Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Procedure
2.2.1. I-MOBID2
2.2.2. CMAI
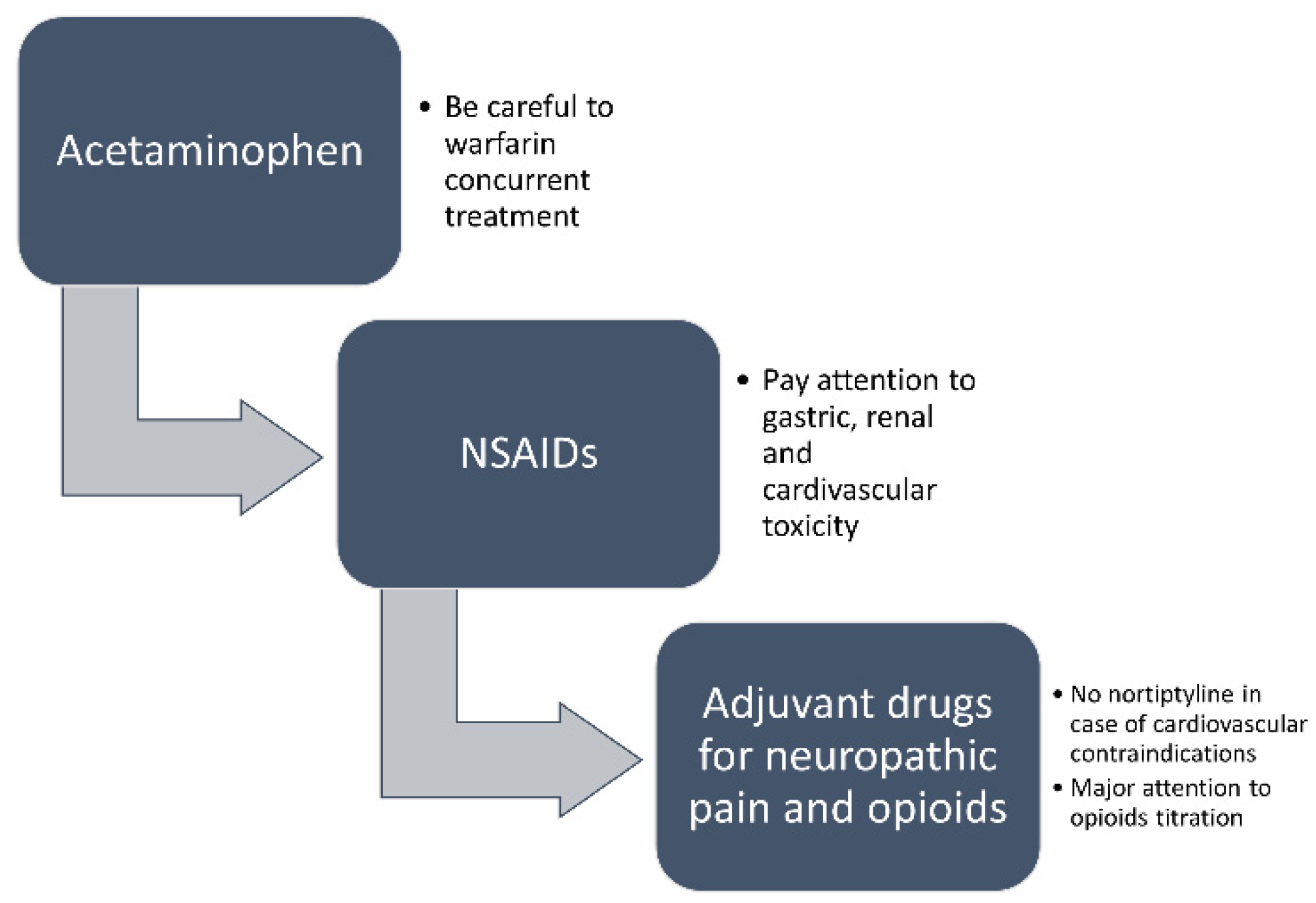
2.3. Pain Treatment
2.4. Inclusion Criteria
2.5. Ethical Approval
2.6. Statistical Analysis
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. Lond. Engl. Alzheimer’s Dis. Int. 2021. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 10 April 2022).
