The Use of Mechanical Ventilation Support at the End of Life in Motor Neurone Disease/Amyotrophic Lateral Sclerosis: A Scoping Review
Abstract
1. Introduction
2. Methods
3. Search Strategy
3.1. Inclusion/Exclusion Criteria
3.2. Search Sources
3.3. Screening and Study Selection
3.4. Data Extraction and Charting
4. Findings
4.1. Type of Study
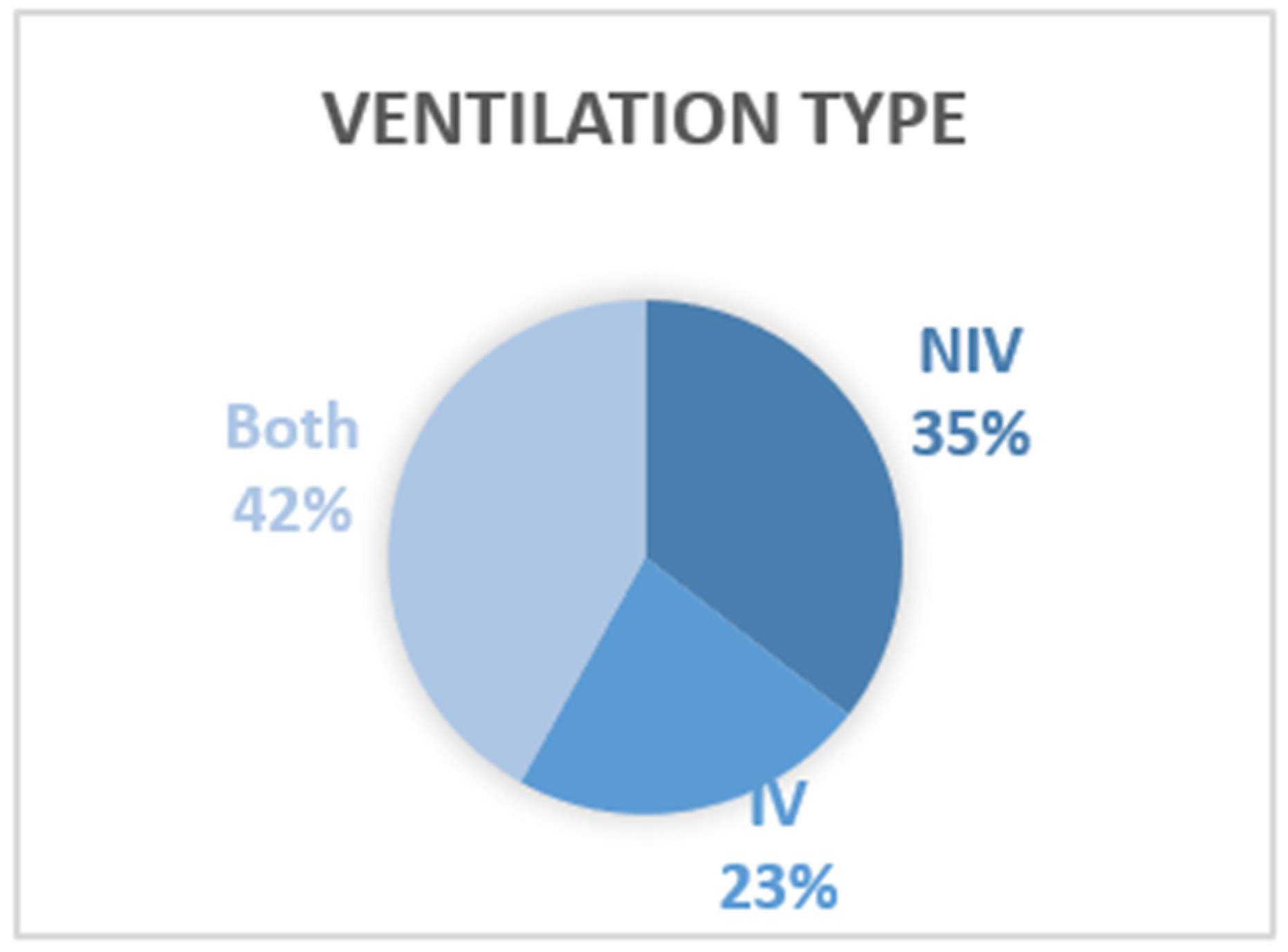
4.2. Type of Ventilation
5. Thematic Evidence
5.1. Place of Death
5.2. Cause of Death
5.3. Comment on Death
- ‘Slight distress and movement’;
- ‘Although thought to probably be comfortable, he had subtle flickering eyelids–not clear if it represented reflex/involuntary’;
- ‘Looked unsettled and family started to get anxious that he was distressed’;
- ‘Laboured breathing, although not obviously distressed’ [46] (Table 2, p3).
