Gross Total Resection Promotes Subsequent Recovery and Further Enhancement of Impaired Natural Killer Cell Activity in Glioblastoma Patients
Abstract
:Highlights
- Natural killer cell activity is dramatically impaired in patients with glioblastoma.
- Surgical resection of glioblastoma promotes redistribution of NK cell subsets and increases NK cell activity 30 days after surgery.
- Gross total resection rather than subtotal resection significantly recovers and further increases the impaired NK cell activity in patients with glioblastoma.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethical Considerations and Surgical Resection of Glioblastoma
2.3. Blood Sampling and Processing
2.4. Determination of NKA and Absolute NK Cell Counts
2.5. Flow Cytometery Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics of Patients with Glioblastoma
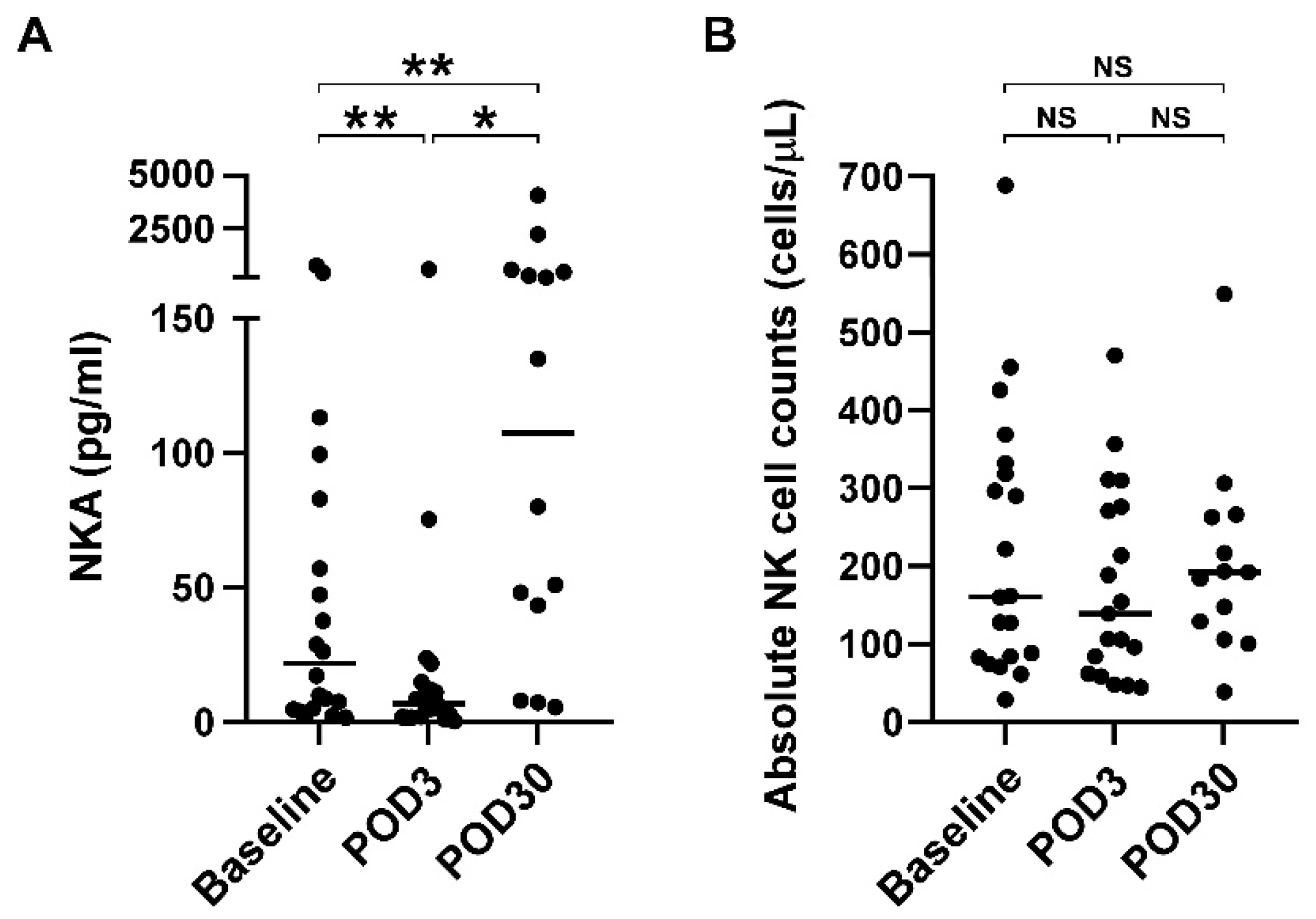
3.2. Impaired NKA Recovered 30 Days after Surgical Resection of Glioblastoma
3.3. Redistribution of NK Cell Subsets but Not T Cell Subsets after Cranial Surgery
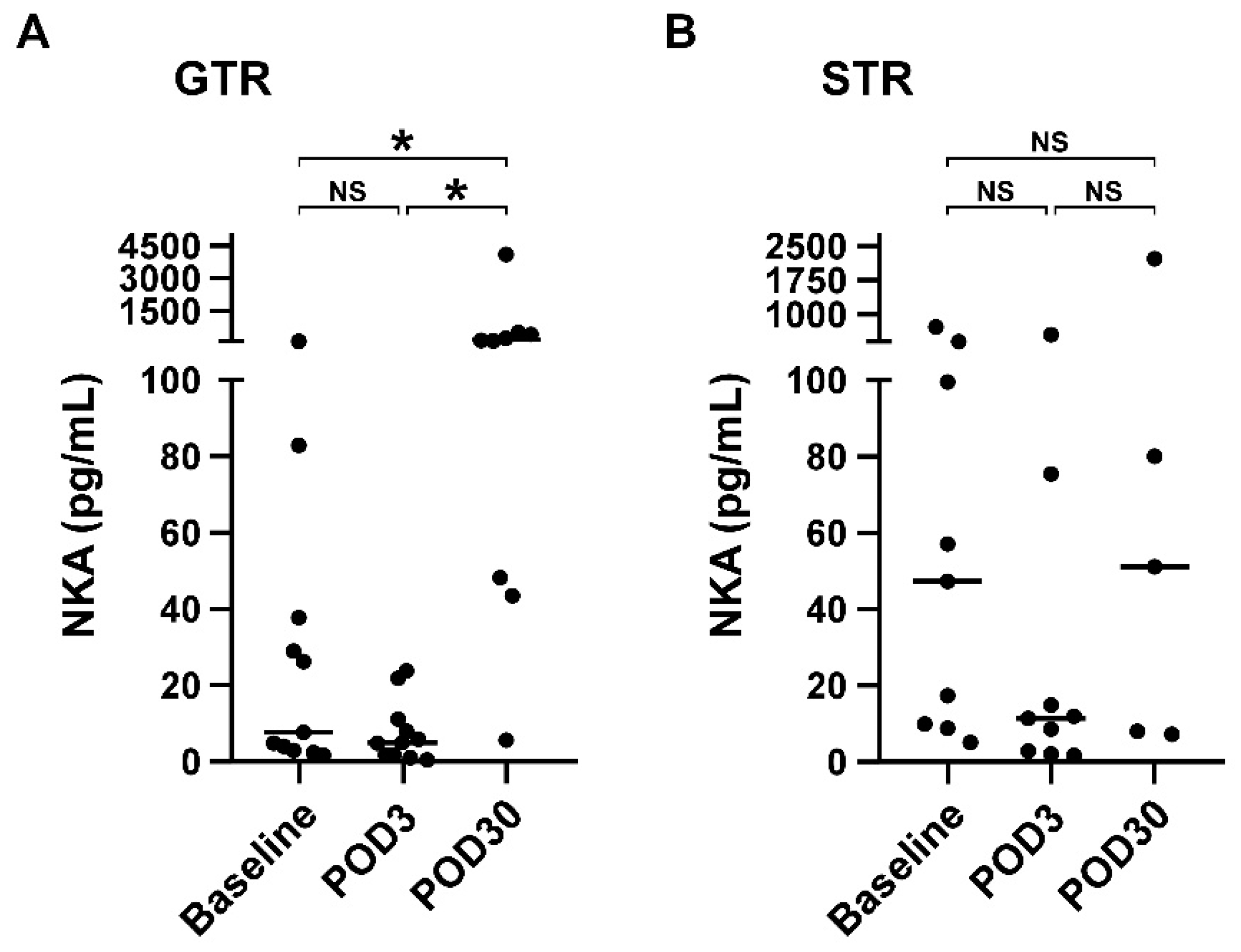
3.4. NKA Is Significantly Increased on POD30 Compared with Baseline in Patients Receiving Gross Total Resection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Trinchieri, G.; Santoli, D.; Koprowski, H. Spontaneous cell-mediated cytotoxicity in humans: Role of interferon and immunoglobulins. J. Immunol. 1978, 120, 1849–1855. [Google Scholar]
- Santoli, D.; Trinchieri, G.; Lief, F.S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J. Immunol. 1978, 121, 526–531. [Google Scholar]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Theresine, M.; Andres, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef]
- Bjorkstrom, N.K.; Ljunggren, H.G.; Michaelsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef]
- Low, J.T.; Ostrom, Q.T.; Cioffi, G.; Neff, C.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. Primary brain and other central nervous system tumors in the United States (2014–2018): A summary of the CBTRUS statistical report for clinicians. Neurooncol. Pract. 2022, 9, 165–182. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neurooncol. 2020, 147, 297–307. [Google Scholar] [CrossRef]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Vybihal, V.; Smrcka, M.; Jancalek, R.; et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front. Oncol. 2020, 10, 840. [Google Scholar] [CrossRef]