- Scuteri, D.; Rombolà, L.; Tridico, L.; Mizoguchi, H.; Watanabe, C.; Sakurada, T.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain-related BPSDs. Curr. Med. Chem. 2019, 26, 3764–3774. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Dunn, B.; Stein, P.; Cavazzoni, P. Approval of Aducanumab for Alzheimer Disease—The FDA’s Perspective. JAMA Intern. Med. 2021, 181, 1276–1278. [Google Scholar] [CrossRef]
- Steinberg, M.; Shao, H.; Zandi, P.; Lyketsos, C.G.; Welsh-Bohmer, K.A.; Norton, M.C.; Breitner, J.C.; Steffens, D.C.; Tschanz, J.T. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int. J. Geriatr. Psychiatry 2008, 23, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Matamala-Gomez, M.; Bottiroli, S.; Corasaniti, M.T.; De Icco, R.; Bagetta, G.; Tonin, P. Pain Assessment and Treatment in Dementia at the Time of Coronavirus Disease COVID-19. Front. Neurol. 2020, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Contrada, M.; Tonin, P.; Corasaniti, M.T.; Nicotera, P.; Bagetta, G. Dementia and COVID-19: A Case Report and Literature Review on Pain Management. Pharmaceuticals 2022, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Nicotera, P.; Bagetta, G. New trends in pharmacological control of neuropsychiatric symptoms of dementia. Curr. Opin. Pharmacol. 2021, 61, 69–76. [Google Scholar] [CrossRef]
- Ismail, Z.; Agüera-Ortiz, L.; Brodaty, H.; Cieslak, A.; Cummings, J.; Fischer, C.E.; Gauthier, S.; Geda, Y.E.; Herrmann, N.; Kanji, J.; et al. The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J. Alzheimer’s Dis. JAD 2017, 56, 929–938. [Google Scholar] [CrossRef]
- Cummings, J. The Role of Neuropsychiatric Symptoms in Research Diagnostic Criteria for Neurodegenerative Diseases. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2021, 29, 375–383. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Aalten, P.; Verhey, F.R.J.; Boziki, M.; Brugnolo, A.; Bullock, R.; Byrne, E.J.; Camus, V.; Caputo, M.; Collins, D.; De Deyn, P.P.; et al. Consistency of Neuropsychiatric Syndromes across Dementias: Results from the European Alzheimer Disease Consortium. Dement. Geriatr. Cogn. Disord. 2008, 25, 1–8. [Google Scholar] [CrossRef]
- Ford, E.; Greenslade, N.; Paudyal, P.; Bremner, S.; Smith, H.E.; Banerjee, S.; Sadhwani, S.; Rooney, P.; Oliver, S.; Cassell, J. Predicting dementia from primary care records: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194735. [Google Scholar] [CrossRef]
- Barnes, D.E.; Alexopoulos, G.S.; Lopez, O.L.; Williamson, J.D.; Yaffe, K. Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the Cardiovascular Health Study. Arch. Gen. Psychiatry 2006, 63, 273–279. [Google Scholar] [CrossRef]
- Savva, G.M.; Zaccai, J.; Matthews, F.E.; Davidson, J.E.; McKeith, I.; Brayne, C. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br. J. Psychiatry J. Ment. Sci. 2009, 194, 212–219. [Google Scholar] [CrossRef]
- Brodaty, H.; Connors, M.H.; Xu, J.; Woodward, M.; Ames, D. The course of neuropsychiatric symptoms in dementia: A 3-year longitudinal study. J. Am. Med. Dir. Assoc. 2015, 16, 380–387. [Google Scholar] [CrossRef]
- Husebo, B.S.; Ballard, C.; Sandvik, R.; Nilsen, O.B.; Aarsland, D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: Cluster randomised clinical trial. BMJ 2011, 343, d4065. [Google Scholar] [CrossRef]
- Scuteri, D.; Vulnera, M.; Piro, B.; Bossio, R.B.; Morrone, L.A.; Sandrini, G.; Tamburin, S.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Pattern of treatment of behavioural and psychological symptoms of dementia and pain: Evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. Eur. J. Clin. Pharmacol. 2021, 77, 241–249. [Google Scholar] [CrossRef]
