Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort
Abstract
:1. Introduction
2. Materials and Methods
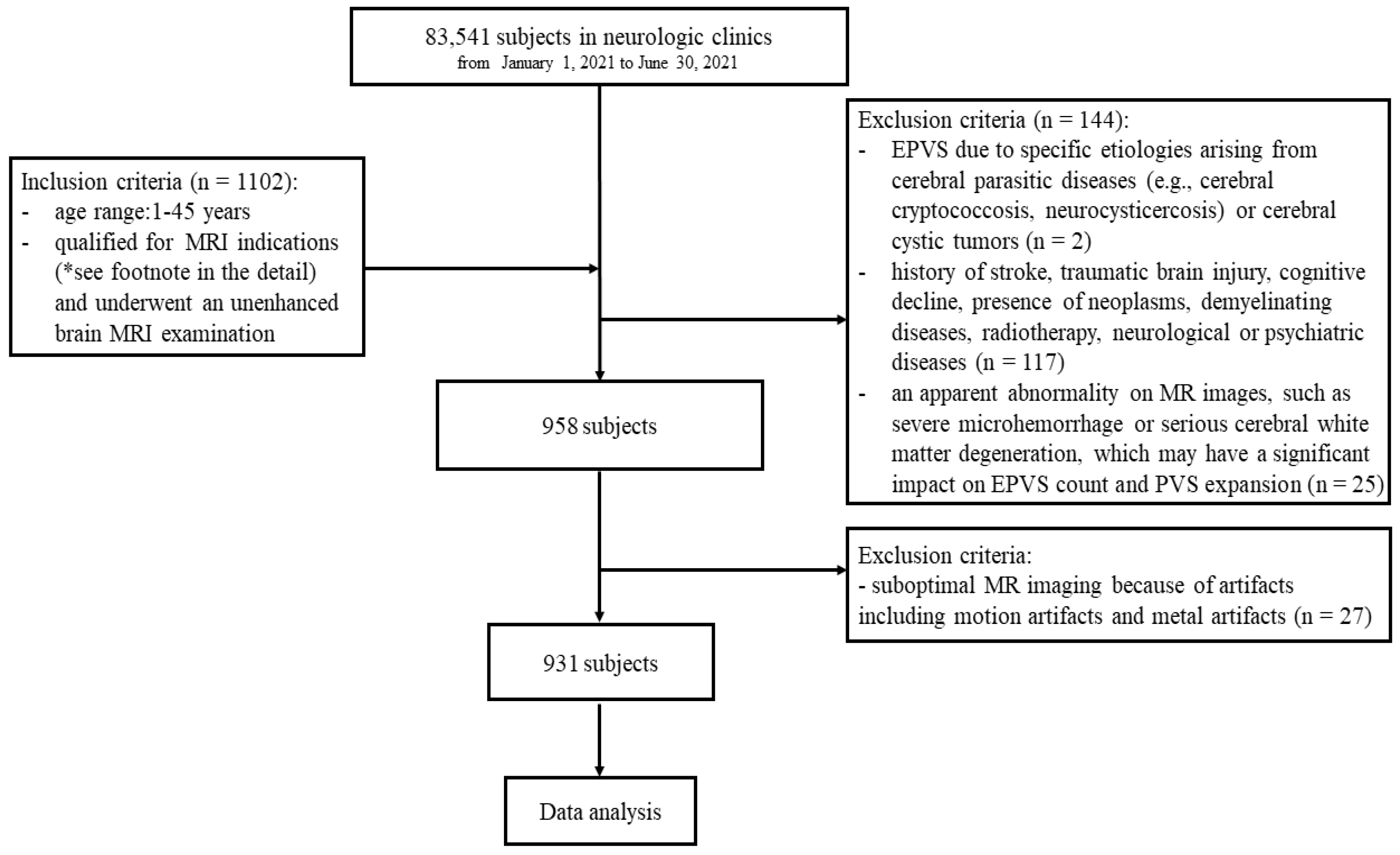
2.1. Study Population
2.2. Definition of Symptoms
2.3. MRI Acquisition
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Features
3.2. Chi-Square Testing
3.3. Logistic Regression Analysis
3.4. Kendall’s Rank Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPVS | enlarged perivascular spaces; |
| CSO | centrum semiovale; |
| BG | basal ganglia; |
| MB | midbrain; |
| PVS | perivascular spaces; |
| CSF | cerebrospinal fluid; |
| CSVD | cerebral small vessel disease; |
| AQP4 | aquaporin-4; |
| CAA | cerebral amyloid angiopathy; |
| BBB | blood–brain barrier; |
| OR | odds ratio; |
| CI | confidence interval. |
References
- Wang, M.-L.; Zhang, X.-X.; Yu, M.-M.; Li, W.-B.; Li, Y.-H. Prevalence of White Matter Hyperintensity in Young Clinical Patients. AJR Am. J. Roentgenol. 2019, 213, 667–671. [Google Scholar] [CrossRef]
- Spengos, K.; Vemmos, K. Risk Factors, Etiology, and Outcome of First-Ever Ischemic Stroke in Young Adults Aged 15 to 45—The Athens Young Stroke Registry. Eur. J. Neurol. 2010, 17, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Holter, K.E.; Kehlet, B.; Devor, A.; Sejnowski, T.J.; Dale, A.M.; Omholt, S.W.; Ottersen, O.P.; Nagelhus, E.A.; Mardal, K.-A.; Pettersen, K.H. Interstitial Solute Transport in 3D Reconstructed Neuropil Occurs by Diffusion Rather than Bulk Flow. Proc. Natl. Acad. Sci. USA 2017, 114, 9894–9899. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef]
- Weller, R.O.; Hawkes, C.A.; Kalaria, R.N.; Werring, D.J.; Carare, R.O. White Matter Changes in Dementia: Role of Impaired Drainage of Interstitial Fluid. Brain Pathol. Zurich Switz. 2015, 25, 63–78. [Google Scholar] [CrossRef]
- Rouhl, R.P.W.; van Oostenbrugge, R.J.; Knottnerus, I.L.H.; Staals, J.E.A.; Lodder, J. Virchow-Robin Spaces Relate to Cerebral Small Vessel Disease Severity. J. Neurol. 2008, 255, 692–696. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Tzourio, C.; Soumaré, A.; Mazoyer, B.; Dufouil, C.; Chabriat, H. Severity of Dilated Virchow-Robin Spaces Is Associated with Age, Blood Pressure, and MRI Markers of Small Vessel Disease: A Population-Based Study. Stroke 2010, 41, 2483–2490. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacLullich, A.M.J.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Rundek, T.; Ekind, M.S.V.; Sacco, R.L.; Wright, C.B. Perivascular Spaces Are Associated with Atherosclerosis: An Insight from the Northern Manhattan Study. AJNR Am. J. Neuroradiol. 2013, 34, 1711–1716. [Google Scholar] [CrossRef] [Green Version]
