Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges
Abstract
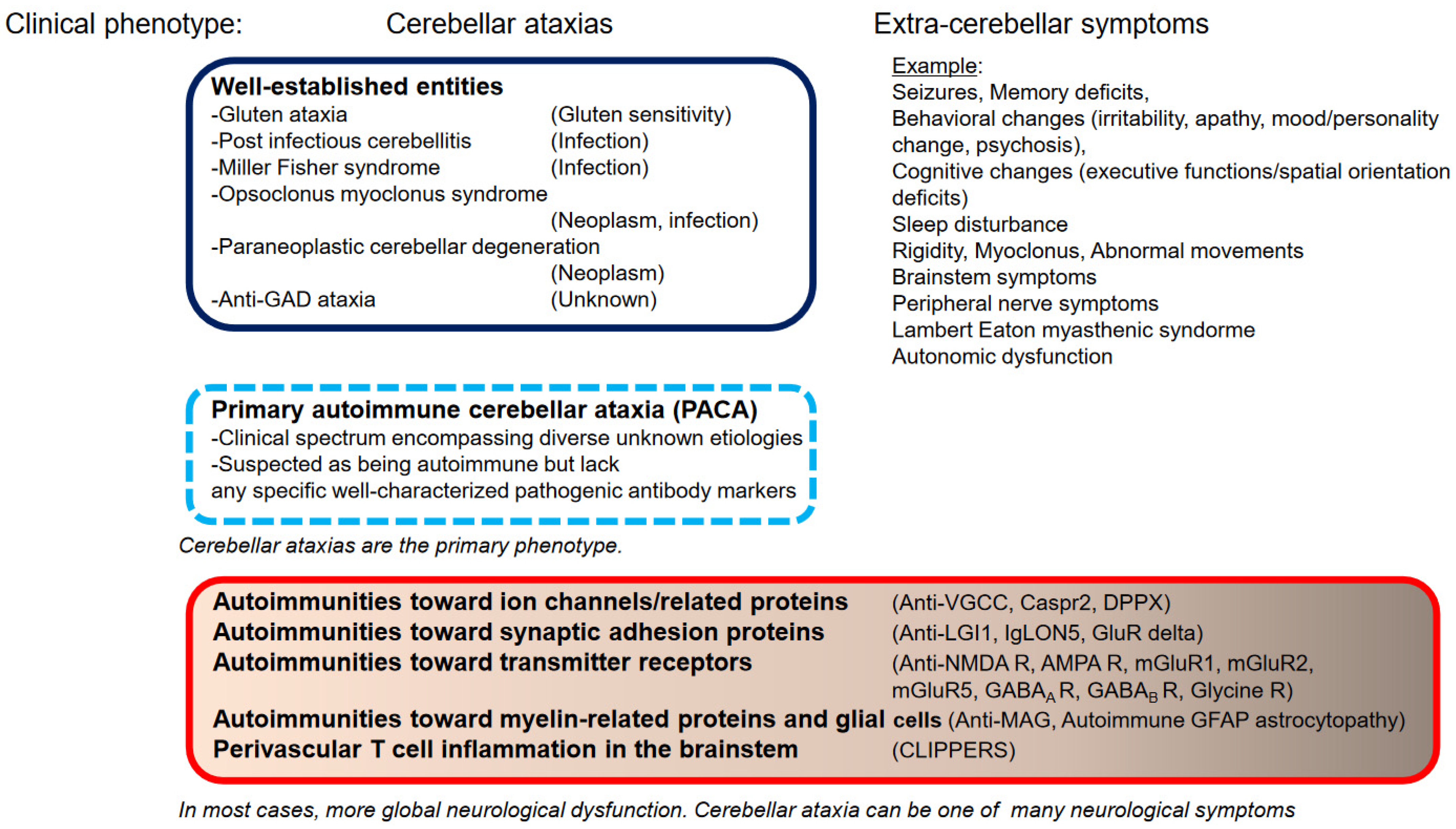
1. Introduction
2. CA Associated with Autoantibodies against Ion Channels and Related Proteins
2.1. Anti-VGCC Ataxia
2.2. Anti-Caspr2 Ataxia
2.3. Anti-DPPX Ataxia
3. CA Associated with Autoantibodies against Synaptic Adhesion/Organizing Proteins
3.1. Anti-LGI1 Ataxia
3.2. Anti-IgLON5 Ataxia
3.3. Anti-GluR Delta Ataxia
4. CA Associated with Autoantibodies against Transmitter Receptors
4.1. Anti-NMDA R and Anti-AMPA R Ataxias
4.2. Anti-mGluR1 Ataxia
4.3. Anti-mGluR2 Ataxia
4.4. Anti-mGluR5 Ataxia
4.5. Anti-GABAA R and anti-GABAB R Ataxias
4.6. Anti-Glycine R Ataxia
5. CA Associated with Autoantibodies against Myelin-Related Proteins
Anti-MAG Ataxia
6. CA Associated with Autoimmunity against Glial Cells and Perivascular T Cells in the Brainstem
6.1. Autoimmune GFAP Astrocytopathy
6.2. CLIPPERS
7. Primary Autoimmune Cerebellar Ataxia (PACA)
7.1. Molecular Characterization of Autoantigens Found in PACA
7.2. Significance of Non-Specific Autoantibodies Commonly Found in Autoimmune Diseases
8. Conclusions
8.1. CAs as Predominant Clinical Feature or Part of a More Diverse Presentation
8.2. Well-Characterized or Poorly Characterized Autoantibodies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hadjivassiliou, M. Immune-mediated acquired ataxias. Handb. Clin. Neurol. 2012, 103, 189–199. [Google Scholar]
- Mitoma, H.; Adhikari, K.; Aeschlimann, D.; Chattopadhyay, P.; Hadjivassiliou, M.; Hampe, C.S.; Honnorat, J.; Joubert, B.; Kakei, S.; Lee, J.; et al. Consensus Paper: Neuroimmune mechanisms of cerebellar ataxias. Cerebellum 2016, 15, 213–232. [Google Scholar] [CrossRef]
- Mitoma, H.; Hadjivassiliou, M.; Honnorat, J. Guidelines for treatment of immune-mediated cerebellar ataxias. Cerebellum Ataxias 2015, 2, 14. [Google Scholar] [CrossRef]
- Joubert, B.; Rotásky, J.; Honnorat, J. Nonparaneoplastic autoimmune cerebellar ataxia. Handb. Clin. Neurol. 2018, 155, 313–332. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M.; Hadjivassiliou, M. Immune-mediated cerebellar ataxias: Clinical diagnosis and treatment based on immunological and physiological mechanisms. J. Mov. Disord. 2021, 14, 10–28. [Google Scholar] [CrossRef]
- Muñiz-Castillo, S.; Honnorat, J. Paraneoplastic neurological syndromes. In Neuroimmune Diseases: From Cells to the Living Brain; Mitoma, H., Manto, M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 439–486. [Google Scholar]
- Hadjivassiliou, M.; Martindale, J.; Shanmugarajah, P.; Grünewald, R.A.; Sarrigiannis, P.G.; Beauchamp, N.; Garrard, K.; Warburton, R.; Sanders, D.S.; Friend, D.; et al. Causes of progressive cerebellar ataxia: Prospective evaluation of 1500 patients. J. Neurol. Neurosurg. Psychiatry 2017, 88, 301–309. [Google Scholar] [CrossRef]
- Klockgether, T. Sporadic ataxia with adult onset: Classification and diagnostic criteria. Lancet Neurol. 2010, 9, 94–104. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Mitoma, H.; Manto, M. Autoimmune ataxia. In Neuroimmune Diseases: From Cells to the Living Brain; Mitoma, H., Manto, M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 599–620. [Google Scholar]
- Graus, F.; Saiz, A.; Dalmau, J. Antibodies and neuronal autoimmune disorders of the CNS. J. Neurol. 2010, 257, 509–517. [Google Scholar] [CrossRef]
- Lancaster, E.; Martinex-Hernandez, E.; Dalmau, J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011, 77, 179–189. [Google Scholar] [CrossRef]
- Dalmau, J.; Rosenfeld, M.R. Autoimmune encephalitis update. Neuro. Oncol. 2014, 16, 771–778. [Google Scholar] [CrossRef]
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to synaptic receptors and neuronal cell surface protein in autoimmune diseases of the central nervous system. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Novo, A.C.; Venegas Pérez, B. Autoimmune glial fibrillary acidic protein astropathy presented as ataxia, myoclonus and bulbar syndrome: A case report and review of the literature. BMJ Neurol. Open 2021, 3, e000142. [Google Scholar] [CrossRef]