‘...he maintained shallow spontaneous respiratory movement but remained comfortable and no additional opioid or sedative was required. He was certified dead 15 min later’.[55] (p263)
“One of the difficulties afterwards was–is he still breathing, because the machine was breathing for him and she used her judgement to make the decision to turn the machine off because that would be a very distressing situation where the machine was breathing for somebody who had passed away”.[13] (HCP6, p520)
5.4. Planning
“A senior doctor should take responsibility for validating the decision to withdraw assisted ventilation and the planning and undertaking of the withdrawal. ...A senior doctor needs to ensure that it is a settled decision of a patient with capacity or that the advance decision is valid and applicable”.[25] (p15)
“Advance care planning is not a single event...Individual preference may change over time and discussion enables people to develop a more considered view concerning assisted ventilation and resuscitation”.[60] (p471)
5.5. Withdrawal
5.5.1. Timing and Reason for Withdrawal
5.5.2. Ethical/Emotional Issues of Withdrawal
‘While the ethical logic is understood, the process of NIV withdrawal, for some at least, feel different to the withdrawal of other treatments’.[41] (p46, emphasis on original)
5.5.3. Practical Issues/Medications Used in Withdrawal
5.5.4. Time until Death
6. Discussion
Strengths and Limitations of the Review
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motor Neurone Disease Association Breathing and Ventilation. 8B—Ventilation for Motor Neurone Disease. 2022. Available online: mndassociation.org (accessed on 19 August 2022).
- Bourke, S.C.; Tomlinson, M.; Williams, T.L.; Bullock, R.E.; Shaw, P.J.; Gibson, G.J. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomized controlled trial. Lancet Neurol. 2006, 5, 140–147. [Google Scholar] [CrossRef]
- Radunovic, A.; Annane, D.; Rafiq, M.K.; Brassington, R.; Mustfa, N. Mechanical ventilation from amyotrophic lateral sclerosis/motor neuron disease (Review). Cochrance Database Syst. Rev. 2017, 10, 1465–1858. [Google Scholar] [CrossRef]
- Bradley, M.D.; Orrell, R.W.; Clarke, J.L.; Davidson, A.C.; Williams, A.; Kullmann, D.; Hirsch, N.P.; Howard, R.S. Outcome of ventilatory support for acute respiratory failure in motor neurone disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.; Messer, B.; Ramsay, M. Tracheostomy ventilation in motor neurone disease: A snapshot of UK practice. Amyotroph. Lateral Scler. Front. Degener. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Holmøy, T.; Indrekvam, S.; Fondenæs, O. Ventilation of patients with amyotrophic lateral sclerosis. Tidsskr Nor Laegeforen 2021, 141. [Google Scholar] [CrossRef]