- Iversen, P.O.; Hjeltnes, N.; Holm, B.; Flatebo, T.; Strom-Gundersen, I.; Ronning, W.; Stanghelle, J.; Benestad, H.B. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood 2000, 96, 2081–2083. [Google Scholar] [CrossRef]
- Tai, L.H.; Zhang, J.; Scott, K.J.; de Souza, C.T.; Alkayyal, A.A.; Ananth, A.A.; Sahi, S.; Adair, R.A.; Mahmoud, A.B.; Sad, S.; et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin. Cancer Res. 2013, 19, 5104–5115. [Google Scholar] [CrossRef]
- Reinhardt, R.; Pohlmann, S.; Kleinertz, H.; Hepner-Schefczyk, M.; Paul, A.; Flohe, S.B. Invasive Surgery Impairs the Regulatory Function of Human CD56 bright Natural Killer Cells in Response to Staphylococcus aureus. Suppression of Interferon-gamma Synthesis. PLoS ONE 2015, 10, e0130155. [Google Scholar] [CrossRef]
- Leaver, H.A.; Craig, S.R.; Yap, P.L.; Walker, W.S. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur. J. Clin. Investig. 2000, 30, 230–238. [Google Scholar] [CrossRef]
- Market, M.; Tennakoon, G.; Auer, R.C. Postoperative Natural Killer Cell Dysfunction: The Prime Suspect in the Case of Metastasis Following Curative Cancer Surgery. Int. J. Mol. Sci. 2021, 22, 11378. [Google Scholar] [CrossRef]
- Pellegatta, S.; Eoli, M.; Frigerio, S.; Antozzi, C.; Bruzzone, M.G.; Cantini, G.; Nava, S.; Anghileri, E.; Cuppini, L.; Cuccarini, V.; et al. The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology 2013, 2, e23401. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Qu, D.; Sun, R.; Zhang, M.; Nan, K. NK cell-produced IFN-gamma regulates cell growth and apoptosis of colorectal cancer by regulating IL-15. Exp. Ther. Med. 2020, 19, 1400–1406. [Google Scholar] [CrossRef]
- Angka, L.; Martel, A.B.; Kilgour, M.; Jeong, A.; Sadiq, M.; de Souza, C.T.; Baker, L.; Kennedy, M.A.; Kekre, N.; Auer, R.C. Natural Killer Cell IFNgamma Secretion is Profoundly Suppressed Following Colorectal Cancer Surgery. Ann. Surg. Oncol. 2018, 25, 3747–3754. [Google Scholar] [CrossRef]
- Perera Molligoda Arachchige, A.S. Human NK cells: From development to effector functions. Innate Immun. 2021, 27, 212–229. [Google Scholar] [CrossRef]
- Lee, S.B.; Cha, J.; Kim, I.K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Lee, Y.K.; Haam, J.H.; Suh, E.; Cho, S.H.; Kim, Y.S. A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection. J. Clin. Med. 2022, 11, 3014. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I.; Lee, J.M.; Kim, T.I. Impact of Smoking on Human Natural Killer Cell Activity: A Large Cohort Study. J. Cancer Prev. 2020, 25, 13–20. [Google Scholar] [CrossRef]
