Music Interventions and Delirium in Adults: A Systematic Literature Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Eligibility Criteria
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment (Risk of Bias)
2.5. Data Analysis
2.5.1. Narrative Synthesis
2.5.2. Meta-Analysis and Statistical Methods
3. Results
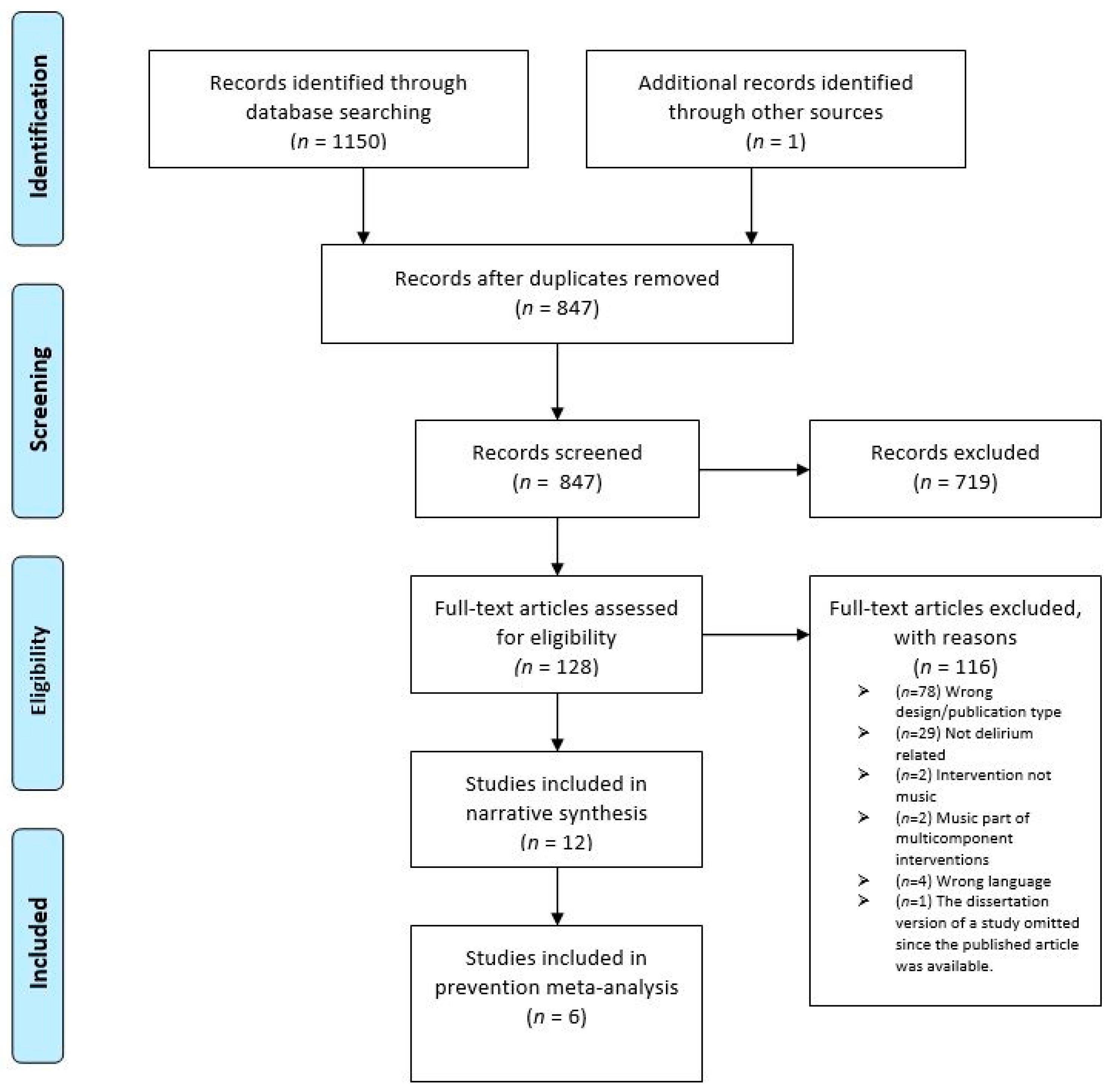
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Research Designs
3.2.2. Samples
3.2.3. Interventions
Music Listening
Music Therapy
3.2.4. Comparators
3.2.5. Outcomes, Tools and Procedures
3.3. Risk of Bias
3.4. Synthesis of Results
3.4.1. Direct Outcomes
Music—No Music (Prevention)
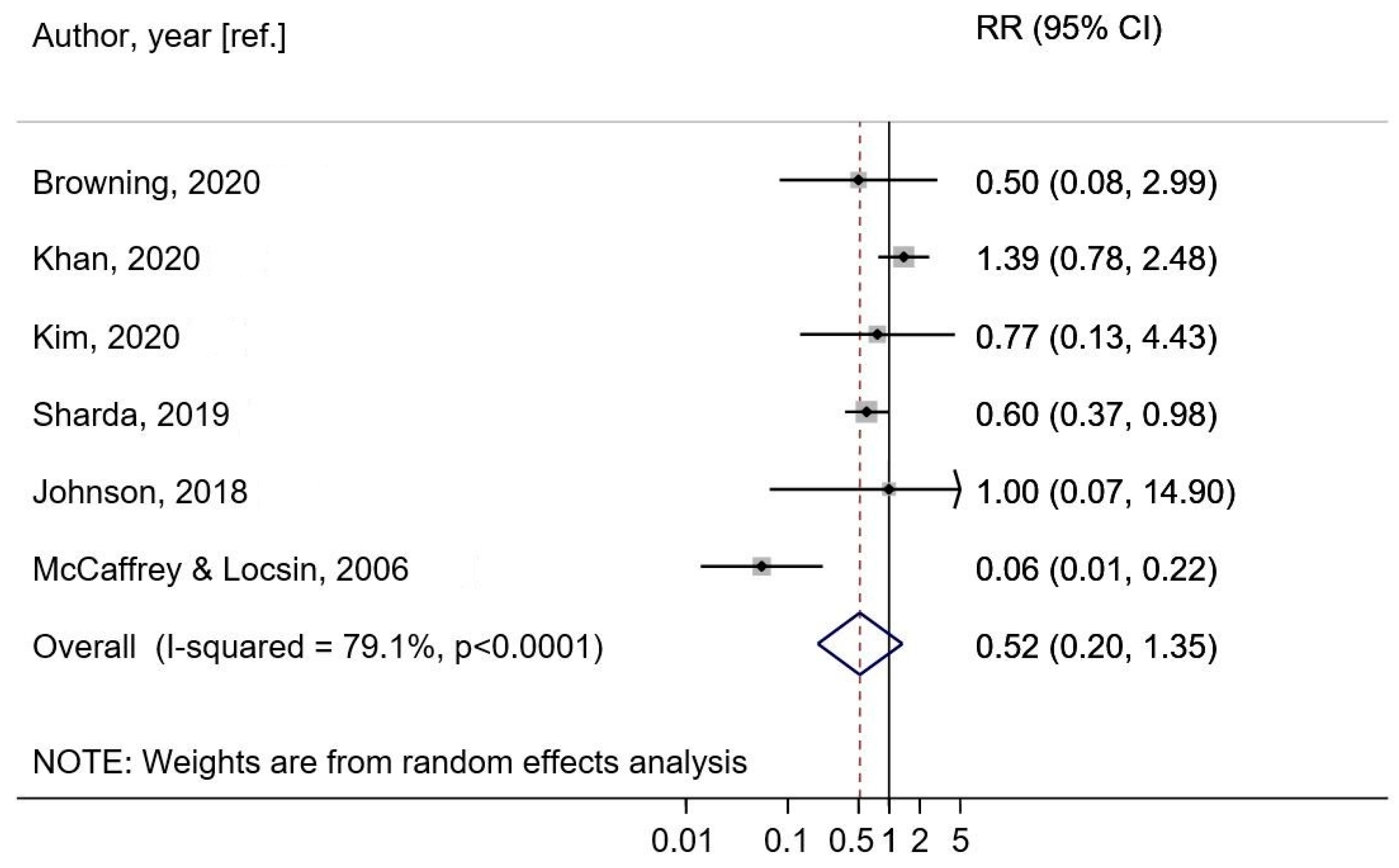
Music—No Music (Prevention Meta-Analysis)
Music—Another Intervention (Treatment)
Music—No Music (Treatment)
3.4.2. Indirect Outcomes
Physiological Measures
Anxiety, Mood, and Engagement
Sleep
4. Discussion
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Marcantonio, E.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Gleason, L.J.; Schmitt, E.M.; Kosar, C.M.; Tabloski, P.; Saczynski, J.S.; Robinson, T.; Cooper, Z.; Rogers, S.O., Jr.; Jones, R.N.; Marcantonio, E.R.; et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015, 150, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Witlox, J.; Eurelings, L.S.; de Jonghe, J.F.; Kalisvaart, K.J.; Eikelenboom, P.; van Gool, W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA 2010, 304, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Krogseth, M.; Wyller, T.B.; Engedal, K.; Juliebø, V. Delirium is a risk factor for institutionalization and functional decline in older hip fracture patients. J. Psychosom. Res. 2013, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.H.J.; Muniz-Terrera, G.; Keage, H.A.D.; Stephan, B.C.M.; Fleming, J.; Ince, P.G.; Matthews, F.E.; Cunningham, C.; Ely, E.W.; MacLullich, A.M.J. Association of delirium with cognitive decline in late life: A neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry 2017, 74, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.G.; Ciampi, A.; Belzile, E.; Dubuc-Sarrasin, M. Subsyndromal Delirium in Older People: A Systematic Review of Frequency, Risk Factors, Course and Outcomes. Focus 2013, 11, 534–543. [Google Scholar] [CrossRef]