- Ushikubo, M. Palliative Care in Japan for Individuals with Amyotrophic Lateral Sclerosis. In Highlights on Several Underestimated Topics in Palliative Care; Cascella, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Spittel, S.; Maier, A.; Kettemann, D.; Walter, B.; Koch, B.; Krause, K.; Norden, J.; Münch, C.; Meyer, T. Non-invasive and tracheostomy invasive ventilation in amyotrophic lateral sclerosis: Utilization and survival rates in a cohort study over 12 years in Germany. Eur. J. Neurol. 2021, 28, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Kuzma-Kozakiewicz, M.; Helczyk, O.; Loose, M.; Keller, J.; Aho-Ozhan, H.; Szejko, N.; Vazquez, C.; Andersen, P.M.; Stenberg, E.; Haggstrom, A.; et al. ALS patients with locked-in syndrome: Quality of life, depression and medical decision making. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 5–6. [Google Scholar]
- Lulé, D.; Zickler, C.; Häcker, S.; Bruno, M.; Demertzi, A.; Pellas, F.; Laureys, S.; Kübler, A. Life can be worth living in locked-in syndrome. Prog. Brain Res. 2009, 177, 339–351. [Google Scholar]
- Dreyer, P.S.; Felding, M.; Klitnæs, C.S.; Lorenzen, C.K. Withdrawal of Invasive Home Mechanical Ventilation in Patients with Advanced Amyotrophic Lateral Sclerosis: Ten Years of Danish Experience. J. Palliat. Med. 2012, 15, 205–209. [Google Scholar] [CrossRef]
- Motor Neurone Disease Association. Withdrawal of Ventilation in MND, in 8C. Motor Neurone Disease Association, England. 2021. Available online: https://www.mndassociation.org/app/uploads/8C-Withdrawal-of-ventilation-with-MND.pdf (accessed on 1 March 2022).
- Baxter, S.K.; Baird, W.; Thompson, S.; Bianchi, S.M.; Walters, S.; Lee, E.; Ahmedzai, S.; Proctor, A.; Shaw, P.; McDermott, C.J. The use of non-invasive ventilation at end of life in patients with motor neurone disease: A qualitative exploration of family carer and health professional experiences. Palliat. Med. 2013, 27, 516–523. [Google Scholar] [CrossRef]
- Oliver, D.J.; Turner, M. Some difficult decisions in ALS/MND. Amyotroph. Lateral Scler. 2010, 11, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Borasio, G.D.; Voltz, R. Discontinuation of mechanical ventilation in patients with amyotrophic lateral sclerosis. J. Neurol. 1998, 245, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Faull, C.; Haynes, C.R.; Oliver, D. Withdrawing non-invasive ventilation (NIV) at the request of a patient with MND: The experiences of doctors in the UK. Amyotroph. Lateral Scler. 2012, 1, 154–155. [Google Scholar]
- Gleeson, A.; Johnson, F. Withdrawal of invasive ventilation in a patient with motor neurone disease and total locked-in syndrome. Pract. Neurol. 2017, 17, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Messer, B.; Armstrong, A.; Doris, T.; Williams, T. Requested withdrawal of mechanical ventilation in six patients with motor neuron disease. BMJ Support. Palliat. Care 2020, 10, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Faull, C. Non-invasive ventilation in amyotrophic lateral sclerosis/motor neurone disease. Minerva Pneumol. 2013, 52, 27–38. [Google Scholar]
- Simonds, A.K. Home Mechanical Ventilation: An Overview. Ann. Am. Thorac. Soc. 2016, 13, 2035–2044. [Google Scholar] [CrossRef]
- Wenzel, D.; Bleazard, L.; Pepper, C.; Faull, C. Symptom Management When Non-Invasive Advanced Respiratory Support is Used During End-of-Life Care: A Systematic Review. MedRxiv. Available online: https://www.medrxiv.org/content/10.1101/2022.03.29.22273098v1 (accessed on 30 March 2022).