- Han, Q.; Liang, H.; Cheng, P.; Yang, H.; Zhao, P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients With High-Grade Glioma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Tang, S.; Liao, J.; Long, Y. Comparative assessment of the efficacy of gross total versus subtotal total resection in patients with glioma: A meta-analysis. Int. J. Surg. 2019, 63, 90–97. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Ahmed, F.I.; Abdullah, K.G.; Durgin, J.; Salinas, R.D.; O’Rourke, D.M.; Brem, S. Evaluating the Association Between the Extent of Resection and Survival in Gliosarcoma. Cureus 2019, 11, e4374. [Google Scholar] [CrossRef] [Green Version]
- Corvino, D.; Kumar, A.; Bald, T. Plasticity of NK cells in Cancer. Front. Immunol. 2022, 13, 888313. [Google Scholar] [CrossRef]
- Wang, L.; Liang, B.; Li, Y.I.; Liu, X.; Huang, J.; Li, Y.M. What is the advance of extent of resection in glioblastoma surgical treatment-a systematic review. Chin. Neurosurg. J. 2019, 5, 2. [Google Scholar] [CrossRef]
- Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 1787. [Google Scholar] [CrossRef]
- Lee, H.A.; Goh, H.G.; Lee, Y.S.; Jung, Y.K.; Kim, J.H.; Yim, H.J.; Lee, M.G.; An, H.; Jeen, Y.T.; Yeon, J.E.; et al. Natural killer cell activity is a risk factor for the recurrence risk after curative treatment of hepatocellular carcinoma. BMC Gastroenterol. 2021, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Porzia, A.; Peruzzi, G.; Birarelli, P.; Milana, B.; Sacco, L.; Dinatale, G.; Peparini, N.; Prezioso, G.; Battella, S.; et al. Effect of surgery on pancreatic tumor-dependent lymphocyte asset: Modulation of natural killer cell frequency and cytotoxic function. Pancreas 2015, 44, 386–393. [Google Scholar] [CrossRef]
- Velasquez, J.F.; Ramirez, M.F.; Ai, D.I.; Lewis, V.; Cata, J.P. Impaired Immune Function in Patients Undergoing Surgery for Bone Cancer. Anticancer Res. 2015, 35, 5461–5466. [Google Scholar]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef]
- Verhoef, C.M.; Van Roon, J.A.; Vianen, M.E.; Glaudemans, C.A.; Lafeber, F.P.; Bijlsma, J.W. Lymphocyte stimulation by CD3-CD28 enables detection of low T cell interferon-gamma and interleukin-4 production in rheumatoid arthritis. Scand. J. Immunol. 1999, 50, 427–432. [Google Scholar] [CrossRef]