- Scuteri, D.; Garreffa, M.R.; Esposito, S.; Bagetta, G.; Naturale, M.D.; Corasaniti, M.T. Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regen. Res. 2018, 13, 1619–1621. [Google Scholar] [CrossRef]
- Scuteri, D.; Piro, B.; Morrone, L.A.; Corasaniti, M.T.; Vulnera, M.; Bagetta, G. The need for better access to pain treatment: Learning from drug consumption trends in the USA. Funct. Neurol. 2017, 22, 229–230. [Google Scholar] [CrossRef]
- Ragneskog, H.; Gerdner, L.A.; Josefsson, K.; Kihlgren, M. Probable Reasons for Expressed Agitation in Persons with Dementia. Clin. Nurs. Res. 1998, 7, 189–206. [Google Scholar] [CrossRef]
- Volicer, L.; Frijters, D.H.; Van der Steen, J.T. Relationship between symptoms of depression and agitation in nursing home residents with dementia. Int. J. Geriatr. Psychiatry 2012, 27, 749–754. [Google Scholar] [CrossRef]
- Rose, K.M.; Beck, C.; Tsai, P.-F.; Liem, P.H.; Davila, D.G.; Kleban, M.; Gooneratne, N.S.; Kalra, G.; Richards, K.C. Sleep disturbances and nocturnal agitation behaviors in older adults with dementia. Sleep 2011, 34, 779–786. [Google Scholar] [CrossRef]
- Lee, D.; Heo, S.H.; Yoon, S.S.; Chang, D.I.; Lee, S.; Rhee, H.Y.; Ku, B.D.; Park, K.C. Sleep disturbances and predictive factors in caregivers of patients with mild cognitive impairment and dementia. J. Clin. Neurol. 2014, 10, 304–313. [Google Scholar] [CrossRef][Green Version]
- Schuster, B.G.; Kosar, L.; Kamrul, R. Constipation in older adults: Stepwise approach to keep things moving. Can. Fam. Physician 2015, 61, 152–158. [Google Scholar]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Management of Neuropsychiatric Symptoms of, D. Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. J. Am. Geriatr. Soc. 2014, 62, 762–769. [Google Scholar] [CrossRef]
- Scuteri, D.; Berliocchi, L.; Rombolà, L.; Morrone, L.A.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Aging on Formalin-Induced Pain Behavior and Analgesic Activity of Gabapentin in C57BL/6 Mice. Front. Pharmacol. 2020, 11, 663. [Google Scholar] [CrossRef]
- Sampson, E.L.; White, N.; Lord, K.; Leurent, B.; Vickerstaff, V.; Scott, S.; Jones, L. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: A longitudinal cohort study. Pain 2015, 156, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Francis, R.M.; Collerton, J.; Davies, K.; Jagger, C.; Kingston, A.; Kirkwood, T.; Robinson, L.; Birrell, F. Prevalence of arthritis and joint pain in the oldest old: Findings from the Newcastle 85+ study. Age Ageing 2011, 40, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, W.P. How can the quality of life of older patients living with chronic pain be improved? Pain Manag. 2019, 9, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, W.P.; Pieper, M.J.; van Dalen-Kok, A.H.; de Waal, M.W.; Husebo, B.S.; Lautenbacher, S.; Kunz, M.; Scherder, E.J.; Corbett, A. Pain management in patients with dementia. Clin. Interv. Aging 2013, 8, 1471–1482. [Google Scholar] [CrossRef]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Scharre, D.W.; Vekeman, F.; Lefebvre, P.; Mody-Patel, N.; Kahler, K.H.; Duh, M.S. Use of Antipsychotic Drugs in Patients with Alzheimer’s Disease Treated with Rivastigmine versus Donepezil. Drugs Aging 2010, 27, 903–913. [Google Scholar] [CrossRef]
- Gauthier, S.; Loft, H.; Cummings, J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: A pooled data analysis. Int. J. Geriatr. Psychiatry 2008, 23, 537–545. [Google Scholar] [CrossRef]
