.J. Altered Anterior Insula Activation during Anticipation and Experience of Painful Stimuli in Expert Meditators. Neuroimage 2013, 64, 538–546. [Google Scholar] [CrossRef]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and Commitment Therapy: Model, Processes and Outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef]
- McCracken, L.M.; Barker, E.; Chilcot, J. Decentering, Rumination, Cognitive Defusion, and Psychological Flexibility in People with Chronic Pain. J. Behav. Med. 2014, 37, 1215–1225. [Google Scholar] [CrossRef]
- Naragon-Gainey, K.; DeMarree, K.G. Structure and Validity of Measures of Decentering and Defusion. Psychol. Assess. 2017, 29, 935–954. [Google Scholar] [CrossRef]
- Lebois, L.A.M.; Papies, E.K.; Gopinath, K.; Cabanban, R.; Quigley, K.S.; Krishnamurthy, V.; Barrett, L.F.; Barsalou, L.W. A Shift in Perspective: Decentering through Mindful Attention to Imagined Stressful Events. Neuropsychologia 2015, 75, 505–524. [Google Scholar] [CrossRef]
- Kross, E.; Ayduk, O.; Mischel, W. When Asking ‘“ Why ”’ Does Not Hurt: Distinguish Rumination From Reflective Processing of Negative Emotions. Psychol. Sci. 2005, 16, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Dörfel, D.; Lamke, J.P.; Hummel, F.; Wagner, U.; Erk, S.; Walter, H. Common and Differential Neural Networks of Emotion Regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A Comparative FMRI Investigation. Neuroimage 2014, 101, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Kross, E. When the Self Becomes Other: Toward an Integrative Understanding of the Processes Distinguishing Adaptive Self-Reflection from Rumination. Ann. N. Y. Acad. Sci. 2009, 1167, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kross, E.; Davidson, M.; Weber, J.; Ochsner, K. Coping with Emotions Past: The Neural Bases of Regulating Affect Associated with Negative Autobiographical Memories. Biol. Psychiatry 2009, 65, 361–366. [Google Scholar] [CrossRef]
- Christian, B.M.; Parkinson, C.; Macrae, C.N.; Miles, L.K.; Wheatley, T. When Imagining Yourself in Pain, Visual Perspective Matters: The Neural and Behavioral Correlates of Simulated Sensory Experiences. J. Cogn. Neurosci. 2015, 27, 866–875. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.; Gross, J.J. For Better or for Worse: Neural Systems Supporting the Cognitive down- and up-Regulation of Negative Emotion. Neuroimage 2004, 23, 483–499. [Google Scholar] [CrossRef]
- Kalisch, R.; Wiech, K.; Critchley, H.D.; Seymour, B.; O’Doherty, J.P.; Oakley, D.A.; Allen, P.; Dolan, R.J. Anxiety Reduction through Detachment: Subjective, Physiological, and Neural Effects. J. Cogn. Neurosci. 2005, 17, 874–883. [Google Scholar] [CrossRef]
- Koenigsberg, H.W.; Fan, J.; Ochsner, K.N.; Liu, X.; Guise, K.; Pizzarello, S.; Dorantes, C.; Tecuta, L.; Guerreri, S.; Goodman, M.; et al. Neural Correlates of Using Distancing to Regulate Emotional Responses to Social Situations. Neuropsychologia 2010, 48, 1813–1822. [Google Scholar] [CrossRef]
- Kross, E.; Ayduk, O. Self-Distancing: Theory, Research, and Current Directions. In In Advances in Experimental Social Psychology; Academic Press: Cambridge, MA, USA, 2017; Volume 55, pp. 81–136. [Google Scholar]
- Vandekerckhove, M.; Wang, Y. Emotion, Emotion Regulation and Sleep: An Intimate Relationship. AIMS Neurosci. 2017, 5, 1–22. [Google Scholar] [CrossRef]
- Falquez, R.; Couto, B.; Ibanez, A.; Freitag, M.T.; Berger, M.; Arens, E.A.; Lang, S.; Barnow, S. Detaching from the Negative by Reappraisal: The Role of Right Superior Frontal Gyrus (BA9/32). Front. Behav. Neurosci. 2014, 8, 165. [Google Scholar] [CrossRef]