- Bertaina, M.; Nuñez-Gil, I.J.; Franchin, L.; Rozas, I.F.; Arroyo-Espliguero, R.; Viana-Llamas, M.C.; Romero, R.; Eid, C.M.; Uribarri, A.; Becerra-Muñoz, V.M.; et al. Non-invasive ventilation for SARS-CoV-2 acute respiratory failure: A subanalysis from the HOPE COVID-19 registry. Emerg. Med. J. 2021, 38, 359–365. [Google Scholar] [CrossRef]
- Mental Capacity Act, Mental Capacity Act (England and Wales). Legislation.gov.uk. 2005. Available online: https://www.legislation.gov.uk/ukpga/2005/9/contents (accessed on 8 April 2022).
- General Medical Council, Treatment and Care towards the End of Life: Good Practice in Decision Making. 2010. Available online: https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/treatment-and-care-towards-the-end-of-life/guidance (accessed on 8 April 2022).
- Association for Palliative Medicine of Great Britain and Ireland (APM). Withdrawal of Assisted Ventilation at the Request of a Patient with Motor Neurone Disease: Guidance for Professionals; Association for Palliative Medicine of Great Britain and Ireland (AMP): Fareham, UK, 2015. [Google Scholar]
- Ito, H.; Itai, K.; Ito, M. Withdrawal of mechanical ventilation in patients with amyotrophic lateral sclerosis: A questionnaire study. J. Neurol. Sci. 2017, 381, 454. [Google Scholar] [CrossRef]
- Gilbar, R.; Karako-Eyal, N. Withdrawal of life-prolonging treatment in the face of severely limited resources: Ethical and legal analysis of the law in Israel. Med. Law Int. 2020, 20, 230–255. [Google Scholar] [CrossRef]
- Young, J.M.; Marshall, C.L.; Anderson, E.J. Amyotrophic Lateral Sclerosis Patients’ Perspectives on use of Mechanical Ventilation. Health Soc. Work 1994, 19, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.; Regen, E.; Oliver, D.; McDermott, C.; Faull, C. Withdrawal of ventilation at the patient’s request in MND: A retrospective exploration of the ethical and legal issues that have arisen for doctors in the UK. BMJ Support. Palliat. Care 2017, 7, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Pollock, D.; Khalil, H.; Alexander, L.; Mclnerney, P.; Godfrey, C.M.; Peters, M.; Tricco, A.C. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Évid. Synth. 2022, 20, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Pollock, D.; Davies, E.L.; Peters, M.D.J.; Tricco, A.C.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H.; Munn, Z. Undertaking a scoping review: A practical guide for nursing and midwifery students, clinicians, researchers, and academics. J. Adv. Nurs. 2021, 77, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Khalil, H.; Larsen, P.; Marnie, C.; Pollock, D.; Tricco, A.C.; Munn, Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Évid. Synth. 2022, 20, 953–968. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Moss, A.; Oppenheimer, E.A.; Casey, P.; Cazzolli, P.A.; Roos, R.P.; Stocking, C.B.; Siegler, M. Patients with Amyotrophic Lateral Sclerosis Receiving Long-term Mechanical Ventilation: Advance Care Planning and Outcomes. Chest 1996, 110, 249–255. [Google Scholar] [CrossRef]
- Kühnlein, P.; Kübler, A.; Raubold, S.; Worrell, M.; Kurt, A.; Gdynia, H.J.; Sperfeld, A.-D.; Ludolph, A.C. Palliative care and circumstances of dying in German ALS patients using non-invasive ventilation. Amyotroph. Lateral Scler. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis. 2008, 9, 91–98. [Google Scholar] [CrossRef]
