Frontostriatal Functional Connectivity Underlies the Association between Punishment Sensitivity and Procrastination
Abstract
:1. Introduction
1.1. The Temporal Difference Model of Procrastination: Theory and Neural Correlates
1.2. The Role of Punishment in Theories of Procrastination
1.3. The Caudate: Nexus between Reward, Punishment, and Cognitive Control of Behavior
1.4. Punishment Sensitivity and Individual Differences in Procrastination
1.5. The Current Study
2. Materials and Method
2.1. Participants and Procedure
2.2. Measures
2.2.1. Procrastination
2.2.2. Punishment Sensitivity
2.2.3. fMRI Data Acquisition
2.3. VBM Analysis
2.3.1. Preprocessing
2.3.2. Second-Level Modeling Analysis
2.4. rsFC Analysis
2.4.1. Preprocessing
2.4.2. Functional Connectivity Analysis
3. Results
3.1. Behavioral Results
3.2. Neuroanatomical Correlates of Punishment Sensitivity
3.3. rsFC Results
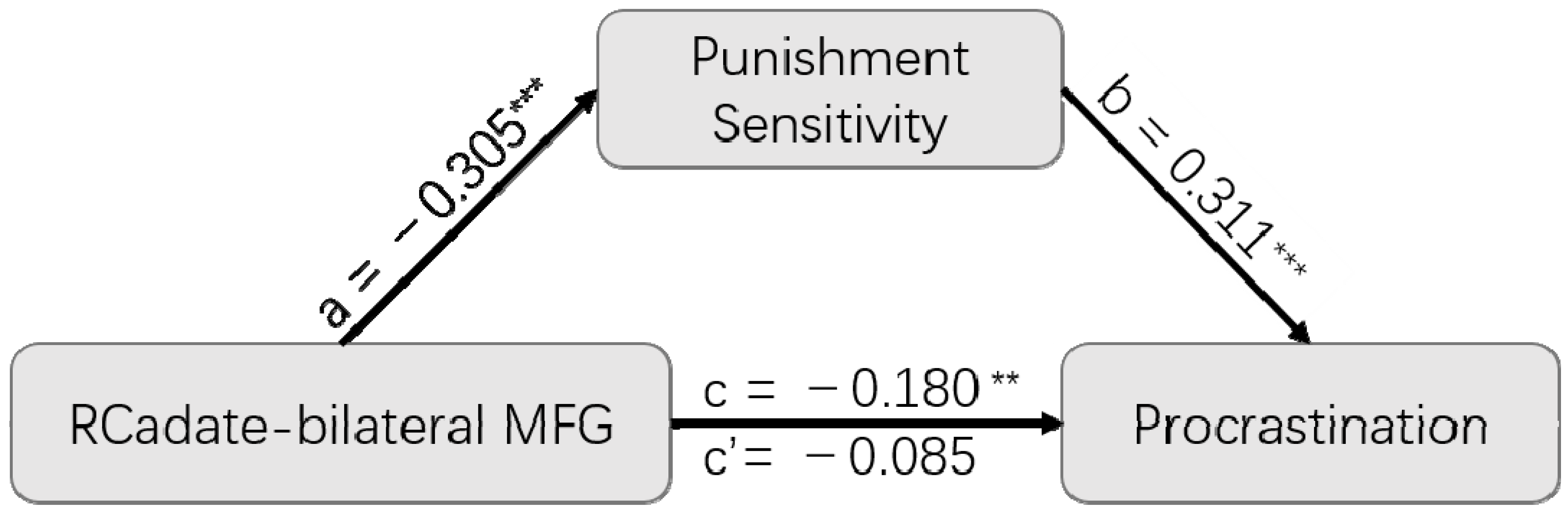
3.4. Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steel, P. The Nature of Procrastination: A Meta-Analytic and Theoretical Review of Quintessential Self-Regulatory Failure. Psychol. Bull. 2007, 133, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Steel, P.; Ferrari, J. Sex, Education and Procrastination: An Epidemiological Study of Procrastinators’ Characteristics from a Global Sample. Eur. J. Pers. 2013, 27, 51–58. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, T. Modeling Procrastination: Asymmetric Decisions to Act Between the Present and the Future. J. Exp. Psychol. Gen. 2019, 149, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, P.; Feng, T. To Do It Now or Later: The Cognitive Mechanisms and Neural Substrates Underlying Procrastination. WIREs Cogn. Sci. 2019, 10, e1492. [Google Scholar] [CrossRef]
- Zhang, S.; Verguts, T.; Zhang, C.; Feng, P.; Chen, Q.; Feng, T. Outcome Value and Task Aversiveness Impact Task Procrastination through Separate Neural Pathways. Cereb. Cortex 2021, 31, 3846–3855. [Google Scholar] [CrossRef]
- Zhang, S.; Becker, B.; Chen, Q.; Feng, T. Insufficient Task-Outcome Association Promotes Task Procrastination through a Decrease of Hippocampal–Striatal Interaction. Hum. Brain Mapp. 2019, 40, 597–607. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Chen, Q.; Feng, T. Neural Basis Responsible for Episodic Future Thinking Effects on Procrastination: The Interaction between the Cognitive Control Pathway and Emotional Processing Pathway. Cortex 2021, 145, 250–263. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, P.; Zhang, C.; Feng, T. Brain Morphological Dynamics of Procrastination: The Crucial Role of the Self-Control, Emotional, and Episodic Prospection Network. Cereb. Cortex 2020, 30, 2834–2853. [Google Scholar] [CrossRef]
- Tice, D.M.; Bratslavsky, E. Giving in to Feel Good: The Place of Emotion Regulation in the Context of General Self-Control. Psychol. Inq. 2000, 11, 149–159. [Google Scholar] [CrossRef]