- Pittock, S.J.; Debruyne, J.; Krecke, K.N.; Giannini, C.; van den Ameele, J.; De Herdt, V.; McKeon, A.; Fealey, R.D.; Weinshenker, B.G.; Aksamit, A.J.; et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2010, 133, 2626–2634. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Boscolo, S.; Tongiorgi, E.; Grunewald, R.A.; Sharrack, B.; Sanders, D.S.; Woodroofe, N.; Davies-Jones, G.A. Cerebellar ataxia as a possible organ specific autoimmune disease. Mov. Disord. 2008, 23, 1270–1277. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Graus, F.; Honnorat, J.; Jarius, S.; Titulaer, M.; Manto, M.; Hoggard, N.; Sarrigiannis, P.; Mitoma, H. Diagnostic criteria for primary autoimmune cerebellar ataxia (PACA)-Guidelines from an International Task Force on Immune Mediated Cerbellar Ataxia. Cerebellum 2020, 19, 605–610. [Google Scholar] [CrossRef]
- Clouston, P.D.; Saper, C.B.; Arbizu, T.; Johnston, I.; Lang, B.; Newsom-Davis, J.; Posner, J.B. Paraneoplastic cerebellar degeneration. III. Cerebellar degeneration, cancer, and the Lambert-Eaton myasthenic syndrome. Neurology 1992, 42, 1944–1950. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. ‘Medusa-head ataxia’: The expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 2: Anti-PKC-gamma, anti-GluR-delta2, anti-Ca/ARHGAP26 and anti-VGCC. J. Neuroinflamm. 2015, 12, 167. [Google Scholar] [CrossRef]
- Graus, F.; Lang, B.; Pozo-Rosich, P.; Saiz, A.; Casamitjana, R.; Vincent, A. P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology 2002, 59, 764–766. [Google Scholar] [CrossRef]
- Mason, W.P.; Graus, F.; Lang, B.; Honnorat, J.; Delattre, J.Y.; Valldeoriola, F.; Antoine, J.C.; Rosenblum, M.K.; Rosenfeld, M.R.; Newsom-Davis, J.; et al. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain 1997, 120, 1279–1300. [Google Scholar] [CrossRef]
- Burk, K.; Wick, M.; Roth, G.; Decker, P.; Voltz, R. Antineuronal antibodies in sporadic late-onset cerebellar ataxia. J. Neurol. 2010, 257, 59–62. [Google Scholar] [CrossRef]
- Liao, Y.J.; Safa, P.; Chen, Y.R.; Sobel, R.A.; Boyden, E.S.; Tsien, R.W. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Saint-Martin, M.; Pieters, A.; Déchelotte, B.; Malleval, C.; Pinatel, D.; Pascual, O.; Karagogeos, D.; Honnorat, J.; Pellier-Monnin, V.; Noraz, N. Impact of anti-CASPR2 autoantibodies from patients with autoimmune encephalitis on CASPR2/TAG-1 interaction and Kv1 expression. J. Autoimmun. 2019, 103, 102284. [Google Scholar] [CrossRef] [PubMed]
- van Sonderen, A.; Ariño, H.; Petit-Pedrol, M.; Leypoldt, F.; Körtvélyessy, P.; Wandinger, K.P.; Lancaster, E.; Wirtz, P.W.; Schreurs, M.W.; Sillevis Smitt, P.A.; et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 2016, 87, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.; Gobert, F.; Thomas, L.; Saint-Martin, M.; Desestret, V.; Convers, P.; Rogemond, V.; Picard, G.; Ducray, F.; Psimaras, D.; et al. Autoimmune episodic ataxia with anti-CASPR2 antibody-associated encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 14, e371. [Google Scholar] [CrossRef]
- Muñiz-Castrillo, S.; Joubert, B.; Elsensohn, M.-H.; Anne-Laurie Pinto, A.-L.; Saint-Martin, M.; Vogrig, A.; Picard, G.; Rogemond, V.; Dubois, V.; Tamouza, R.; et al. Anti-CASPR2 clinical phenotypes correlate with HLA and immunological features. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1076–1084. [Google Scholar] [CrossRef]
- Patterson, K.R.; Dalmalu, J.; Lancaster, E. Mechanisms of Caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Ann. Neurol. 2018, 83, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Tobin, W.O.; Lennon, V.A.; Komorowski, L.; Probst, C.; Clardy, S.L.; Aksamit, A.J.; Lucchinetti, C.F.; Matsumoto, J.Y.; Pittock, S.J.; Sandroni, P.; et al. DPPX potassium channel antibody; frequency, clinical accompaniments and outcomes in 20 patients. Neurology 2014, 83, 1797–1803. [Google Scholar] [CrossRef]