- Meyer, T.; Dullinger, J.S.; Münch, C.; Keil, J.P.; Hempel, E.; Rosseau, S.; Borisow, N.; Linke, P. Elective termination of respiratory therapy in amyotrophic lateral sclerosis. Nervenarzt 2008, 79, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Escarrabill, J.; Vianello, A.; Farrero, E.; Ambrosino, N.; Llorens, J.M.; Vitacca, M. Place of death in patients with amyotrophic lateral sclerosis. Rev. Port. Pneumol. 2014, 20, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Faull, C.; Haynes, C.R.; Oliver, D. Issues for palliative medicine doctors surrounding the withdrawal of non-invasive ventilation at the request of a patient with motor neurone disease: A scoping study: Table 1. BMJ Support. Palliat. Care 2014, 4, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, C.; Neuwirth, C.; Sommacal, A.; Andersen, P.M.; Weber, M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS ONE 2017, 12, e0177555. [Google Scholar] [CrossRef] [PubMed]
- Kettemann, D.; Funke, A.; Maier, A.; Rosseau, S.; Meyer, R.; Spittel, S.; Münch, C.; Meyer, T. Clinical characteristics and course of dying in patients with amyotrophic lateral sclerosis withdrawing from long-term ventilation. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Markovic, N.; Povitz, M.; Smith, J.; Leasa, D.; Shoesmith, C.; Gofton, T.E. Patterns of Non-Invasive Ventilation in Amyotrophic Lateral Sclerosis. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2018, 45, 445–450. [Google Scholar] [CrossRef]
- Thurn, T.; Borasio, G.D.; Chiò, A.; Galvin, M.; McDermott, C.J.; Mora, G.; Sermeus, W.; Winkler, A.S.; Anneser, J. Physicians’ attitudes toward end-of-life decisions in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 74–81. [Google Scholar] [CrossRef]
- Faull, C.; Wenzel, D. Mechanical ventilation withdrawal in motor neuron disease: An evaluation of practice. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef]
- Cazzolli, P.A.; Oppenheimer, E.A. Home mechanical ventilation for amyotrophic lateral sclerosis: Nasal compared to tracheostomy-intermittent positive pressure ventilation. J. Neurol. Sci. 1996, 139, 123–128. [Google Scholar] [CrossRef]
- Ushikubo, M. Comparison Between Home and Hospital as the Place of Death for Individuals with Amyotrophic Lateral Sclerosis in the Last Stages of Illness. Am. J. Hosp. Palliat. Med. 2014, 32, 417–426. [Google Scholar] [CrossRef]
- Ushikubo, M. Circumstances and Signs of Approaching Death in Patients with Amyotrophic Lateral Sclerosis Undergoing Noninvasive Ventilation in Home Care Settings. J. Neurosci. Nurs. 2018, 50, 182–186. [Google Scholar] [CrossRef]
- Veronese, S.; Valle, A.; Chio, A.; Calvo, A.; Oliver, D. The last months of life of people with amyotrophic lateral sclerosis in mechanical invasive ventilation: A qualitative study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.; McDermott, C.; Oliver, D. Withdrawal of Assisted Ventilation at the Patient’s Request in MND: A Retrospective Exploration of the Ethical and Legal Issues Concerning Relatives, Nurses and Allied Health Care Professionals. medRxiv 2022. [Google Scholar] [CrossRef]
- Chapman, C.; Bayes, S.; Sim, M. Communication surrounding initiation and withdrawal of non-invasive ventilation in adults with Motor Neuron(e) Disease: Clinicians’ and family members’ perspectives. Int. J. Care Coord. 2021, 24, 96–106. [Google Scholar] [CrossRef]
- Berger, J.T. Preemptive Use of Palliative Sedation and Amyotrophic Lateral Sclerosis. J. Pain Symptom Manag. 2012, 43, 802–805. [Google Scholar] [CrossRef]
- Gannon, C. A request for hospice admission from hospital to withdraw ventilation. J. Med. Ethics 2005, 31, 383–384. [Google Scholar] [CrossRef] [PubMed]
- LeBon, B.; Fisher, S. Case report: Maintaining and withdrawing long-term invasive ventilation in a patient with MND/ALS in a home setting. Palliat. Med. 2011, 25, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D. Ventilation in motor neuron disease: Difficult decisions in difficult circumstances. Amyotroph. Lateral Scler. 2004, 5, 6–8. [Google Scholar] [CrossRef]