- Benvenuto, F.; Voci, A.; Carminati, E.; Gualandi, F.; Mancardi, G.; Uccelli, A.; Vergani, L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem. Cell Res. Ther. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.F.; Zhang, Y.N.; Yang, B.Y.; Wu, C.Y. Human memory, but not naive, CD4+ T cells expressing transcription factor T-bet might drive rapid cytokine production. J. Biol. Chem. 2014, 289, 35561–35569. [Google Scholar] [CrossRef] [PubMed]
- Ryken, T.C.; Kuo, J.S.; Prabhu, R.S.; Sherman, J.H.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Steroids in the Treatment of Adults With Metastatic Brain Tumors. Neurosurgery 2019, 84, E189–E191. [Google Scholar] [CrossRef] [Green Version]
- Capellino, S.; Claus, M.; Watzl, C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol. Immunol. 2020, 17, 705–711. [Google Scholar] [CrossRef]
- Chitadze, G.; Fluh, C.; Quabius, E.S.; Freitag-Wolf, S.; Peters, C.; Lettau, M.; Bhat, J.; Wesch, D.; Oberg, H.H.; Luecke, S.; et al. In-depth immunophenotyping of patients with glioblastoma multiforme: Impact of steroid treatment. Oncoimmunology 2017, 6, e1358839. [Google Scholar] [CrossRef]
- Vitale, C.; Chiossone, L.; Cantoni, C.; Morreale, G.; Cottalasso, F.; Moretti, S.; Pistorio, A.; Haupt, R.; Lanino, E.; Dini, G.; et al. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur. J. Immunol. 2004, 34, 3028–3038. [Google Scholar] [CrossRef]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef]
- Furue, H.; Matsuo, K.; Kumimoto, H.; Hiraki, A.; Suzuki, T.; Yatabe, Y.; Komori, K.; Kanemitsu, Y.; Hirai, T.; Kato, T.; et al. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis 2008, 29, 316–320. [Google Scholar] [CrossRef]
- Charap, A.J.; Enokida, T.; Brody, R.; Sfakianos, J.; Miles, B.; Bhardwaj, N.; Horowitz, A. Landscape of natural killer cell activity in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e001523. [Google Scholar] [CrossRef]
- Borg, M.; Wen, S.W.C.; Hansen, T.F.; Jakobsen, A.; Andersen, R.F.; Hilberg, O.; Weinreich, U.M.; Nederby, L. Natural killer cell activity as a biomarker for the diagnosis of lung cancer in high-risk patients. J. Int. Med. Res. 2022, 50, 3000605221108924. [Google Scholar] [CrossRef]