- Hilal, S.; Tan, C.S.; Adams, H.H.H.; Habes, M.; Mok, V.; Venketasubramanian, N.; Hofer, E.; Ikram, M.K.; Abrigo, J.; Vernooij, M.W.; et al. Enlarged Perivascular Spaces and Cognition: A Meta-Analysis of 5 Population-Based Studies. Neurology 2018, 91, e832–e842. [Google Scholar] [CrossRef]
- Granberg, T.; Moridi, T.; Brand, J.S.; Neumann, S.; Hlavica, M.; Piehl, F.; Ineichen, B.V. Enlarged Perivascular Spaces in Multiple Sclerosis on Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. J. Neurol. 2020, 267, 3199–3212. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.; Li, C.; Wang, R.; Chen, M.; Chen, H.; Su, W. Diffusion Tensor Imaging Along the Perivascular Space Index in Different Stages of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 773951. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Kim, H.J.; Fox, Z.; Jäger, H.R.; Wilson, D.; Charidimou, A.; Na, H.K.; Na, D.L.; Seo, S.W.; Werring, D.J. MRI-Visible Perivascular Space Location Is Associated with Alzheimer’s Disease Independently of Amyloid Burden. Brain J. Neurol. 2017, 140, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wei, G.; Cheng, M.; Jiang, H. Association between Enlarged Perivascular Spaces and Internal Carotid Artery Stenosis: A Study in Patients Diagnosed by Digital Subtraction Angiography. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104635. [Google Scholar] [CrossRef]
- Best, J.G.; Barbato, C.; Ambler, G.; Du, H.; Banerjee, G.; Wilson, D.; Shakeshaft, C.; Cohen, H.; Yousry, T.A.; Al-Shahi Salman, R.; et al. Association of Enlarged Perivascular Spaces and Anticoagulant-Related Intracranial Hemorrhage. Neurology 2020, 95, e2192–e2199. [Google Scholar] [CrossRef]
- Yang, S.; Qin, W.; Yang, L.; Fan, H.; Li, Y.; Yin, J.; Hu, W. The Relationship between Ambulatory Blood Pressure Variability and Enlarged Perivascular Spaces: A Cross-Sectional Study. BMJ Open 2017, 7, e015719. [Google Scholar] [CrossRef]
- Saeki, N.; Sato, M.; Kubota, M.; Uchino, Y.; Murai, H.; Nagai, Y.; Ishikura, H.; Nomura, S.; Matsuura, I.; Yamaura, A. MR Imaging of Normal Perivascular Space Expansion at Midbrain. AJNR Am. J. Neuroradiol. 2005, 26, 566–571. [Google Scholar]
- Yakushiji, Y.; Charidimou, A.; Hara, M.; Noguchi, T.; Nishihara, M.; Eriguchi, M.; Nanri, Y.; Nishiyama, M.; Werring, D.J.; Hara, H. Topography and Associations of Perivascular Spaces in Healthy Adults: The Kashima Scan Study. Neurology 2014, 83, 2116–2123. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular Spaces in the Brain: Anatomy, Physiology and Pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Hurford, R.; Charidimou, A.; Fox, Z.; Cipolotti, L.; Jager, R.; Werring, D.J. MRI-Visible Perivascular Spaces: Relationship to Cognition and Small Vessel Disease MRI Markers in Ischaemic Stroke and TIA. J. Neurol. Neurosurg. Psychiatry 2014, 85, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Pantoni, L. Cerebral Small Vessel Disease: From Pathogenesis and Clinical Characteristics to Therapeutic Challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, R.; Jiaerken, Y.; Wang, S.; Yu, W.; Hong, H.; Lian, C.; Li, K.; Zeng, Q.; Luo, X.; et al. Deep White Matter Hyperintensity Is Associated with the Dilation of Perivascular Space. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 2370–2380. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Dufouil, C.; Mazoyer, B.; Soumaré, A.; Ricolfi, F.; Tzourio, C.; Chabriat, H. Frequency and Location of Dilated Virchow-Robin Spaces in Elderly People: A Population-Based 3D MR Imaging Study. AJNR Am. J. Neuroradiol. 2011, 32, 709–713. [Google Scholar] [CrossRef]
- Francis, F.; Ballerini, L.; Wardlaw, J.M. Perivascular Spaces and Their Associations with Risk Factors, Clinical Disorders and Neuroimaging Features: A Systematic Review and Meta-Analysis. Int. J. Stroke Off. J. Int. Stroke Soc. 2019, 14, 359–371. [Google Scholar] [CrossRef]
- Chavda, V.; Chaurasia, B.; Fiorindi, A.; Umana, G.E.; Lu, B.; Montemurro, N. Ischemic Stroke and SARS-CoV-2 Infection: The Bidirectional Pathology and Risk Morbidities. Neurol. Int. 2022, 14, 391–405. [Google Scholar] [CrossRef]
- Dunbar, M.; Kirton, A. Perinatal Stroke. Semin. Pediatr. Neurol. 2019, 32, 100767. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Contin. Minneap. Minn 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Choi, E.Y.; Park, Y.W.; Lee, M.; Kim, M.; Lee, C.S.; Ahn, S.S.; Kim, J.; Lee, S.-K. Magnetic Resonance Imaging-Visible Perivascular Spaces in the Basal Ganglia Are Associated With the Diabetic Retinopathy Stage and Cognitive Decline in Patients With Type 2 Diabetes. Front. Aging Neurosci. 2021, 13, 666495. [Google Scholar] [CrossRef]
- Ergul, A.; Kelly-Cobbs, A.; Abdalla, M.; Fagan, S.C. Cerebrovascular Complications of Diabetes: Focus on Stroke. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 148–158. [Google Scholar] [CrossRef]
- Sink, K.M.; Divers, J.; Whitlow, C.T.; Palmer, N.D.; Smith, S.C.; Xu, J.; Hugenschmidt, C.E.; Wagner, B.C.; Williamson, J.D.; Bowden, D.W.; et al. Cerebral Structural Changes in Diabetic Kidney Disease: African American-Diabetes Heart Study MIND. Diabetes Care 2015, 38, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance Systems in the Brain-Implications for Alzheimer Disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Lysen, T.S.; Yilmaz, P.; Dubost, F.; Ikram, M.A.; de Bruijne, M.; Vernooij, M.W.; Luik, A.I. Sleep and Perivascular Spaces in the Middle-Aged and Elderly Population. J. Sleep Res. 2022, 31, e13485. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Del Brutto, V.J.; Castillo, P.R. Enlarged Basal Ganglia Perivascular Spaces and Sleep Parameters. A Population-Based Study. Clin. Neurol. Neurosurg. 2019, 182, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kylkilahti, T.M.; Berends, E.; Ramos, M.; Shanbhag, N.C.; Töger, J.; Markenroth Bloch, K.; Lundgaard, I. Achieving Brain Clearance and Preventing Neurodegenerative Diseases-A Glymphatic Perspective. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 2137–2149. [Google Scholar] [CrossRef]
- Qi, Y.; Lin, M.; Yang, Y.; Li, Y. Relationship of Visceral Adipose Tissue With Dilated Perivascular Spaces. Front. Neurosci. 2020, 14, 583557. [Google Scholar] [CrossRef]
- Ballerini, L.; Lovreglio, R.; Valdés Hernández, M.D.C.; Ramirez, J.; MacIntosh, B.J.; Black, S.E.; Wardlaw, J.M. Perivascular Spaces Segmentation in Brain MRI Using Optimal 3D Filtering. Sci. Rep. 2018, 8, 2132. [Google Scholar] [CrossRef] [Green Version]
| 0–15 years (n = 58) | 16–30 years (n = 258) | 31–45 years (n = 615) | ||
|---|---|---|---|---|
| Gender | Male, n (%) | 29 (50.0%) | 119 (46.1%) | 298 (48.5%) |
| Female, n (%) | 29 (50.0%) | 139 (53.9%) | 317 (51.5%) | |
| Vascular risk factors | Diabetes, n (%) | 0 (0.0%) | 3 (1.2%) | 34 (5.5%) |
| Hypertension, n (%) | 1 (1.7%) | 11 (4.3%) | 72 (11.7%) | |
| Hyperlipidemia, n (%) | 0 (0.0%) | 1 (0.4%) | 31 (5.0%) | |
| Symptoms | Absent, n (%) | 13 (22.4%) | 14 (5.4%) | 39 (6.3%) |
| Headache, n (%) | 16 (27.6%) | 81 (31.4%) | 147 (23.9%) | |
| Dizziness, n (%) | 4 (6.9%) | 43 (16.7%) | 124 (20.2%) | |
| Vertigo, n (%) | 0 (0.0%) | 12 (4.7%) | 24 (3.9%) | |
| Syncope, n (%) | 2 (3.4%) | 9 (3.5%) | 8 (1.3%) | |
| Light-headedness, n (%) | 1 (1.7%) | 6 (2.3%) | 23 (3.7%) | |
| Somatic symptoms, n (%) | 0 (0.0%) | 16 (6.2%) | 53 (8.6%) | |
| Hearing disturbances, n (%) | 2 (3.4%) | 7 (2.7%) | 23 (3.7%) | |
| Visual disturbances, n (%) | 1 (1.7%) | 5 (1.9%) | 14 (2.3%) | |
| Convulsions, n (%) | 1 (1.7%) | 18 (7.0%) | 30 (4.9%) | |
| Tremors, n (%) | 0 (0.0%) | 8 (3.1%) | 13 (2.1%) | |
| Sleeping disturbances, n (%) | 1 (1.7%) | 8 (3.1%) | 18 (2.9%) | |
| Unspecified, n (%) | 17 (29.3%) | 31 (12.0%) | 99 (16.1%) | |
| Prevalence | CSO-EPVS, n (%) | 43 (74.1%) | 255 (98.8%) | 614 (99.8%) |
| BG-EPVS, n (%) | 39 (67.2%) | 224 (86.8%) | 579 (94.1%) | |
| MB-EPVS, n (%) | 38 (65.5%) | 135 (52.3%) | 370 (60.2%) | |
| CSO-EPVS score | 0, n (%) | 15 (25.9%) | 3 (1.2%) | 1 (0.2%) |
| 1, n (%) | 29 (50.0%) | 150 (58.1%) | 306 (49.8%) | |
| 2, n (%) | 13 (22.4%) | 90 (34.9%) | 255 (41.5%) | |