- Hare, T.A.; Tottenham, N.; Davidson, M.C.; Glover, G.H.; Casey, B.J. Contributions of Amygdala and Striatal Activity in Emotion Regulation. Biol. Psychiatry 2005, 57, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural Systems Supporting Interoceptive Awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior Insular Cortex and Emotional Awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef] [PubMed]
- Fish, A.M.; Nadig, A.; Seidlitz, J.; Reardon, P.K.; Mankiw, C.; Mcdermott, C.L.; Blumenthal, J.D.; Clasen, L.S.; Lalonde, F.; Lerch, J.P.; et al. Sex-Biased Trajectories of Amygdalo-Hippocampal Morphology Change over Human Development. Neuroimage 2020, 204, 116122. [Google Scholar] [CrossRef]
- Mcrae, K.; Ochsner, K.N.; Mauss, I.B.; Gabrieli, J.J.D.; Gross, J.J. Gender Differences in Emotion Regulation: An FMRI Study of Cognitive Reappraisal. Gr. Process. Intergr. Relat. 2008, 11, 143–162. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P. The International Affective Picture System (IAPS) in the study of emotion and attention. Handb. Emot. Elicitation Assess. 2007, 29, 70–73. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Bunge, S.A.; Gross, J.J.; Gabrieli, J.D.E. Rethinking Feelings: An FMRI Study of the Cognitive Regulation of Emotion. J. Cogn. Neurosci. 2002, 14, 1215–1229. [Google Scholar] [CrossRef]
- Phan, K.L.; Fitzgerald, D.A.; Nathan, P.J.; Moore, G.J.; Uhde, T.W.; Tancer, M.E. Neural Substrates for Voluntary Suppression of Negative Affect: A Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2005, 57, 210–219. [Google Scholar] [CrossRef]
- Banks, S.J.; Eddy, K.T.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala Frontal Connectivity during Emotion Regulation. Soc. Cogn. Affect. Neurosci. 2007, 2, 303–312. [Google Scholar] [CrossRef]
- McLaren, D.G.; Ries, M.L.; Xu, G.; Johnson, S.C. A Generalized Form of Context-Dependent Psychophysiological Interactions (GPPI): A Comparison to Standard Approaches. Neuroimage 2012, 61, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J. Emotion Regulation: Current Status and Future Prospects. Psychol. Inq. 2015, 26, 1–26. [Google Scholar] [CrossRef]
- van der Kolk, B.A.; Herron, N.; Hostetler, A. The history of trauma in psychiatry. Psychiatr. Clin. North Am. 1994, 17, 583–600. [Google Scholar] [CrossRef]
- Southward, M.W.; Wilson, A.C.; Cheavens, J.S. On What Do Therapists Agree? Assessing Therapist Evaluations of Emotion Regulation Strategy Effectiveness. Psychol. Psychother. Theory Res. Pract. 2020, 94, 231–246. [Google Scholar] [CrossRef]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Webe, J.; Ochsner, K.N. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb. Cortex 2014, 24, 2981–2990. [Google Scholar] [CrossRef]
- Gross, J.J. Handbook of Emotion Regulation, 2nd ed.; Gross, J.J., Ed.; The Guilford Press: New York, NY, USA, 2014. [Google Scholar]
- Scherer, K.R. The Dynamic Architecture of Emotion: Evidence for the Component Process Model. Cogn. Emot. 2009, 23, 1307–1351. [Google Scholar] [CrossRef]
- Augustine, A.A.; Hemenover, S.H. On the Relative Effectiveness of Affect Regulation Strategies: A Meta-Analysis. Cogn. Emot. 2009, 23, 1181–1220. [Google Scholar] [CrossRef]
- Bishop, S.R.; Lau, M.; Shapiro, S.; Carlson, L.; Anderson, N.D.; Carmody, J.; Segal, Z.V.; Abbey, S.; Speca, M.; Velting, D.; et al. Mindfulness: A Proposed Operational Definition. Clin. Psychol. Sci. Pract. 2004, 11, 230–241. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and Emotional Influences in Anterior Cingulate Cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Andrews-hanna, J.R.; Smallwood, J.; Spreng, R.N. The Default Network and Self-Generated Thought: Component Processes, Dynamic Control, and Clinical Relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, J.; Van Tol, M.J.; Goerlich-Dobre, K.S.; Gromann, P.M.; Swart, M.; De Haan, L.; Wiersma, D.; Bruggeman, R.; Krabbendam, L.; Aleman, A. Dissociable Morphometric Profiles of the Affective and Cognitive Dimensions of Alexithymia. Cortex 2014, 54, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, B.P.; Pospos, S.; Lavretsky, H. The Neural Mechanisms of Meditative Practices: Novel Approaches for Healthy Aging. Curr. Behav. Neurosci. Rep. 2016, 3, 328–339. [Google Scholar] [CrossRef]