- Boronat, A.; Gelfand, J.M.; Gresha-Arribas, N.; Jeong, H.-Y.; Walsh, M.; Roberts, K.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R.; Graus, F.; et al. Encephalitis and antibodies to DPPX, a subunit of Kv4.2 potassium channels. Ann. Neurol. 2013, 73, 120–128. [Google Scholar] [CrossRef]
- Kaulin, Y.A.; De Santiago-Castillo, J.A.; Rocha, C.A.; Nadal, M.S.; Rudy, B.; Covarrubias, M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4. 2 channels. J. Neurosci. 2009, 29, 3242–3251. [Google Scholar] [CrossRef]
- Petit-Pedrol, M.; Sell, J.; Planagumà, J.; Mannara, F.; Radosevic, M.; Haselmann, H.; Ceanga, M.; Sabater, L.; Spatola, M.; Soto, D.; et al. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain 2018, 141, 3144–3159. [Google Scholar] [CrossRef]
- Herranz-Pérez, V.; Olucha-Bordonau, F.E.; Morante-Redolat, J.M.; Pérez-Tur, J. Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res. 2010, 1307, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.R.; Alexander, S.; Waters, P.; Kleopa, K.A.; Pettingill, P.; Zuliani, L.; Peles, E.; Buckley, C.; Lang, B.; Vincent, A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010, 133, 2734–2748. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.; Kas, A.; Apartis, E.; Chami, L.; Rogemond, V.; Levy, P.; Psimaras, D.; Habert, M.O.; Baulac, M.; Delattre, J.Y.; et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 2016, 139, 1079–1093. [Google Scholar] [PubMed]
- Steriade, C.; Day, G.S.; Lee, L.; Murray, B.J.; Fritzler, M.J.; Keith, J. LGI1 autoantibodies associated with cerebellar degeneration. Neuropathol. Appl. Neurobiol. 2014, 40, 645–649. [Google Scholar] [CrossRef]
- Gaig, C.; Graus, F.; Compta, Y.; Högl, B.; Bataller, L.; Brüggemann, N.; Giordana, C.; Heidbreder, A.; Kotschet, K.; Lewerenz, J.; et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 2017, 88, 1736–1743. [Google Scholar] [CrossRef]
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef]
- Gaig, C.; Compta, Y. Neurological profiles beyond the sleep disorder in patients with anti-IgLON5 disease. Curr. Opin. Neurol. 2019, 32, 493–499. [Google Scholar] [CrossRef]
- Shambrook, P.; Hesters, A.; Marois, C.; Zemba, D.; Servan, J.; Gaymard, B.; Pico, F.; Delorme, C.; Lubetzki, C.; Arnulf, I.; et al. Delyed benefit from aggressive immunotherapy in waxing and waning anti-IgLON5 disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1009. [Google Scholar] [CrossRef]
- Yuzaki, M. Two classes of secreted synaptic organizers in the central nervous system. Annu. Rev. Physiol. 2018, 80, 243–262. [Google Scholar] [CrossRef]
- Shimokaze, T.; Kato, M.; Yoshimura, Y.; Takahashi, Y.; Hayasaka, K. A case of acute cerebellitis accompanied by autoantibodies against glutamate receptor δ2. Brain Dev. 2007, 29, 224–226. [Google Scholar] [CrossRef]
- Shiihara, T.; Kato, M.; Konno, A.; Takahashi, Y.; Hayasaka, K. Acute cerebellar ataxia and consecutive cerebellitis produced by glutamate receptor δ2 autoantibody. Brain Dev. 2007, 29, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Launey, T.; Mikawa, S.; Torashima, T.; Yanagihara, D.; Kasaura, T.; Miyamoto, A.; Yuzaki, M. New role of δ2-glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat. Neurosci. 2003, 6, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Lancaster, E.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011, 10, 63–74. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef]