- Ballard, C.G.; Gauthier, S.; Cummings, J.L.; Brodaty, H.; Grossberg, G.T.; Robert, P.; Lyketsos, C.G. Management of agitation and aggression associated with Alzheimer disease. Nat. Rev. Neurol. 2009, 5, 245–255. [Google Scholar] [CrossRef]
- Rajkumar, A.P.; Ballard, C.; Fossey, J.; Orrell, M.; Moniz-Cook, E.; Woods, R.T.; Murray, J.; Whitaker, R.; Stafford, J.; Knapp, M.; et al. Epidemiology of Pain in People With Dementia Living in Care Homes: Longitudinal Course, Prevalence, and Treatment Implications. J. Am. Med. Dir. Assoc. 2017, 18, 453.e451–453.e456. [Google Scholar] [CrossRef]
- Kales, H.C.; Lyketsos, C.G.; Miller, E.M.; Ballard, C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int. Psychogeriatr. 2019, 31, 83–90. [Google Scholar] [CrossRef]
- Corbett, A.; Burns, A.; Ballard, C. Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ 2014, 349, g6420. [Google Scholar] [CrossRef]
- Corbett, A.; Husebo, B.S.; Achterberg, W.P.; Aarsland, D.; Erdal, A.; Flo, E. The importance of pain management in older people with dementia. Br. Med. Bull. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Hadjistavropoulos, T.; Herr, K.; Turk, D.C.; Fine, P.G.; Dworkin, R.H.; Helme, R.; Jackson, K.; Parmelee, P.A.; Rudy, T.E.; Lynn Beattie, B.; et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin. J. Pain 2007, 23, S1–S43. [Google Scholar] [CrossRef]
- Husebo, B.S.; Strand, L.I.; Moe-Nilssen, R.; Husebo, S.B.; Ljunggren, A.E. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) Pain Scale in a clinical setting. Scand. J. Caring Sci. 2010, 24, 380–391. [Google Scholar] [CrossRef]
- Scuteri, D.; Contrada, M.; Loria, T.; Sturino, D.; Cerasa, A.; Tonin, P.; Sandrini, G.; Tamburin, S.; Bruni, A.C.; Nicotera, P.; et al. Pain and agitation treatment in severe dementia patients: The need for Italian Mobilization–Observation–Behaviour–Intensity–Dementia (I-MOBID2) Pain Scale translation, adaptation and validation with psychometric testing. Biomed. Pharmacother. 2022, 150, 113013. [Google Scholar] [CrossRef]
- Corbett, A.; Husebo, B.; Malcangio, M.; Staniland, A.; Cohen-Mansfield, J.; Aarsland, D.; Ballard, C. Assessment and treatment of pain in people with dementia. Nat. Rev. Neurol. 2012, 8, 264–274. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Marx, M.S.; Rosenthal, A.S. A description of agitation in a nursing home. J. Gerontol. 1989, 44, M77–M84. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J. Conceptualization of agitation: Results based on the Cohen-Mansfield Agitation Inventory and the Agitation Behavior Mapping Instrument. Int. Psychogeriatr. 1996, 8 (Suppl. 3), 309–315. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2010, 19, 539–549. [Google Scholar] [CrossRef]
- Husebo, B.S.; Ostelo, R.; Strand, L.I. The MOBID-2 pain scale: Reliability and responsiveness to pain in patients with dementia. Eur. J. Pain 2014, 18, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Husebo, B.S.; Ballard, C.; Fritze, F.; Sandvik, R.K.; Aarsland, D. Efficacy of pain treatment on mood syndrome in patients with dementia: A randomized clinical trial. Int. J. Geriatr. Psychiatry 2014, 29, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, S.; Barisch-Fritz, B.; Scharpf, A.; Bossers, W.; Meinzer, M.; Steib, S.; Stein, T.; Bös, K.; Stahn, A.; Niessner, C.; et al. Recommendations for assessing motor performance in individuals with dementia: Suggestions of an expert panel—A qualitative approach. Eur. Rev. Aging Phys. Act. 2019, 16, 5. [Google Scholar] [CrossRef]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual; Lippincott Williams & Wilkins, Inc.: Philadelphia, PA, USA, 1983. [Google Scholar]
- Margolis, R.B.; Chibnall, J.T.; Tait, R.C. Test-retest reliability of the pain drawing instrument. Pain 1988, 33, 49–51. [Google Scholar] [CrossRef]