- Tice, D.M.; Bratslavsky, E.; Baumeister, R.F. Emotional Distress Regulation Takes Precedence over Impulse Control: If You Feel Bad, Do It! J. Pers. Soc. Psychol. 2001, 80, 53–67. [Google Scholar] [CrossRef]
- Sirois, F.; Pychyl, T. Procrastination and the Priority of Short-Term Mood Regulation: Consequences for Future Self. Soc. Pers. Psychol. Compass 2013, 7, 115–127. [Google Scholar] [CrossRef]
- Sirois, F.M. Out of Sight, out of Time? A Meta-Analytic Investigation of Procrastination and Time Perspective. Eur. J. Pers. 2014, 28, 511–520. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Gray, J.A. A Critique of Eysenck’s Theory of Personality. In A Model for Personality; Springer: Berlin/Heidelberg, Germany, 1981; pp. 246–276. [Google Scholar]
- Samejima, K.; Ueda, Y.; Doya, K.; Kimura, M. Neuroscience: Representation of Action-Specific Reward Values in the Striatum. Science 2005, 310, 1337–1340. [Google Scholar] [CrossRef]
- Lau, B.; Glimcher, P.W. Value Representations in the Primate Striatum during Matching Behavior. Neuron 2008, 58, 451–463. [Google Scholar] [CrossRef]
- Santacruz, S.R.; Rich, E.L.; Wallis, J.D.; Carmena, J.M. Caudate Microstimulation Increases Value of Specific Choices. Curr. Biol. 2017, 27, 3375–3383.e3. [Google Scholar] [CrossRef]
- Hariri, A.R.; Brown, S.M.; Williamson, D.E.; Flory, J.D.; De Wit, H.; Manuck, S.B. Preference for Immediate over Delayed Rewards Is Associated with Magnitude of Ventral Striatal Activity. J. Neurosci. 2006, 26, 13213–13217. [Google Scholar] [CrossRef]
- Pessiglione, M.; Seymour, B.; Flandin, G.; Dolan, R.J.; Frith, C.D. Dopamine-Dependent Prediction Errors Underpin Reward-Seeking Behaviour in Humans. Nature 2006, 442, 1042–1045. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The Role of the Basal Ganglia in Learning and Memory: Neuropsychological Studies. Behav. Brain Res. 2009, 199, 53–60. [Google Scholar] [CrossRef]
- Seger, C.A. How Do the Basal Ganglia Contribute to Categorization? Their Roles in Generalization, Response Selection, and Learning via Feedback. Neurosci. Biobehav. Rev. 2008, 32, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Yoon, H.S.; Kim, H.; Hamann, S. Individual Differences in Sensitivity to Reward and Punishment and Neural Activity during Reward and Avoidance Learning. Soc. Cogn. Affect. Neurosci. 2015, 10, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Palminteri, S.; Justo, D.; Jauffret, C.; Pavlicek, B.; Dauta, A.; Delmaire, C.; Czernecki, V.; Karachi, C.; Capelle, L.; Durr, A.; et al. Critical Roles for Anterior Insula and Dorsal Striatum in Punishment-Based Avoidance Learning. Neuron 2012, 76, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Ide, J.S.; Li, H.T.; Chen, Y.; Le, T.M.; Li, C.S.P.; Zhornitsky, S.; Li, C.S.R. Gray Matter Volumetric Correlates of Behavioral Activation and Inhibition System Traits in Children: An Exploratory Voxel-Based Morphometry Study of the ABCD Project Data. Neuroimage 2020, 220, 117085. [Google Scholar] [CrossRef]