- Milisen, K.; Foreman, M.D.; Abraham, I.L.; De Geest, S.; Godderis, J.; Vandermeulen, E.; Fischler, B.; Delooz, H.H.; Spiessens, B.; Broos, P.L.O. A Nurse-Led Interdisciplinary Intervention Program for Delirium in Elderly Hip-Fracture Patients. J. Am. Geriatr. Soc. 2001, 49, 523–532. [Google Scholar] [CrossRef]
- Asghar, A.; Siddiqui, K.M.; Ahsan, K.; Chughtai, S. Postoperative delirium after cardiac surgery; incidence, management and prevention. Anaesth. Pain Intensive Care 2017, 21, 109–112. [Google Scholar]
- Ludolph, P.; Stoffers-Winterling, J.; Kunzler, A.M.; Rösch, R.; Geschke, K.; Vahl, C.F.; Lieb, K. Non-pharmacologic multicomponent interventions preventing delirium in hospitalized people. J. Am. Geriatr. Soc. 2020, 68, 1864–1871. [Google Scholar] [CrossRef]
- Oh, E.S.; Fong, T.G.; Hshieh, T.T.; Inouye, S.K. Delirium in older persons: Advances in diagnosis and treatment. JAMA 2017, 318, 1161–1174. [Google Scholar] [CrossRef]
- Brancatisano, O.; Baird, A.; Thompson, W.F. Why is music therapeutic for neurological disorders? The Therapeutic Music Capacities Model. Neurosci. Biobehav. Rev. 2020, 112, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Ridder, H.M.; Stige, B.; Qvale, L.G.; Gold, C. Individual music therapy for agitation in dementia: An exploratory randomized controlled trial. Aging Ment. Health 2013, 17, 667–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vink, A.C.; Zuidersma, M.; Boersma, F.; de Jonge, P.; Zuidema, S.U.; Slaets, J.P. Effect of music therapy versus recreational activities on neuropsychiatric symptoms in elderly adults with dementia: An exploratory randomized controlled trial. J. Am. Geriatr. Soc. 2014, 62, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wang, Y.; Zhao, X.; Zhang, Z.; Ding, C. Does music therapy affect the global cognitive function of patients with dementia? A meta-analysis. NeuroRehabilitation 2021, 48, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Morales, C.; Calero, R.; Moreno-Morales, P.; Pintado, C. Music Therapy in the Treatment of Dementia: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, J.T.; Smaling, H.J.; van der Wouden, J.C.; Bruinsma, M.S.; Scholten, R.J.; Vink, A.C. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst. Rev. 2018, 7, Cd003477. [Google Scholar] [CrossRef] [Green Version]