- Kragel, P.A.; Labar, K.S. Somatosensory Representations Link the Perception of Emotional Expressions and Somatosensory Representations Link the Perception of Emotional Expressions and Sensory Experience. eNeuro 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Baur, V.; Hänggi, J.; Langer, N.; Jäncke, L. Resting-State Functional and Structural Connectivity within an Insula—Amygdala Route Specifically Index State and Trait Anxiety. Biol. Psychiatry 2013, 73, 85–92. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A Link between the Systems: Functional Differentiation and Integration within the Human Insula Revealed by Meta-Analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300. [Google Scholar] [CrossRef]
- Craig, A.D. How Do You Feel? Interoception: The Sense of the Physiological Condition of the Body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing Your Own Heart: Distinguishing Interoceptive Accuracy from Interoceptive Awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interoception and Emotion. Curr. Opin. Psychol. 2017, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Dusek, A.; Benson, H.; Mcgarvey, M.; Brian, T. Meditation Experience Is Associated with Increased Cortical Thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Ott, U.; Gard, T.; Hempel, H.; Weygandt, M.; Morgen, K.; Ho, B.K.; Vaitl, D. Investigation of Mindfulness Meditation Practitioners with Voxel-Based Morphometry. Soc. Cogn. Affect. Neurosci. 2008, 3, 55–61. [Google Scholar] [CrossRef]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural Network of Cognitive Emotion Regulation—An ALE Meta-Analysis and MACM Analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef]
- Morawetz, C.; Bode, S.; Derntl, B.; Heekeren, H.R. The Effect of Strategies, Goals and Stimulus Material on the Neural Mechanisms of Emotion Regulation: A Meta-Analysis of FMRI Studies. Neurosci. Biobehav. Rev. 2017, 72, 111–128. [Google Scholar] [CrossRef]
- Lutz, J.; Herwig, U.; Opialla, S.; Hittmeyer, A.; Jancke, L.; Rufer, M.; Grosse Holtforth, M.; Bruhl, A.B. Mindfulness and Emotion Regulation--an FMRI Study. Soc. Cogn. Affect. Neurosci. 2014, 9, 776–785. [Google Scholar] [CrossRef]
- Morawetz, C.; Bode, S.; Baudewig, J.; Heekeren, H.R. Effective Amygdala-Prefrontal Connectivity Predicts Individual Differences in Successful Emotion Regulation. Soc. Cogn. Affect. Neurosci. 2017, 12, 569–585. [Google Scholar] [CrossRef]
- Ledoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Baxter, M.G.; Murray, E.A.; Hall, W.J. The Amygdala and Reward. Nat. Rev. Neurosci. 2002, 3, 563. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The Cognitive Control of Emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Quirk, G.J.; Beer, J.S. Prefrontal Involvement in the Regulation of Emotion: Convergence of Rat and Human Studies. Curr. Opin. Neurobiol. 2006, 16, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.J. Trait Anxiety and Impoverished Prefrontal Control of Attention. Nat. Neurosci. 2009, 12, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Caldara, R.; Seghier, M.; Schuller, A.M.; Lazeyras, F.; Mayer, E. A Network of Occipito-Temporal Face-Sensitive Areas besides the Right Middle Fusiform Gyrus Is Necessary for Normal Face Processing. Brain 2003, 126, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.V.; Downing, P.E. The Neural Basis of Visual Body Perception. Nat. Rev. Neurosci. 2007, 8, 636–648. [Google Scholar] [CrossRef]