- Sadeh, T.; Shohamy, D.; Levy, D.R.; Reggev, N.; Maril, A. Cooperation between the Hippocampus and the Striatum during Episodic Encoding. J. Cogn. Neurosci. 2011, 23, 1597–1608. [Google Scholar] [CrossRef]
- Braunlich, K.; Seger, C. The Basal Ganglia. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 135–148. [Google Scholar] [CrossRef]
- Kim, D.; Park, G.Y.; O′Doherty, J.P.; Lee, S.W. Task Complexity Interacts with State-Space Uncertainty in the Arbitration between Model-Based and Model-Free Learning. Nat. Commun. 2019, 10, 5738. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A. Précis of The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Behav. Brain Sci. 1982, 5, 469–484. [Google Scholar] [CrossRef]
- Hewig, J.; Hagemann, D.; Seifert, J.; Naumann, E.; Bartussek, D. The Relation of Cortical Activity and BIS/BAS on the Trait Level. Biol. Psychol. 2006, 71, 42–53. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Tops, M.; Kostermans, E.; De Cremer, D. Sensitivity to Punishment and Reward Omission: Evidence from Error-Related ERP Components. Biol. Psychol. 2008, 79, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Bacon, A.M. At Long Last—A Reinforcement Sensitivity Theory Explanation of Procrastination. J. Individ. Differ. 2019, 40, 234–241. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, R.; Xu, T.; Zhou, F.; Feng, T. The Effect of Conscientiousness on Procrastination: The Interaction between the Self-Control and Motivation Neural Pathways. Hum. Brain Mapp. 2021, 42, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Svartdal, F.; Steel, P. Irrational Delay Revisited: Examining Five Procrastination Scales in a Global Sample. Front. Psychol. 2017, 8, 1927. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.H. At Last, My Research Article on Procrastination. J. Res. Pers. 1986, 20, 474–495. [Google Scholar] [CrossRef]
- McCown, W.; Johnson, J.; Petzel, T. Procrastination, a Principal Components Analysis. Pers. Individ. Differ. 1989, 10, 197–202. [Google Scholar] [CrossRef]
- Mann, L.; Burnett, P.; Radford, M.; Ford, S. The Melbourne Decision Making Questionnaire: An Instrument for Measuring Patterns for Coping with Decisional Conflict. J. Behav. Decis. Mak. 1997, 10, 1–19. [Google Scholar] [CrossRef]
- Steel, P. Arousal, Avoidant and Decisional Procrastinators: Do They Exist? Pers. Individ. Differ. 2010, 48, 926–934. [Google Scholar] [CrossRef]
- Corr, P.J. Reinforcement Sensitivity Theory of Personality Questionnaires: Structural Survey with Recommendations. Pers. Individ. Differ. 2016, 89, 60–64. [Google Scholar] [CrossRef]