- Leypoldt, F.; Armangue, T.; Dalmau, J. Autoimmune encephalopathies. Ann. N. Y. Acad. Sci. 2015, 1338, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Poorthuis, M.H.F.; van Rooij, J.L.M.; Koch, A.H.; Verdonkschot, A.E.M.; Leembruggen, M.M.; Titulaer, M.J. Cerebellar ataxia as a presenting symptom in a patient with anti-NMDA receptor encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e579. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.A.; Gleichman, A.J.; Lynch, D.R. Glutamatergic autoencephalitides: An emerging field. J. Neural Transm. 2014, 121, 957–968. [Google Scholar] [CrossRef][Green Version]
- Höftberger, R.; van Sonderen, A.; Leypoldt, F.; Houghton, D.; Geschwind, M.; Gelfand, J.; Paredes, M.; Sabater, L.; Saiz, A.; Titulaer, M.J.; et al. Encephalitis and AMPA receptor antibodies. Neurology 2015, 84, 2403–2412. [Google Scholar] [CrossRef]
- Lai, M.; Hughes, E.G.; Peng, X.; Zhou, L.; Gleichman, A.J.; Shu, H.; Matà, S.; Kremens, D.; Vitaliani, R.; Geschwind, M.D.; et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 2009, 65, 424–434. [Google Scholar] [CrossRef]
- Sillevis Smitt, P.; Kinoshita, A.; De Leeuw, B.; Moll, W.; Coesmans, M.; Jaarsma, D.; Henzen-Logmans, S.; Vecht, C.; De Zeeuw, C.; Sekiyama, N.; et al. Paraneoplastic cerebellar ataxia due to antibodies toward against a glutamate receptor. N. Engl. J. Med. 2000, 342, 21–27. [Google Scholar] [CrossRef]
- Marignier, R.; Chenevier, F.; Rogemond, V.; Sillevis Smitt, P.; Renoux, C.; Cavillon, G.; Androdias, G.; Vukusic, S.; Graus, F.; Honnorat, J.; et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: A primary autoimmune disease? Arch. Neurol. 2010, 67, 627–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spatola, M.; Pedrol, M.P.; Maudes, E.; Simabukuro, M.; Muñiz-Castrillo, S.; Pinto, A.L.; Wandinger, K.P.; Spiegler, J.; Schramm, P.; Dutra, L.A.; et al. Clinical features prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology 2020, 95, e3012–e3025. [Google Scholar] [PubMed]
- Ruiz-García, R.; Martínez-Hernández, E.; Joubert, B.; Petit-Pedrol, M.; Pajarón-Boix, E.; Fernández, V.; Salais, L.; Del Pozo, M.; Armangué, T.; Sabater, L.; et al. Paraneoplastic cerebellar ataxia and antibodies to metabotropic glutamate receptor 2. Neurol. Neuroimmunol. Neuroinflamm. 2019, 7, e658. [Google Scholar] [CrossRef] [PubMed]
- Spatola, M.; Sabater, L.; Planagumà, J.; Martínez-Hernandez, E.; Armangué, T.; Prüss, H.; Iizuka, T.; Caparó Oblitas, R.L.; Antoine, J.C.; Li, R.; et al. Encephalitis with mGluR5 antibodies: Symptoms and antibody effects. Neurology 2018, 90, e1964–e1972. [Google Scholar] [CrossRef]
- Petit-Pedrol, M.; Armangue, T.; Peng, X.; Bataller, L.; Cellucci, T.; Davis, R.; McCracken, L.; Martinez-Hernandez, E.; Mason, W.P.; Kruer, M.C.; et al. Encephalitis with refractory seizures, status epilepticus and antibodies to the GABA(A) receptor: A case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014, 13, 276–286. [Google Scholar] [CrossRef]
- Spatola, M.; Petit-Pedrol, M.; Simabukuro, M.M.; Armangue, T.; Castro, F.J.; Barcelo Artigues, M.I.; Julià Benique, M.R.; Benson, L.; Gorman, M.; Felipe, A.; et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology 2017, 88, 1012–1020. [Google Scholar]
- Lancaster, E.; Lai, M.; Peng, X.; Hughes, E.; Constantinescu, R.; Raizer, J.; Friedman, D.; Skeen, M.B.; Grisold, W.; Kimura, A.; et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: Case series and characterization of the antigen. Lancet Neurol. 2010, 9, 67–76. [Google Scholar] [CrossRef]
- Jarius, S.; Steinmeyer, F.; Knobel, A.; Streitberger, K.; Hotter, B.; Horn, S.; Heuer, H.; Schreiber, S.J.; Wilhelm, T.; Trefzer, U.; et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J. Neuroimmunol. 2013, 256, 94–96. [Google Scholar]
- Hutchinson, M.; Waters, P.; McHugh, J.; Gorman, G.; O’Riordan, S.; Connolly, S.; Hager, H.; Yu, P.; Becker, C.M.; Vincent, A. Progressive encephalomyelitis, rigidity, and myoclonus: A novel glycine receptor antibody. Neurology 2008, 71, 1291–1292. [Google Scholar]