- Moro, V.; Urgesi, C.; Pernigo, S.; Lanteri, P.; Pazzaglia, M.; Aglioti, S.M. The Neural Basis of Body form and Body Action Agnosia. Neuron 2008, 60, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Taylor, S.F.; Fig, L.M.; Decker, L.R.; Koeppe, R.A.; Minoshima, S. Limbic Activation and Psychophysiologic Responses to Aversive Visual Stimuli: Interaction with Cognitive Task. Neuropsychopharmacology 2000, 23, 508–516. [Google Scholar] [CrossRef]
- Goldin, P.R.; McRae, K.; Ramel, W.; Gross, J.J. The Neural Bases of Emotion Regulation: Reappraisal and Suppression of Negative Emotion. Biol. Psychiatry 2008, 63, 577–586. [Google Scholar] [CrossRef]
- Wang, M.; Su, J.; Zhang, J.; Zhao, Y.; Yao, Q.; Zhang, Q. Visual Cortex and Cerebellum Hyperactivation during Negativen Picture Stimuli in Migraine Patients. Sci. Rep. 2017, 7, 41919. [Google Scholar] [CrossRef]
- Astafiev, S.V.; Stanley, C.M.; Shulman, G.L.; Corbetta, M. Extrastriate Body Area in Human Occipital Cortex Responds to the Performance of Motor Actions. Nat. Neurosci. 2004, 7, 542–548. [Google Scholar] [CrossRef]
- Masuda, A.; Sackett, C.F. Cognitive Defusion and Self-Relevant Negative Thoughts: Examining the Impact of a Ninety Year Old Technique. Behav. Res. Ther. 2004, 42, 477–485. [Google Scholar] [CrossRef]
- Blackledge, J.T.; Hayes, S.C. Emotion Regulation in Acceptance and Commitment Therapy. J. Clin. Psychol. 2001, 57, 243–255. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Hitchman, G.; Lei, X. Module Number of Default Mode Network: Inter-Subject Variability and Effects of Sleep Deprivation. Brain Res. 2015, 1596, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, N.; Liu, Y. Unconscious Emotion Regulation: Nonconscious Reappraisal Decreases Emotion- Related Physiological Reactivity during Frustration Unconscious Emotion Regulation: Nonconscious Reappraisal Decreases Emotion-Related Physiological Reactivity during Frustratio. Cogn. Emot. 2014, 29, 1042–1053. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hari, R.; Hietanen, J.K.; Glerean, E. Maps of Subjective Feelings. Proc. Natl. Acad. Sci. USA 2018, 115, 9198–9203. [Google Scholar] [CrossRef]
- Said, C.P.; Moore, C.D.; Engell, A.D.; Haxby, J.V. Distributed Representations of Dynamic Facial Expressions in the Superior Temporal Sulcus. J. Vis. 2010, 10, 11. [Google Scholar] [CrossRef]
- Saarimäki, H.; Gotsopoulos, A.; Jääskeläinen, I.P.; Lampinen, J.; Vuilleumier, P.; Hari, R.; Sams, M.; Nummenmaa, L. Discrete Neural Signatures of Basic Emotions. Cereb. Cortex 2016, 26, 2563–2573. [Google Scholar] [CrossRef]
| Watch Negative | Experiential Emotion Regulation | Cognitive Defusion | Watch Neutral | |
|---|---|---|---|---|
| Negative Rating | 3.44 (0.51) | 2.86 (0.53) | 3.18 (0.53) | 4.16 (0.40) |
| Valence Rating | 3.76 (1.03) | 3.34 (0.67) | 3.52 (0.72) | 4.39 (0.91) |
| Arousal Rating | 4.24 (1.35) | 4.39 (0.89) | 4.50 (1.10) | 3.61 (1.35) |
| Left Amygdala | 0.111(0.22) | 0.044 (0.20) | −0.024 (0.17) | −0.040 (0.16) |
| Right Amygdala | 0.045 (0.09) | 0.037 (0.09) | 0.006 (0.10) | 0.020 (0.11) |
| Brain Area | H | BA | MNI Coordinates | Cluster Size | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive activation for the rating response across all the conditions | |||||||
| Lingual Gyrus | R | 18 | 14 | −90 | −18 | 149 | 5.07 |
| L | −6 | −70 | 6 | 200 | 4.97 | ||
| Precentral Gyrus | L | 6 | −40 | 2 | 40 | 100 | 5.23 |
| Inferior Occipital Gyrus | L | 17 | −16 | −92 | −8 | 161 | 5.51 |
| Inferior Parietal Lobule | R | 7 | −28 | −56 | 44 | 103 | 5.30 |