- Margolis, R.B.; Tait, R.C.; Krause, S.J. A rating system for use with patient pain drawings. Pain 1986, 24, 57–65. [Google Scholar] [CrossRef]
- Rose, V.L. Guidelines from the American Geriatric Society target management of chronic pain in older persons. Am. Fam. Physician 1998, 58, 1213–1214, 1217. [Google Scholar]
- Ickowicz, E.; Ferrell, B.; Casarett, D.; Epplin, J.; Fine, P.; Gloth, M.; Herr, K.; Katz, P.; Keefe, F.; Koo, P.J. The management of persistent pain in older persons. J. Am. Geriatr. Soc. 2002, 50, S205–S224. [Google Scholar] [CrossRef]
- Franceschi, M.; Scarcelli, C.; Niro, V.; Seripa, D.; Pazienza, A.M.; Pepe, G.; Colusso, A.M.; Pacilli, L.; Pilotto, A. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf. 2008, 31, 545–556. [Google Scholar] [CrossRef]
- Lanza, F.; Rack, M.F.; Doucette, M.; Ekholm, B.; Goldlust, B.; Wilson, R. An endoscopic comparison of the gastroduodenal injury seen with salsalate and naproxen. J. Rheumatol. 1989, 16, 1570–1574. [Google Scholar]
- Ruschitzka, F.; Borer, J.S.; Krum, H.; Flammer, A.J.; Yeomans, N.D.; Libby, P.; Lüscher, T.F.; Solomon, D.H.; Husni, M.E.; Graham, D.Y.; et al. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: The PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement) Trial. Eur. Heart J. 2017, 38, 3282–3292. [Google Scholar] [CrossRef]
- Zhang, Q.; Bal-dit-Sollier, C.; Drouet, L.; Simoneau, G.; Alvarez, J.C.; Pruvot, S.; Aubourg, R.; Berge, N.; Bergmann, J.F.; Mouly, S.; et al. Interaction between acetaminophen and warfarin in adults receiving long-term oral anticoagulants: A randomized controlled trial. Eur. J. Clin. Pharmacol. 2011, 67, 309–314. [Google Scholar] [CrossRef]
- Sandvik, R.K.; Selbaek, G.; Seifert, R.; Aarsland, D.; Ballard, C.; Corbett, A.; Husebo, B.S. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: A cluster randomized trial. Eur. J. Pain 2014, 18, 1490–1500. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Lu, Y.; Detke, M.J.; Lee, T.C.; Iyengar, S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005, 116, 109–118. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. Morb. Mortal. Wkly. Report. Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- Allan, L.; Hays, H.; Jensen, N.H.; de Waroux, B.L.; Bolt, M.; Donald, R.; Kalso, E. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ 2001, 322, 1154–1158. [Google Scholar] [CrossRef]
- Fine, P.G. Chronic pain management in older adults: Special considerations. J. Pain Symptom Manag. 2009, 38, S4–S14. [Google Scholar] [CrossRef]
- Manietta, C.; Labonté, V.; Thiesemann, R.; Sirsch, E.G.; Möhler, R. Algorithm-based pain management for people with dementia in nursing homes. Cochrane Database Syst. Rev. 2022, 4, Cd013339. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Reisberg, B. The GDS/FAST staging system. Int. Psychogeriatr. 1997, 9 (Suppl. 1), 167–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Won, C.W.; Kim, S.; Choi, H.R.; Kim, B.S.; Jeon, S.Y.; Kim, S.Y.; Park, K.W. Five items differentiate mild to severe dementia from normal to minimal cognitive impairment—Using the Global Deterioration Scale. J. Clin. Gerontol. Geriatr. 2016, 7, 1–5. [Google Scholar] [CrossRef][Green Version]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ Br. Med. J. 2013, 346, e7586. [Google Scholar] [CrossRef]
- Won, A.B.; Lapane, K.L.; Vallow, S.; Schein, J.; Morris, J.N.; Lipsitz, L.A. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J. Am. Geriatr. Soc. 2004, 52, 867–874. [Google Scholar] [CrossRef]