- Polkey, M.I.; Lyall, R.A.; Davidson, A.C.; Leigh, P.N.; Moxham, J. Ethical and clinical issues in the use of home non-invasive mechanical ventilation for the palliation of breathlessness in motor neurone disease. Thorax 1999, 54, 367–371. [Google Scholar] [CrossRef][Green Version]
- Faull, C.; Oliver, D. Withdrawal of ventilation at the request of a patient with motor neurone disease: Guidance for professionals. BMJ Support. Palliat. Care 2016, 6, 144–146. [Google Scholar] [CrossRef]
- Tripodoro, V.; Rabec, C.; De Vito, E. Withdrawing noninvasive ventilation at end-of-life care: Is there a right time? Curr. Opin. Support. Palliat. Care 2019, 13, 344–350. [Google Scholar] [PubMed]
- Turner, M.R.; Faull, C.; McDermott, C.J.; Nickol, A.H.; Palmer, J.; Talbot, K. Tracheostomy in motor neurone disease. Pract. Neurol. 2019, 19, 467–475. [Google Scholar] [PubMed]
- Eng, D. Management guidelines for motor neurone disease patients on non-invasive ventilation at home. Palliat. Med. 2006, 20, 69–79. [Google Scholar] [PubMed]
- National Institute of Health and Care Excellence (NICE). Motor neurone disease: Assessment and management. In NICE Guideline; National Institute of Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]



| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Patients with MND and experiences of using ventilation Family caregiver with experience of supporting someone with MND in using ventilation Health professional with experience of working with someone with MND using ventilation | Publications not including patients with MND/ALS |
| Concept | Use of ventilation Withdrawal of ventilation Dying with ventilation in place Non-invasive ventilation Invasive or tracheostomy ventilation Mechanics of ventilation Decision making Medications for withdrawal of ventilation/symptom management | Publications not involving ventilation at the end of life for patients with MND |
| Context | Health care settings Home Worldwide | |
| Types of evidence sources | ||
| Study Design | Qualitative, quantitative, mixed methods, observational, experimental, clinical trials, quasi experimental studies, case studies, reviews | Letters and conference abstracts |
| Publication Type | Peer-reviewed publications, conference proceedings where a full report is available, textbook chapters, books, reports, preprint repositories, UK national guidance | Unable to obtain full text Local guidance provided by NHS Trusts |
| Language | English or those that can be translated into English using online translation tools | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, E.; Lee, J.-S.; Wenzel, D.; Faull, C. The Use of Mechanical Ventilation Support at the End of Life in Motor Neurone Disease/Amyotrophic Lateral Sclerosis: A Scoping Review. Brain Sci. 2022, 12, 1162. https://doi.org/10.3390/brainsci12091162
Wilson E, Lee J-S, Wenzel D, Faull C. The Use of Mechanical Ventilation Support at the End of Life in Motor Neurone Disease/Amyotrophic Lateral Sclerosis: A Scoping Review. Brain Sciences. 2022; 12(9):1162. https://doi.org/10.3390/brainsci12091162
Chicago/Turabian StyleWilson, Eleanor, Jeong-Su Lee, David Wenzel, and Christina Faull. 2022. "The Use of Mechanical Ventilation Support at the End of Life in Motor Neurone Disease/Amyotrophic Lateral Sclerosis: A Scoping Review" Brain Sciences 12, no. 9: 1162. https://doi.org/10.3390/brainsci12091162
APA StyleWilson, E., Lee, J.-S., Wenzel, D., & Faull, C. (2022). The Use of Mechanical Ventilation Support at the End of Life in Motor Neurone Disease/Amyotrophic Lateral Sclerosis: A Scoping Review. Brain Sciences, 12(9), 1162. https://doi.org/10.3390/brainsci12091162