- Li, J.; Tian, Y.; Ding, L.; Zou, H.; Ren, Z.; Shi, L.; Feathers, D.; Wang, N. Simulating Extreme Environments: Ergonomic Evaluation of Chinese Pilot Performance and Heat Stress Tolerance. Work 2015, 51, 215–222. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Chen, Z. Effects of the Behavioral Inhibition System (BIS), Behavioral Activation System (BAS), and Emotion Regulation on Depression: A One-Year Follow-up Study in Chinese Adolescents. Psychiatry Res. 2015, 230, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Unified Segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Peelle, J.E.; Cusack, R.; Henson, R.N.A. Adjusting for Global Effects in Voxel-Based Morphometry: Gray Matter Decline in Normal Aging. Neuroimage 2012, 60, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Zhang, D.; Snyder, A.Z.; Raichle, M.E. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J. Neurophysiol. 2009, 101, 3270–3283. [Google Scholar] [CrossRef]
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. PLoS ONE 2011, 6, e25031. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Schwarzkopf, D.S.; Kanai, R.; Rees, G. Reciprocal Anatomical Relationship between Primary Sensory and Prefrontal Cortices in the Human Brain. J. Neurosci. 2011, 31, 9472–9480. [Google Scholar] [CrossRef]
- Corr, P.J. Reinforcement Sensitivity Theory and Personality. Neurosci. Biobehav. Rev. 2004, 28, 317–332. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Feng, T. Identifying the Neural Substrates of Procrastination: A Resting-State FMRI Study. Sci. Rep. 2016, 6, 33203. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, P.; Guo, Y.; Feng, T. The Neural Substrates of Procrastination: A Voxel-Based Morphometry Study. Brain Cogn. 2018, 121, 11–16. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-Analytic Evidence for a Superordinate Cognitive Control Network Subserving Diverse Executive Functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Peters, J.; Büchel, C. The Neural Mechanisms of Inter-Temporal Decision-Making: Understanding Variability. Trends Cogn. Sci. 2011, 15, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.H.; Gopalakrishnan, A.; Hazlett, C.J.; Woldorff, M.G. Dorsal Anterior Cingulate Cortex Resolves Conflict from Distracting Stimuli by Boosting Attention toward Relevant Events. Cereb. Cortex 2005, 15, 229–237. [Google Scholar] [CrossRef]
- Koechlin, E.; Corrado, G.; Pietrini, P.; Grafman, J. Dissociating the Role of the Medial and Lateral Anterior Prefrontal Cortex in Human Planning. Proc. Natl. Acad. Sci. USA 2000, 97, 7651–7656. [Google Scholar] [CrossRef] [PubMed]
- Benoit, R.G.; Gilbert, S.J.; Frith, C.D.; Burgess, P.W. Rostral Prefrontal Cortex and the Focus of Attention in Prospective Memory. Cereb. Cortex 2012, 22, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Momennejad, I.; Haynes, J.D. Human Anterior Prefrontal Cortex Encodes the “what” and “When” of Future Intentions. Neuroimage 2012, 61, 139–148. [Google Scholar] [CrossRef]
- Volman, I.; Roelofs, K.; Koch, S.; Verhagen, L.; Toni, I. Anterior Prefrontal Cortex Inhibition Impairs Control over Social Emotional Actions. Curr. Biol. 2011, 21, 1766–1770. [Google Scholar] [CrossRef]