- Horgas, A.L.; Tsai, P.F. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs. Res. 1998, 47, 235–242. [Google Scholar] [CrossRef]
- Scuteri, D.; Mantovani, E.; Tamburin, S.; Sandrini, G.; Corasaniti, M.T.; Bagetta, G.; Tonin, P. Opioids in Post-stroke Pain: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 587050. [Google Scholar] [CrossRef]
- Bayer, A.; Tadd, W. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: Descriptive study. BMJ 2000, 321, 992–993. [Google Scholar] [CrossRef]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; De Francesco, A.E.; Esposito, S.; Zito, M.; Morrone, L.A.; Bagetta, G.; Tonin, P.; et al. Pattern of triptans use: A retrospective prescription study in Calabria, Italy. Neural Regen. Res. 2020, 15, 1340–1343. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Bagetta, G. Eptinezumab for the treatment of migraine. Drugs Today 2019, 55, 695–703. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Nicotera, P.; Bagetta, G. Role of CGRP pathway polymorphisms in migraine: A systematic review and impact on CGRP mAbs migraine therapy. J. Headache Pain 2021, 22, 87. [Google Scholar] [CrossRef]
- Herrero, S.; Guerrero, A.L.; Ruiz, M.; Pedraza, M.I.; Mulero, P.; Barón, J.; Irene, I.; De la Cruz, C.; Peñas, M.L. Migraine in the elderly: Clinical characteristics in a series of 71 cases. J. Headache Pain 2013, 14, P152. [Google Scholar] [CrossRef][Green Version]
- McLachlan, A.J.; Hilmer, S.N.; Le Couteur, D.G. Variability in response to medicines in older people: Phenotypic and genotypic factors. Clin. Pharmacol. Ther. 2009, 85, 431–433. [Google Scholar] [CrossRef]
- Nørgaard, A.; Jensen-Dahm, C.; Gasse, C.; Hansen, E.S.; Waldemar, G. Psychotropic Polypharmacy in Patients with Dementia: Prevalence and Predictors. J. Alzheimer’s Dis. 2017, 56, 707–716. [Google Scholar] [CrossRef]
- Riedl, L.; Kiesel, E.; Hartmann, J.; Fischer, J.; Roßmeier, C.; Haller, B.; Kehl, V.; Priller, J.; Trojan, M.; Diehl-Schmid, J. A bitter pill to swallow—Polypharmacy and psychotropic treatment in people with advanced dementia. BMC Geriatr. 2022, 22, 214. [Google Scholar] [CrossRef]
- Letinier, L.; Pujade, I.; Duthoit, P.; Evrard, G.; Salvo, F.; Gil-Jardine, C.; Pariente, A. Emergency department admissions induced by drug-drug interactions in the elderly: A cross-sectional study. Clin. Transl. Sci. 2022. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef]
- Scuteri, D.; Crudo, M.; Rombolà, L.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Sakurada, T.; Greco, R.; Corasaniti, M.T.; Morrone, L.A.; et al. Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 2018, 129, 20–24. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Watanabe, C.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Role of 5-HT1A Receptor in the Anxiolytic-Relaxant Effects of Bergamot Essential Oil in Rodent. Int. J. Mol. Sci. 2020, 21, 2597. [Google Scholar] [CrossRef]
- Scuteri, D.; Sandrini, G.; Tamburin, S.; Corasaniti, M.T.; Nicotera, P.; Tonin, P.; Bagetta, G. Bergamot rehabilitation AgaINst agitation in dementia (BRAINAID): Study protocol for a randomized, double-blind, placebo-controlled trial to assess the efficacy of furocoumarin-free bergamot loaded in a nanotechnology-based delivery system of the essential oil in the treatment of agitation in elderly affected by severe dementia. Phytother. Res. PTR 2021, 35, 5333–5338. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Cassano, R.; Trombino, S.; Russo, R.; Mizoguchi, H.; Watanabe, C.; Hamamura, K.