| 3, n (%) | 1 (1.7%) | 12 (4.7%) | 49 (8.0%) | |
| 4, n (%) | 0 (0.0%) | 0 (0.0%) | 4 (0.7%) | |
| BG-EPVS score | 1, n (%) | 57 (98.3%) | 223 (86.4%) | 456 (74.1%) |
| 2, n (%) | 1 (1.7%) | 34 (13.2%) | 147 (23.9%) | |
| 3, n (%) | 0 (0.0%) | 0 (0.0%) | 7 (1.1%) | |
| 4, n (%) | 0 (0.0%) | 1 (0.4%) | 5 (0.8%) | |
| MB-EPVS score | 0, n (%) | 20 (34.5%) | 123 (47.7%) | 245 (39.8%) |
| 1, n (%) | 38 (65.5%) | 135 (52.3%) | 370 (60.2%) |
| CSO-EPVS | p | BG-EPVS | p | MB-EPVS | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low: 0–1 (n = 519) | High: 2–4 (n = 412) | Low: 1 (n = 734) | High: 2-4 (n = 197) | Low: 0 (n = 388) | High: 1 (n = 543) | ||||
| Age | <0.001 | <0.001 | 0.052 | ||||||
| 1–15 y, n (%) | 44 (8.5) [3.2] | 14 (3.4) [−3.2] | 57 (7.8) [3.7] | 1 (0.5) [−3.7] | 20 (5.2) [−1.1] | 38 (7.0) [1.1] | |||
| 16–30 y, n (%) | 156 (30.1) [1.8] | 102 (24.8) [−1.8] | 223 (30.4) [3.5] | 35 (17.8) [−3.5] | 123 (31.7) [2.3] | 135 (24.9) [−2.3] | |||
| 31–45 y, n (%) | 319 (61.5) [−3.3] | 296 (71.8) [3.3] | 454 (61.9) [−5.2] | 161 (81.7) [5.2] | 245 (63.1) [−1.6] | 370 (68.1) [1.6] | |||
| Male, n (%) | 235 (45.3) | 211 (51.2) | 0.072 | 342 (46.6) | 104 (52.8) | 0.122 | 180 (46.4) | 266 (49.0) | 0.434 |
| Diabetes, n (%) | 16 (3.1) | 21 (5.1) | 0.118 | 22 (3.0) | 15 (7.6) | 0.003 | 7 (1.8) | 30 (5.5) | 0.004 |
| Hypertension, n (%) | 32 (6.2) | 52 (12.6) | 0.001 | 46 (6.3) | 38 (19.3) | <0.001 | 13 (3.4) | 71 (13.1) | <0.001 |
| Hyperlipidemia, n (%) | 13 (2.5) | 19 (4.6) | 0.080 | 21 (2.9) | 11 (5.6) | 0.063 | 10 (2.6) | 22 (4.1) | 0.223 |
| CSO-EPVS | p | BG-EPVS | p | MB-EPVS | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low: 0–1 (n = 519) | High: 2–4 (n = 412) | Low: 1 (n = 734) | High: 2–4 (n = 197) | Low: 0 (n = 388) | High: 1 (n = 543) | ||||
| Absent, n (%) | 36 (6.9) | 30 (7.3) | 0.839 | 53 (7.2) | 13 (6.6) | 0.763 | 32 (8.2) | 34 (6.3) | 0.244 |
| Headache, n (%) | 128 (24.7) | 116 (28.2) | 0.229 | 195 (26.6) | 49 (24.9) | 0.631 | 83 (21.4) | 161 (29.7) | 0.005 |
| Dizziness, n (%) | 83 (16.0) | 88 (21.4) | 0.036 | 125 (17.0) | 46 (23.4) | 0.042 | 58 (14.9) | 113 (20.8) | 0.023 |
| Vertigo, n (%) | 22 (4.2) | 14 (3.4) | 0.509 | 30 (4.1) | 6 (3.0) | 0.501 | 21 (5.4) | 15 (2.8) | 0.039 |
| Syncope, n (%) | 11 (2.1) | 8 (1.9) | 0.849 | 16 (2.2) | 3 (1.5) | 0.563 | 7 (1.8) | 12 (2.2) | 0.666 |
| Light-headedness, n (%) | 15 (2.9) | 15 (3.6) | 0.519 | 21 (2.9) | 9 (4.6) | 0.228 | 11 (2.8) | 19 (3.5) | 0.572 |
| Somatic symptoms, n (%) | 40 (7.7) | 29 (7.0) | 0.699 | 51 (6.9) | 18 (9.1) | 0.298 | 35 (9.0) | 34 (6.3) | 0.113 |
| Hearing disturbance, n (%) | 20 (3.9) | 12 (2.9) | 0.434 | 25 (3.4) | 7 (3.6) | 0.920 | 18 (4.6) | 14 (2.6) | 0.089 |