- Baler, R.D.; Volkow, N.D. Drug Addiction: The Neurobiology of Disrupted Self-Control. Trends Mol. Med. 2006, 12, 559–566. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Wagner, D.D. Cognitive Neuroscience of Self-Regulation Failure. Trends Cogn. Sci. 2011, 15, 132–139. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The Cognitive Control of Emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Hare, T.A.; Camerer, C.F.; Rangel, A. Self-Control in Decision-Making Involves Modulation of the VmPFC Valuation System. Science 2009, 324, 646–648. [Google Scholar] [CrossRef] [Green Version]
- Mano, H.; Yoshida, W.; Shibata, K.; Zhang, S.; Koltzenburg, M.; Kawato, M.; Seymour, B. Thermosensory Perceptual Learning Is Associated with Structural Brain Changes in Parietal-Opercular (SII) Cortex. J. Neurosci. 2017, 37, 9380–9388. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Aihara, T.; Shimokawa, T.; Yamashita, O. Large-Scale Brain Network Associated with Creative Insight: Combined Voxel-Based Morphometry and Resting-State Functional Connectivity Analyses. Sci. Rep. 2018, 8, 6477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, Z.; Liu, P.; Feng, T. The Neural Substrates Responsible for How Trait Anxiety Affects Delay Discounting: Right Hippocampal and Cerebellar Connectivity with Bistable Right Inferior Parietal Lobule. Psychophysiology 2020, 57, e13495. [Google Scholar] [CrossRef] [PubMed]

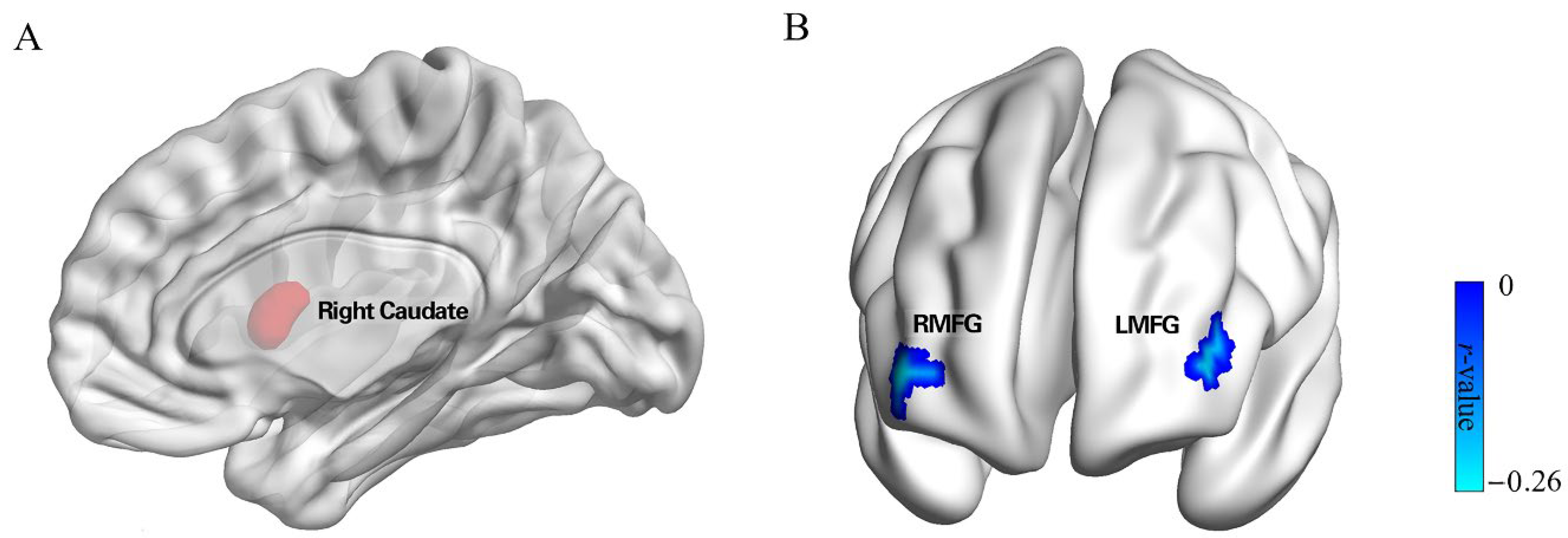
| Seed | Region | MNI | Voxels | Peak Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Punishment Sensitivity | Bilateral Cerebellum | 4.5 | −51 | −3 | 966 | 3.725 |
| Right Caudate | 13.5 | 7.5 | 10.5 | 655 | 3.622 | |
| Left Fusiform | −40.5 | −55.5 | −12 | 728 | −3.34 | |
| Seed | Region | MNI | Voxels | Peak Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right Caudate | Right MFG | 39 | 54 | 3 | 41 | −0.258 |
| Left MFG | −42 | 48 | 6 | 45 | −0.250 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Luo, J.; Huo, H.; Seger, C.A.; Chen, Q. Frontostriatal Functional Connectivity Underlies the Association between Punishment Sensitivity and Procrastination. Brain Sci. 2022, 12, 1163. https://doi.org/10.3390/brainsci12091163
Dong W, Luo J, Huo H, Seger CA, Chen Q. Frontostriatal Functional Connectivity Underlies the Association between Punishment Sensitivity and Procrastination. Brain Sciences. 2022; 12(9):1163. https://doi.org/10.3390/brainsci12091163
Chicago/Turabian StyleDong, Wenshan, Jie Luo, Hangfeng Huo, Carol A. Seger, and Qi Chen. 2022. "Frontostriatal Functional Connectivity Underlies the Association between Punishment Sensitivity and Procrastination" Brain Sciences 12, no. 9: 1163. https://doi.org/10.3390/brainsci12091163
APA StyleDong, W., Luo, J., Huo, H., Seger, C. A., & Chen, Q. (2022). Frontostriatal Functional Connectivity Underlies the Association between Punishment Sensitivity and Procrastination. Brain Sciences, 12(9), 1163. https://doi.org/10.3390/brainsci12091163