- Carvajal-González, A.; Leite, M.I.; Waters, P.; Woodhall, M.; Coutinho, E.; Balint, B.; Lang, B.; Pettingill, P.; Carr, A.; Sheerin, U.M.; et al. Glycine receptor antibodies in PERM and related syndromes: Characteristics, clinical features and outcomes. Brain 2014, 137, 2178–2192. [Google Scholar] [CrossRef]
- DeBellard, M.E.; Tang, S.; Mukhopadhyay, G.; Shen, Y.J.; Filbin, M.T. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. Cell Neurosci. 1996, 7, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Zis, P.; Rao, D.G.; Hoggard, N.; Sarrigiannis, P.G.; Hadjivasiliou, M. Anti-MAG associated cerebellar ataxia and response to rituximab. J. Neurol. 2018, 265, 115–118. [Google Scholar] [CrossRef]
- Fang, B.; McKeon, A.; Hinson, S.R.; Kryzer, T.J.; Pittock, S.J.; Aksamit, A.J.; Lennon, V.A. Autoimmune glial fibrillary acidic protein astrocytopathy: A novel meningoencephalomyelitis. JAMA Neurol. 2016, 73, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Kunchok, A.; Zekeridou, A.; McKeon, A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr. Opin. Neurol. 2019, 32, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Zarkali, A.; Cousins, O.; Athauda, D.; Moses, S.; Moran, N.; Harikrishnan, S. Glial fibrillary acidic protein antibody-positive meningoencephalomyelitis. Pract. Neurol. 2018, 18, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Dudesek, A.; Rimmele, E.; Tesar, S.; Kolbaske, S.; Rommer, P.S.; Benecke, R.; Zettl, U.K. CLIPPERS: Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Review of an increasingly recognized entity within the spectrum of inflammatory central nervous system disorders. Clin. Exp. Immunol. 2014, 175, 385–396. [Google Scholar] [CrossRef]
- Berzero, G.; Hacohen, Y.; Komorowski, L.; Scharf, M.; Dehais, C.; Leclercq, D.; Fourchotte, V.; Buecher, B.; Honnorat, J.; Graus, F.; et al. Paraneoplastic cerebellar degeneration associated with anti-ITPR1 antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e326. [Google Scholar] [CrossRef]
- Alfugham, N.; Gadoth, A.; Lennon, V.A.; Komorowski, L.; Scharf, M.; Hinson, S.; McKeon, A.; Pittock, S.J. ITPR1 autoimmunity: Frequency, neurologic phenotype, and cancer association. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e418. [Google Scholar] [CrossRef]
- Zuliani, L.; Sabater, L.; Saiz, A.; Baiges, J.J.; Giometto, B.; Graus, F. Homer 3 autoimmunity in subacute idiopathic cerebellar ataxia. Neurology 2007, 68, 239–240. [Google Scholar] [CrossRef]
- Hoftberger, R.; Sabater, L.; Ortega, A.; Dalmau, J.; Graus, F. Patient with homer-3 antibodies and cerebellitis. JAMA Neurol. 2013, 70, 506–509. [Google Scholar] [CrossRef]
- Bataller, L.; Sabater, L.; Saiz, A.; Serra, C.; Claramonte, B.; Grausm, F. Carbonic anhydrase-related protein VIII: Autoantigen in paraneoplastic cerebellar degeneration. Ann. Neurol. 2004, 56, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, R.; Sabater, L.; Velasco, F.; Ciordia, R.; Dalmau, J.; Graus, F. Carbonic anhydrase-related protein VIII antibodies and paraneoplastic cerebellar degeneration. Neuropathol. Appl. Neurobiol. 2014, 40, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Prevezianou, A.; Tzartos, J.S.; Dagklis, I.E.; Bentenidi, E.; Angelopoulos, P.; Bostantjopoulou, S. Paraneoplastic cerebellar degeneration in a patient with breast cancer associated with carbonic anhydrase-related protein VIII autoantibodies. J Neuroimmunol. 2020, 344, 577242. [Google Scholar] [CrossRef] [PubMed]
- Sabater, L.; Bataller, L.; Carpentier, A.F.; Aguirre-Cruz, M.L.; Saiz, A.; Benyahia, B.; Dalmau, J.; Graus, F. Protein kinase Cgamma autoimmunity in paraneoplastic cerebellar degeneration and non-small-cell lung cancer. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1359–1362. [Google Scholar] [CrossRef]
- Höftberger, R.; Kovacs, G.G.; Sabater, L.; Nagy, P.; Racz, G.; Miquel, R.; Dalmau, J.; Graus, F. Protein kinase Cγ antibodies and paraneoplastic cerebellar degeneration. J. Neuroimmunol. 2013, 256, 91–93. [Google Scholar] [CrossRef]