| Visual disturbance, n (%) | 12 (2.3) | 8 (1.9) | 0.699 | 18 (2.5) | 2 (1.0) | 0.217 | 10 (2.6) | 10 (1.8) | 0.445 |
| Convulsion, n (%) | 29 (5.6) | 20 (4.9) | 0.619 | 41 (5.6) | 8 (4.1) | 0.395 | 25 (6.4) | 24 (4.4) | 0.173 |
| Tremor, n (%) | 14 (2.7) | 7 (1.7) | 0.308 | 17 (2.3) | 4 (2.0) | 0.811 | 9 (2.3) | 12 (2.2) | 0.912 |
| Sleep disturbance, n (%) | 18 (3.5) | 9 (2.2) | 0.246 | 24 (3.3) | 3 (1.5) | 0.195 | 14 (3.6) | 13 (2.4) | 0.276 |
| Unspecified, n (%) | 91 (17.5) | 56 (13.6) | 0.101 | 118 (16.1) | 29 (14.7) | 0.643 | 62 (16.0) | 85 (15.7) | 0.893 |
| OR | 95% CI | p | |
|---|---|---|---|
| CSO-EPVS | |||
| Age | 0.005 | ||
| 16–30 years | 1.910 | 0.991–3.682 | 0.053 |
| 31–45 years | 2.544 | 1.356–4.772 | 0.004 |
| Female | 0.857 | 0.655–1.120 | 0.258 |
| Diabetes | 1.307 | 0.651–2.624 | 0.451 |
| Hypertension | 1.824 | 1.128–2.951 | 0.014 |
| Hyperlipidemia | 1.260 | 0.590–2.691 | 0.550 |
| Dizziness | 1.225 | 0.865–1.734 | 0.253 |
| Unspecified | 0.778 | 0.533–1.137 | 0.195 |
| BG-EPVS | |||
| Age | <0.001 | ||
| 16–30 years | 8.466 | 1.133–63.266 | 0.037 |
| 31–45 years | 17.021 | 2.329–124.382 | 0.005 |
| Female | 0.921 | 0.659–1.288 | 0.632 |
| Diabetes | 1.829 | 0.888–3.768 | 0.101 |
| Hypertension | 2.766 | 1.695–4.514 | <0.001 |
| Hyperlipidemia | 0.991 | 0.442–2.223 | 0.983 |
| Dizziness | 1.253 | 0.840–1.870 | 0.268 |
| Sleep disturbance | 0.505 | 0.147–1.729 | 0.276 |
| MB-EPVS | |||
| Age | 0.087 | ||
| 16–30 years | 0.533 | 0.290–0.982 | 0.043 |
| 31–45 years | 0.676 | 0.378–1.210 | 0.187 |
| Diabetes | 2.938 | 1.214–6.956 | 0.014 |
| Hypertension | 4.137 | 2.324–8.018 | <0.001 |
| Headache | 1.807 | 1.272–2.556 | 0.001 |
| Dizziness | 1,574 | 1.059–2.340 | 0.025 |
| Vertigo | 0.667 | 0.327–1.360 | 0.265 |
| Somatic symptoms | 0.727 | 0.421–1.258 | 0.255 |
| Hearing disturbance | 0.632 | 0.298–1.341 | 0.232 |
| Convulsion | 0.883 | 0.478–1.631 | 0.691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Q.; Wang, M.; Wei, X.; Li, W. Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort. Brain Sci. 2022, 12, 1164. https://doi.org/10.3390/brainsci12091164
Zou Q, Wang M, Wei X, Li W. Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort. Brain Sciences. 2022; 12(9):1164. https://doi.org/10.3390/brainsci12091164
Chicago/Turabian StyleZou, Qiaoqiao, Mingliang Wang, Xiaoer Wei, and Wenbin Li. 2022. "Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort" Brain Sciences 12, no. 9: 1164. https://doi.org/10.3390/brainsci12091164
APA StyleZou, Q., Wang, M., Wei, X., & Li, W. (2022). Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort. Brain Sciences, 12(9), 1164. https://doi.org/10.3390/brainsci12091164