- Sibanda, A.; Carnes, D.; Visentin, D.; Cleary, M. A systematic review of the use of music interventions to improve outcomes for patients undergoing hip or knee surgery. J. Adv. Nurs. 2019, 75, 502–516. [Google Scholar] [CrossRef]
- Guerra, G.G.; Almeida, L.; Zorzela, L.; King-Jones, S.; Joffe, A.R.; Hartling, L.; Jou, H.; Vohra, S.; Canadian Critical Care Trials, G. Efficacy of music on sedation, analgesia and delirium in critically ill patients. A systematic review of randomized controlled trials. J. Crit. Care 2019, 53, 75–80. [Google Scholar] [CrossRef]
- Sherriff, C.-A.; Mathews, J.; Reynish, E.L.; Shenkin, S.D. Music therapy for neuropsychiatric symptoms in the general hospital: A systematic literature review. Music Med. 2017, 9, 217–226. [Google Scholar] [CrossRef]
- Khan, S.H.; Kitsis, M.; Golovyan, D.; Wang, S.; Chlan, L.L.; Boustani, M.; Khan, B.A. Effects of music intervention on inflammatory markers in critically ill and post-operative patients: A systematic review of the literature. Heart Lung 2018, 47, 489–496. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; TP Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097-6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the conduct of narrative synthesis in systematic reviews. Prod. ESRC Methods Program. Version 2006, 1, b92. [Google Scholar]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- StataCorp LP. Stata data analysis and statistical Software. Spec. Ed. Release 2007, 10, 733. [Google Scholar]
- Khan, S.H.; Xu, C.; Purpura, R.; Durrani, S.; Lindroth, H.; Wang, S.; Gao, S.; Heiderscheit, A.; Chlan, L.; Boustani, M.; et al. Decreasing Delirium Through Music: A Randomized Pilot Trial. Am. J. Crit. Care 2020, 29, e31–e38. [Google Scholar] [CrossRef] [Green Version]
- Giovagnoli, A.R.; Manfredi, V.; Schifano, L.; Paterlini, C.; Parente, A.; Tagliavini, F. Combining drug and music therapy in patients with moderate Alzheimer’s disease: A randomized study. Neurol. Sci. 2018, 39, 1021–1028. [Google Scholar] [CrossRef]