- Marcon, F.; Zuo, J.; Pearce, H.; Nicol, S.; Margielewska-Davies, S.; Farhat, M.; Mahon, B.; Middleton, G.; Brown, R.; Roberts, K.J.; et al. NK cells in pancreatic cancer demonstrate impaired cytotoxicity and a regulatory IL-10 phenotype. Oncoimmunology 2020, 9, 1845424. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.; Song, A.Y.; Choi, J.W.; Lee, H.H.; Kim, M.Y.; Ko, D.H.; Kang, H.J.; Kim, S.W.; Bryceson, Y.; Kim, S.C.; et al. Progressive Impairment of NK Cell Cytotoxic Degranulation Is Associated With TGF-beta1 Deregulation and Disease Progression in Pancreatic Cancer. Front. Immunol. 2019, 10, 1354. [Google Scholar] [CrossRef] [PubMed]


| Variable | Glioblastoma Patients | |
|---|---|---|
| Age, years, median (IQR) | 61.5 (52.3–68.8) | |
| Sex | ||
| Male | 9 (45.0%) | |
| Female | 11 (55.0%) | |
| Glioblastoma | ||
| Primary | 5 (25.0%) | |
| Recurrent | 15 (75.0%) | |
| Extent of resection | ||
| GTR | 11 (55.0%) | |
| STR | 9 (45.0%) | |
| Tumor location | ||
| Frontal lobe | 11 (55.0%) | |
| Parietal lobe | 6 (30.0%) | |
| Temporal lobe | 4 (20.0%) | |
| Insular | 1 (5.0%) | |
| Cerebellum | 1 (5.0%) | |
| Comorbidity | ||
| Diabetes | 5 (25.0%) | |
| Dyslipidemia | 3 (15.0%) | |
| Hypertension | 3 (15.0%) | |
| Asthma | 1 (5.0%) | |
| Polyovarian syndrome | 1 (5.0%) | |
| CSDH | 1 (5.0%) | |
| Gout | 1 (5.0%) | |
| HBV Carrier | 1 (5.0%) | |
| Hepatitis C | 1 (5.0%) | |
| Thyroid goiters | 1 (5.0%) | |
| Time points of blood samples | ||
| Baseline | 20 (100%) | |
| POD3 | 20 (100%) | |
| POD30 | 14 (70.0%) | |
| Baseline | POD3 | POD30 | p-Value † | ||
|---|---|---|---|---|---|
| NKA (pg/mL) | 21.8 (4.9, 76.5) | 7.0 (1.9, 14.2) | 107.6 (34.7, 457.9) | 0.001 | |
| Absolute NK counts (cells/l) | 160.99 (83.7, 325.36) | 138.85 (62.39, 276.13) | 192 (129.06, 262.89) | 0.652 | |
| NK cell subset (n) | |||||
| CD56brightCD16− NK cell (%) | 1 (0.7, 3) | 0.5 (0.3, 1.6) | 0.7 (0.5, 1.2) | 0.095 | |
| CD56dimCD16+ NK cell (%) | 1 (0.7, 3) | 0.5 (0.3, 1.6) | 0.7 (0.5, 1.2) | 0.095 | |
| T cell subset (n) | |||||
| CD4+CD8− T cell (%) | 41.3 (32.3, 57) | 43.4 (29.8, 57.1) | 39.5 (29.2, 68.6) | 0.704 | |
| CD4−CD8+ T cell (%) | 5.4 (3.1, 16.6) | 7.5 (3.1, 16.8) | 5.4 (2.6, 22.3) | 0.382 | |
| CD56+ T cell (%) | 50 (30.7, 55.5) | 46.2 (33.2, 59.4) | 48.7 (26.7, 56.1) | 0.342 | |
| GTR (n = 11) | STR (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | POD3 | POD30 | p-Value † | Baseline | POD3 | POD30 | p-Value † | |
| NKA (pg/mL) | 7.7 (3, 37.8) | 5.0 (1.8, 11.2) | 153.5 (45.9, 482.4) | 0.001 | 47.3 (9.4, 250.2) | 11.4 (2.5, 45.2) | 51.2 (7.7, 1156.8) | 0.316 |