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; et al. Development and Translation of NanoBEO, a Nanotechnology-Based Delivery System of Bergamot Essential Oil Deprived of Furocumarins, in the Control of Agitation in Severe Dementia. Pharmaceutics 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Crudo, M.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; et al. Preclinical Characterization of Antinociceptive Effect of Bergamot Essential Oil and of Its Fractions for Rational Translation in Complementary Therapy. Pharmaceutics 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Ernst, E. Complementary and alternative approaches to the treatment of persistent musculoskeletal pain. Clin. J. Pain 2004, 20, 244–255. [Google Scholar] [CrossRef]
- Blyth, F.M.; March, L.M.; Brnabic, A.J.; Jorm, L.R.; Williamson, M.; Cousins, M.J. Chronic pain in Australia: A prevalence study. Pain 2001, 89, 127–134. [Google Scholar] [CrossRef]
- Pharmacological management of persistent pain in older persons. J. Am. Geriatr. Soc. 2009, 57, 1331–1346. [CrossRef]
- Landi, F.; Onder, G.; Cesari, M.; Gambassi, G.; Steel, K.; Russo, A.; Lattanzio, F.; Bernabei, R. Pain management in frail, community-living elderly patients. Arch. Intern. Med. 2001, 161, 2721–2724. [Google Scholar] [CrossRef]
| STUDY PERIOD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Allocation | Post-Allocation:Interventions | ||||||||
| TIMEPOINT | -within 2 weeks | Behavioral Baseline Assessment 1 week | Baseline 1 week | week1 | week2 | week3 | week4 | week5 | week6 | week7 | week8 |
| ENROLMENT: | X | ||||||||||
| Eligibility screen | X | ||||||||||
| Informed consent | X | ||||||||||
| Physical examination | X | ||||||||||
| Allocation | X | ||||||||||
| INTERVENTIONS: | |||||||||||
| Analgesic treatment upon assessment |  | ||||||||||
| Usual treatment |  | ||||||||||
| ASSESSMENTS: | |||||||||||
| I-MOBID-2 | baseline observation | X | X | X | X | X | X | X | X | ||
| Timed “Up and Go” (TUG) | baseline observation | X | X | X | X | X | X | X | X | ||
| STUDY PERIOD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-Allocation:Interventions | ||||||||
| TIMEPOINT | -within 2 weeks | Baseline 2 weeks | week1 | week2 | week3 | week4 | week5 | week6 | week7 | week8 |
| ENROLMENT: | X | |||||||||
| Eligibility screen | X | |||||||||
| Informed consent | X | |||||||||
| Physical examination | X | |||||||||
| Allocation | X | |||||||||
| INTERVENTIONS: | ||||||||||
| Analgesic treatment upon assessment |  | |||||||||
| Usual treatment |  | |||||||||
| ASSESSMENTS: | ||||||||||
| CMAI | Baseline observation | X | X | X | X | X | X | X | X | |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuteri, D.; Contrada, M.; Loria, T.; Tonin, P.; Sandrini, G.; Tamburin, S.; Nicotera, P.; Bagetta, G.; Corasaniti, M.T. Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol. Brain Sci. 2022, 12, 573. https://doi.org/10.3390/brainsci12050573
Scuteri D, Contrada M, Loria T, Tonin P, Sandrini G, Tamburin S, Nicotera P, Bagetta G, Corasaniti MT. Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol. Brain Sciences. 2022; 12(5):573. https://doi.org/10.3390/brainsci12050573
Chicago/Turabian StyleScuteri, Damiana, Marianna Contrada, Teresa Loria, Paolo Tonin, Giorgio Sandrini, Stefano Tamburin, Pierluigi Nicotera, Giacinto Bagetta, and Maria Tiziana Corasaniti. 2022. "Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol" Brain Sciences 12, no. 5: 573. https://doi.org/10.3390/brainsci12050573
APA StyleScuteri, D., Contrada, M., Loria, T., Tonin, P., Sandrini, G., Tamburin, S., Nicotera, P., Bagetta, G., & Corasaniti, M. T. (2022). Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol. Brain Sciences, 12(5), 573. https://doi.org/10.3390/brainsci12050573