- Kim, J.; Choi, D.; Yeo, M.S.; Yoo, G.E.; Kim, S.J.; Na, S. Effects of patient-directed interactive music therapy on sleep quality in postoperative elderly patients: A randomized-controlled trial. Nat. Sci. Sleep 2020, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Fleury, J.; McClain, D. Music intervention to prevent delirium among older patients admitted to a trauma intensive care unit and a trauma orthopaedic unit. Intensive Crit. Care Nurs. 2018, 47, 7–14. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, R. The effect of music on acute confusion in older adults after hip or knee surgery. Appl. Nurs. Res. 2009, 22, 107–112. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, R.; Locsin, R. The effect of music on pain and acute confusion in older adults undergoing hip and knee surgery. Holist. Nurs. Pract. 2006, 20, 218–224. [Google Scholar] [CrossRef]
- McCaffrey, R.; Locsin, R. The effect of music listening on acute confusion and delirium in elders undergoing elective hip and knee surgery. J. Clin. Nurs. 2004, 13, 91–96. [Google Scholar] [CrossRef]
- Sharda, N.; Mattoon, E.; Matters, L.; Prewitt, J.; McDonald, S.; Sloane, R.; Cassas, C.; White, H. Bach to the Basics: Implementation and Impact of a Postoperative, Inpatient Personalized Music Program for Older Adults. J. Perianesth. Nurs. 2019, 34, 347–353. [Google Scholar] [CrossRef]
- Correâ, L.; Caparrol, A.J.D.S.; Martins, G.; Pavarini, S.C.I.; Grataõ, A.C.M. Effects of music on body and facial expressions and psychological and behavioral symptoms of older adults. Braz. J. Occup. Ther. 2020, 28, 539–553. [Google Scholar]
- Cheong, C.Y.; Tan, J.A.; Foong, Y.L.; Koh, H.M.; Chen, D.Z.; Tan, J.J.; Ng, C.J.; Yap, P. Creative Music Therapy in an Acute Care Setting for Older Patients with Delirium and Dementia. Dement. Geriatr. Cogn. Dis. Extra 2016, 6, 268–275. [Google Scholar] [CrossRef]
- Helmes, E.; Wiancko, D.C. Effects of music in reducing disruptive behavior in a general hospital. J. Am. Psychiatr. Nurses Assoc. 2006, 12, 37–44. [Google Scholar] [CrossRef]