| Absolute NK count (cells/mL) | 222.2 (83.2, 426.2) | 105.7 (62.4, 270.7) | 166.1 (117.4, 205.1) | 0.619 | 160.3 (84.2, 296.5) | 163.8 (77.3, 310.8) | 262.9 (192.0, 266.4) | 0.836 |
| NK cell subset | ||||||||
| CD56brightCD16− NK cell (%) | 1.0 (0.5, 1.9) | 0.5 (0.3, 1.8) | 0.7 (0.6, 1.4) | 0.260 | 1.4 (0.8, 3.9) | 0.5 (0.4, 2.6) | 0.4 (0.3, 1.6) | 0.160 |
| CD56dimCD16+ NK cell (%) | 88.5 (74.2, 92.7) | 89.4 (83, 93.3) | 84.9 (82.0, 87.0) | 0.478 | 80.9 (69.9, 90.4) | 85.1 (73.2, 91.8) | 75.4 (50.6, 89) | 0.600 |
| T cell subset | ||||||||
| CD4+CD8− T cell (%) | 42.8 (30.6, 72.5) | 42.7 (22.9, 67.3) | 39.8 (34.2, 69.3) | 0.984 | 39.8 (33.9, 54.5) | 44.1 (29.9, 52.1) | 36.4 (29.2, 52.0) | 0.956 |
| CD4−CD8+ T cell (%) | 47.7 (25.6, 54.5) | 40.8 (30.1, 51.1) | 46.8 (26.7, 55.7) | 0.946 | 52.7 (36.2, 62.3) | 50.6 (42, 60.7) | 55.2 (43.2, 57.9) | 0.998 |
| CD56+ T cell (%) | 7.4 (3, 23.3) | 8.2 (3.2, 19.4) | 10.9 (4.6, 23.9) | 0.886 | 3.5 (3.1, 10.3) | 3.9 (2.2, 12.4) | 2.6 (2.5, 3.5) | 0.664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; You, J.-F.; Wang, Y.-C.; Lan, S.-W.; Wei, K.-C.; Chen, K.-T.; Huang, Y.-C.; Wu, T.-W.E.; Huang, A.P.-H. Gross Total Resection Promotes Subsequent Recovery and Further Enhancement of Impaired Natural Killer Cell Activity in Glioblastoma Patients. Brain Sci. 2022, 12, 1144. https://doi.org/10.3390/brainsci12091144
Lee C-C, You J-F, Wang Y-C, Lan S-W, Wei K-C, Chen K-T, Huang Y-C, Wu T-WE, Huang AP-H. Gross Total Resection Promotes Subsequent Recovery and Further Enhancement of Impaired Natural Killer Cell Activity in Glioblastoma Patients. Brain Sciences. 2022; 12(9):1144. https://doi.org/10.3390/brainsci12091144
Chicago/Turabian StyleLee, Cheng-Chi, Jeng-Fu You, Yu-Chi Wang, Shao-Wei Lan, Kuo-Chen Wei, Ko-Ting Chen, Yin-Cheng Huang, Tai-Wei Erich Wu, and Abel Po-Hao Huang. 2022. "Gross Total Resection Promotes Subsequent Recovery and Further Enhancement of Impaired Natural Killer Cell Activity in Glioblastoma Patients" Brain Sciences 12, no. 9: 1144. https://doi.org/10.3390/brainsci12091144
APA StyleLee, C.-C., You, J.-F., Wang, Y.-C., Lan, S.-W., Wei, K.-C., Chen, K.-T., Huang, Y.-C., Wu, T.-W. E., & Huang, A. P.-H. (2022). Gross Total Resection Promotes Subsequent Recovery and Further Enhancement of Impaired Natural Killer Cell Activity in Glioblastoma Patients. Brain Sciences, 12(9), 1144. https://doi.org/10.3390/brainsci12091144