| Brain Area | H | BA | MNI Coordinates | T | Cluster Size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Watch Negative Versus Watch Neutral | |||||||
| Parahippocampal Gyrus, posterior division | R | 35 | 20 | −28 | −18 | 5.81 | 75 |
| Lingual Gyrus | R | 14 | −42 | −12 | 4.87 | 76 | |
| Hippocampus | L | −26 | −22 | −10 | 5.41 | 234 | |
| Amygdala | R | 26 | −2 | −14 | 3.63 | 69 | |
| Thalamus | R | 8 | −8 | −2 | 5.25 | 198 | |
| Watch Neutral Versus Watch Negative | |||||||
| Angular cortex | R | 40 | 62 | −52 | 36 | 7.00 | 74 |
| Superior Occipital Cortex | R | 19 | 31 | −74 | 40 | 4.62 | 70 |
| Precuneus | R | 23 | 22 | −62 | 42 | 4.65 | 103 |
| Brain Area | H | BA | MNI Coordinates | T | Cluster Size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Experiential Emotion Regulation versus Watch Negative | |||||||
| Occipital Fusiform Gyrus | R | 18 | 20 | −82 | −16 | 5.78 | 170 |
| Fusiform | R | 37 | 36 | −50 | −16 | 4.74 | 78 |
| Inferior Frontal Gyrus, pars opercularis | R | 46 | 48 | 20 | 26 | 4.76 | 151 |
| Angular Gyrus | R | 40 | 60 | −46 | 32 | 7.49 | 196 |
| Postcentral Gyrus | L | 3 | −58 | −18 | 34 | 6.33 | 303 |
| Cingulate Gyrus, anterior division | L | 32/9/24 | −6 | 18 | 36 | 5.36 | 151 |
| Paracingulate Gyrus | L | −6 | 30 | 34 | 5.36 | 96 | |
| Lateral Occipital Cortex, Superior division | R | 7/19 | 30 | −72 | 40 | 5.69 | 173 |
| L | −26 | −74 | 42 | 5.28 | 191 | ||
| Cognitive Defusion versus Watch Negative | |||||||
| Temporal Occipital Fusiform Cortex | R | 37 | 36 | −48 | −14 | 7.16 | 180 |
| Occipital pole | R | 17 | 12 | −94 | 6 | 5.00 | 137 |
| Watch Negative versus Cognitive Defusion | |||||||
| Hippocampus | L | −28 | −22 | −10 | 5.20 | 74 | |
| Brainstem | L | −10 | −27 | −11 | 5.20 | 96 | |
| Thalamus | R | 5 | −7 | −4 | 5.85 | 113 | |
| Experiential Emotion Regulation versus Cognitive Defusion | |||||||
| Angular Gyrus | L | 40 | −53 | −51 | 33 | 5.28 | 85 |
| Brain Area | H | BA | MNI Coordinates | Cluster Size | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| gPPI for experiential emotion regulation with the left amygdala as the seed region | |||||||
| Frontal Pole | L | 10/46 | −38 | 44 | 20 | 132 | 5.95 |
| Inferior Frontal Gyrus, pars triangularis | L | 10 | −39 | 29 | 17 | 46 | 3.97 |
| Insular Cortex | L | 13 | −34 | 8 | 6 | 64 | 5.74 |
| gPPI for cognitive defusion with left amygdala as the seed region | |||||||
| Occipital Fusiform Gyrus | L | 18 | −30 | −86 | −16 | 84 | 6.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Vantieghem, I.; Dong, D.; Nemegeer, J.; De Mey, J.; Van Schuerbeek, P.; Marinazzo, D.; Vandekerckhove, M. Approaching or Decentering? Differential Neural Networks Underlying Experiential Emotion Regulation and Cognitive Defusion. Brain Sci. 2022, 12, 1215. https://doi.org/10.3390/brainsci12091215
Wang Y, Vantieghem I, Dong D, Nemegeer J, De Mey J, Van Schuerbeek P, Marinazzo D, Vandekerckhove M. Approaching or Decentering? Differential Neural Networks Underlying Experiential Emotion Regulation and Cognitive Defusion. Brain Sciences. 2022; 12(9):1215. https://doi.org/10.3390/brainsci12091215
Chicago/Turabian StyleWang, Yulin, Iris Vantieghem, Debo Dong, Johan Nemegeer, Johan De Mey, Peter Van Schuerbeek, Daniele Marinazzo, and Marie Vandekerckhove. 2022. "Approaching or Decentering? Differential Neural Networks Underlying Experiential Emotion Regulation and Cognitive Defusion" Brain Sciences 12, no. 9: 1215. https://doi.org/10.3390/brainsci12091215
APA StyleWang, Y., Vantieghem, I., Dong, D., Nemegeer, J., De Mey, J., Van Schuerbeek, P., Marinazzo, D., & Vandekerckhove, M. (2022). Approaching or Decentering? Differential Neural Networks Underlying Experiential Emotion Regulation and Cognitive Defusion. Brain Sciences, 12(9), 1215. https://doi.org/10.3390/brainsci12091215