- Weihua, Z.; Haitao, R.; Fang, F.; Xunzhe, Y.; Jing, W.; Hongzhi, G. Neurochondrin antibody serum positivity in three cases of autoimmune cerebellar ataxia. Cerebellum 2019, 18, 1137–1142. [Google Scholar] [CrossRef]
- Barrea, C.; Depierreux, F. Reccurent ataxia and dystonia with anti-neurochondrin autoantibodies. Neuropediatrics. 2021, 52, 228–229. [Google Scholar]
- Bartels, F.; Prüss, H.; Finke, C. Anti-ARHGAP26 Autoantibodies Are Associated With Isolated Cognitive Impairment. Front. Neurol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Honorat, J.A.; Lopez-Chiriboga, A.S.; Kryzer, T.J.; Fryer, J.P.; Devine, M.; Flores, A.; Lennon, V.A.; Pittock, S.J.; McKeon, A. Autoimmune septin-5 cerbellar ataxia. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e474. [Google Scholar] [CrossRef]
- Honorat, J.A.; Lopez-Chiriboga, A.S.; Kryzer, T.J.; Komorowski, L.; Scharf, M.; Hinson, S.R.; Lennon, V.A.; Pittock, S.J.; Klein, C.J.; McKeon, A. Autoimmune gait disturbance accompanying adaptor protein-3B2-IgG. Neurology 2019, 93, e954–e963. [Google Scholar] [CrossRef]
- Hansen, N.; Fitzner, D.; Stöcker, W.; Wiltfang, J.; Bartels, C. Mild cognitive impairment in chronic brain injury associated with serum anti-AP3B2 autoantibodies: Report and literature review. Brain Sci. 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Do, L.D.; Gupton, S.L.; Tanji, K.; Bastien, J.; Brugière, S.; Couté, Y.; Quadrio, I.; Rogemond, V.; Fabien, N.; Desestret, V.; et al. TRIM9 and TRIM67 Are New Targets in Paraneoplastic Cerebellar Degeneration. Cerebellum 2019, 18, 245–254. [Google Scholar] [CrossRef]
- van Coevorden-Hameete, M.H.; van Beuningen, S.F.B.; Perrenoud, M.; Will, L.M.; Hulsenboom, E.; Demonet, J.F.; Sabater, L.; Kros, J.M.; Verschuuren, J.J.G.M.; Titulaer, M.J.; et al. Antibodies to TRIM46 are associated with paraneoplastic neurological syndromes. Ann. Clin. Transl. Neurol. 2017, 4, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Basal, E.; Zalewski, N.; Kryzer, T.J.; Hinson, S.R.; Guo, Y.; Dubey, D.; Benarroch, E.E.; Lucchinetti, C.F.; Pittock, S.J.; Lennon, V.A.; et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology 2018, 91, e1677–e1689. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Grunewald, R.A.; Shanmugarajah, P.D.; Sarrigiannis, P.G.; Zis, P.; Skarlatou, V.; Hoggard, N. Treatment of primary autoimmune cerebellar ataxia with mycophenolate. Cerebellum 2020, 19, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Saiz, A.; Dalmau, J. GAD antibodies in neurological disorders-insights and challenges. Nat. Rev. Neurol. 2020, 16, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, A.; Ikawa, M.; Fujii, A.; Nakamoto, Y.; Kuriyama, M.; Yoneda, M. Hashimoto’s encephalopathy as a treatable adult-onset cerebellar ataxia mimicking spinocerebellar degeneration. Eur. Neurol. 2013, 69, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Fujii, A.; Ito, A.; Yokoyama, H.; Nakagawa, H.; Kuriyama, M. High prevalence of serum autoantibodies against the amino terminal of α-enolase in Hashimoto’s encephalopathy. J. Neuroimmunol. 2007, 185, 195–200. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Sarrigiannis, P.G.; Shanmugarajah, P.D.; Sanders, D.S.; Grünewald, R.A.; Zis, P.; Hoggard, N. Clinical characteristics and management of 50 patients with anti-GAD ataxia: Gluten-free diet has a major impact. Cerebellum. 2021, 20, 179–185. [Google Scholar] [CrossRef]
| Associated Autoantibody | Suggested Specific Etiology in Immune-Mediated Cerebellar Ataxias |
|---|---|
| Anti-gliadin, TG 2, 6 | Gluten ataxia |
| Anti-GQ1b | Miller Fisher syndrome |
| Anti-PCA-1/Yo | PCDs; Breast, uterus and ovarian carcinomas |
| Anti-ANNA-1/Hu | PCDs; Small cell lung carcinoma |
| Anti-CV2/CRMP5 | PCDs; Small cell lung carcinoma, thymoma |
| Anti-ANNA2/Ri | PCDs and Paraneoplastic OMS; Breast carcinoma |
| Anti-Tr/DNER | PCDs; Hodgkin’s lymphoma |
| Anti-Ma2 | PCDs; Testis and lung carcinoma |
| Anti-AGNA/SOX1 | PCDs; Small cell lung carcinoma |
| Anti-ZIC4 | PCDs; Small cell lung carcinoma |
| Anti-amphiphysin | PCDs; Small cell lung and breast carcinomas |
| Anti-PCA-2/MAP1B | PCDs; Small cell lung carcinoma, non-small cell lung carcinoma |
| Anti-ANNA-3/DACH1 | PCDs; Small cell lung carcinoma |
| Anti-KLHL11 | PCDs; testicular carcinoma |
| Associated Autoantibodies | Main Phenotypes | Cerebellar Ataxias | Trigger of Autoimmunity | Therapeutic Outcome |
|---|---|---|---|---|
| Autoantibodies against ion channels and related proteins | ||||
| Anti-VGCC | CAs or/and Lambert-Eaton myasthenic syndrome | Main phenotype | Paraneoplastic (SCLC). Sometimes non-paraneoplastic | Mostly good of not paraneoplastic |
| Anti-Caspr2 | Multi focal symptoms: cognitive disturbance (79%) and epilepsy (53%), CA (35%), insomnia (68%), neuropathic pain (61%), peripheral nerve hyperexcitability (54%), autonomic dysfunction (44%), and weight loss (58%) | Sometimes as one of multitude of features (35%). Pure CA or episodic ataxia | Non-paraneoplastic. Infrequent paraneoplastic (20%, mostly thymoma) | Variable. Good recovery in 50% of patients |
| Anti-DPPX | Multi focal symptoms. Amnesia (80%), brainstem disorder (75%), sleep disturbances (45%), delirium (40%), myoclonus (40%), CA (35%) | Sometimes as one of multitude of features (35%). Few, pure CA | Non-paraneoplastic. Infrequent paraneoplastic (10%, lymphoma) | Mostly good |
| Autoantibodies against synaptic adhesion/organizing molecules | ||||
| Anti-LGI1 | Limbic encephalitis. Amnesia, confusion/disorientation, seizures | Rare, as one of multitude of features | Mostly non-paraneoplastic | Mostly good |
| Anti-IgLON5 | Sleep disorders (36%), bulbar syndrome (27%), syndrome resembling progressive supranuclear palsy (23%), and cognitive decline (14%) | Gait instability might be attributed to ataxia in some patients? | Mostly non-paraneoplastic. Association of taupathy in the hypothalamus and brainstem tegmentum | Poor |
| Anti-GluR delta | CA | Main phenotype | Infection | Good |
| Autoantibodies against transmitter receptors | ||||
| Anti-NMDA R | Multi focal symptoms. Psychosis, memory deficits, seizures, and language disintegration, followed by a state of unresponsiveness | Rare, as one of multitude of features | Non-paraneoplastic. Paraneoplastic, depending on age, 10–45% | Mostly good. Good recovery in 75% of patients |
| Anti-AMPA R | Multi focal symptoms. Distinctive limbic encephalitis (short-term memory loss, confusion, abnormal behavior, and seizures), limbic dysfunction and multifocal encephalopathy (seizures, psychiatric manifestations, CA, abnormal movements), limbic encephalopathy preceded by motor deficits, and psychosis | Infrequent, as one of multitude of features (14%) | Paraneoplastic (70%, lung, breast, thymoma). Sometimes non-paraneoplastic | Variable |
| Anti-mGluR1 | CA (86%) associated with behavioral changes (irritability, apathy, mood, personality change, psychosis with hallucinations, and catatonia), cognitive changes (memory problems, executive functions and spatial orientation deficits) or dysgeusia | Main phenotype | Non-paraneoplastic. Infrequent paraneoplastic (10%, lymphoma) | Variable. Good recovery in 50% of patients |
| Anti-mGluR2 | CAs | Main phenotype | Paraneoplastic | Variable |
| Anti-mGluR5 | Multi focal symptoms. Psychiatric (91%), cognitive (91%), movement disorders (64%), sleep dysfunction (64%), and seizures (55%) | Infrequent, as one of multitude of features (18%) | About the same. Paraneoplastic and Non-paraneoplastic | Mostly good |
| Anti-GABAA R | Multi focal symptoms. seizures, memory and cognitive deficits, behavioral changes, and psychosis | Rare, as one of multitude of features | Mostly non-paraneoplastic | Good |
| Anti-GABAB R | Multi focal symptoms. Behavioral changes or psychosis | Rare, as one of multitude of features | About the same. Paraneoplastic and Non-paraneoplastic | Variable |
| Anti-Glycine R | Progressive encephalomyelitis with rigidity and myoclonus, sometimes associated with brainstem symptoms, brainstem myoclonus, excessive startle, and CA | Infrequent, as one of multitude of features (13%) | Non-paraneoplastic. Infrequent, paraneoplastic (20%) | Mostly good |
| Targeted Antigens | Nature of Autoantigens | Frequency of Cerebellar Ataxia | Extracerebellar Symptoms | Association with Neoplasia | References |
|---|---|---|---|---|---|
| Sj/ITPR-1 | ITPR-1 triggers Ca2+ release from smooth ER, as the main intracellular Ca2+ store in response to stimulation of mGluR1. | Sometimes, as one of multitude of clinical features (8/22) | Peripheral neuropathy, encephalitis, myelopathy | Sometimes (breast and others carcinomas) | [69,70] |
| Homer-3 | Homer-3 cross-links cytoplasmic C-terminus of mGluR1. | Main phenotype (2/2) | - | No reports | [71,72] |
| CARP VIII | CARP VIII reduces ITPR1 affinity for IP3 by binding to the modulatory domain (residues 1387 to 1647) of that receptor | Main phenotype (3/3) | - | Mostly (melanoma, ovary carcinoma) | [73,74,75] |
| PKC-γ | Upon binding of cytosolic Ca2+ released by ITPR1, PKC-γtranslocates to the plasma membrane to modulate the function of other proteins. | Main phenotype (2/2) | - | Mostly (non-small cell lung carcinoma) | [76,77] |
| Neurochondrin | Neurochondrin negatively regulates phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and functions as a mediator of neurite growth and synaptic plasticity | Main phenotype (3/3) | Dystonia | No reports | [78,79] |
| Ca/ARGHAP26 | ARGHAP26 is involved in clathrin-dependent endocytosis. | Main phenotype (7/10) | Cognitive impairment, hyperekplexia | Sometimes (diverse carcinomas, B-cell lymphoma, melanoma) | [80] |
| Septin-5 | Septin-5 regulates exocytosis. | Main phenotype (4/4) | Prominent eye movement symptoms (oscillopsia or vertigo). | No reports | [81] |
| Nb/AP3B2 | AP3B2 regulates the levels of selected membrane proteins in synaptic vesicles. | Sometimes, as one of multitude of clinical features (4/9) | Sensory ataxia, paresthesia, weakness | No reports | [82,83] |
| TRIM9/67 | TRIM9 interacts with membrane-associated SNARE protein, SNAP25, and acts as negative regulator of exocytosis during axonal branching and growth. | Main phenotype (2/2) | - | Mostly (non-small cell lung carcinoma) | [84] |
| TRIM46 | TRIM46 localizes to the axon initial segment and plays an instructive role in the initial polarization of neuronal cells. | Sometimes, main phenotype (1/3) | Encephalomyelitis, dementia | Mostly, (small cell lung carcinoma) | [85] |
| Neuronal intermediate filament (light chain) | Neuronal intermediate filament is cytoskeletal structural components in large-diameter myelinated axons. | 50% (11/21) | Encephalopathy, myelopathy | Mostly (neuroendocrine carcinomas) | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjivassiliou, M.; Manto, M.; Mitoma, H. Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges. Brain Sci. 2022, 12, 1165. https://doi.org/10.3390/brainsci12091165
Hadjivassiliou M, Manto M, Mitoma H. Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges. Brain Sciences. 2022; 12(9):1165. https://doi.org/10.3390/brainsci12091165
Chicago/Turabian StyleHadjivassiliou, Marios, Mario Manto, and Hiroshi Mitoma. 2022. "Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges" Brain Sciences 12, no. 9: 1165. https://doi.org/10.3390/brainsci12091165
APA StyleHadjivassiliou, M., Manto, M., & Mitoma, H. (2022). Rare Etiologies in Immune-Mediated Cerebellar Ataxias: Diagnostic Challenges. Brain Sciences, 12(9), 1165. https://doi.org/10.3390/brainsci12091165