- Browning, S.G.; Watters, R.; Thomson-Smith, C. Impact of therapeutic music listening on intensive care unit patients: A pilot study. Nurs. Clin. 2020, 55, 557–569. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Twiss, E.; Seaver, J.; McCaffrey, R. The effect of music listening on older adults undergoing cardiovascular surgery. Nurs. Crit. Care 2006, 11, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, G.L.; Schwab, R.J.; Watson, P.L.; Patil, N.; Vaccaro, B.; Pandharipande, P.; Ely, E.W. Bench-to-bedside review: Delirium in ICU patients—Importance of sleep deprivation. Crit. Care 2009, 13, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Primers 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
| Study 1 and Design | Setting and Participants | Mean Age (±SD) 2,3 | Enrolment Criteria (Delirium-Related) | Number of Participants |
|---|---|---|---|---|
| Khan et al., 2020 [31] RCT (3 gr.) | Medical and surgical ICU (mechanically ventilated patients) | Total: 57.4 (±14.2) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 56 Data analyzed: 52 |
| Giovagnoli et al., 2018 [32] RCT (2 gr.) | LTC facilities or outpatient hospitals (moderate Alzheimer’s patients) | M-AMT: 74.3 (±5.7) M:72.0 (±7.3) | Probable dementia, (delirium symptom of advancing dementia) | Enrolled: n = 45 Data analyzed: 43 |
| McCaffrey and Locsin, 2006 [36] RCT (2 gr.) | Postoperative orthopedic unit (hip/knee patients) | Total: 75.7 (±6.1) EG:76.8 (±5.1) CG:77.3 (±5.4) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 126 Data analyzed: 124 |
| McCaffrey 2009 [35] RCT (2 gr.) | Postoperative orthopedic unit (hip/knee patients) | EG:74.5 (±4.8) CG:75.9 (±1.2) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 22 Data analyzed: 22 |
| Kim et al., 2020 [33] RCT (3 gr.) | Postoperative ICU (postsurgical patients) | IMT:74.6 (±5.2) PML:72.3 (±4.7) CG:74.1 (±6.7) | Delirium risk (not diagnosed at enrolment) | Enrolled: 147 Data analyzed: 133 |
| Johnson et al., 2018 [34] RCT (2 gr.) | TICU and TOU (postsurgical patients) | Total: 71.8 (±9.2) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 40 Data analyzed: 40 |
| Browning et al., 2020 [42] Prospective cohort study (2 gr.) | Medical ICU (mechanically ventilated patients) | MLG: 64 (±12.96) CG:71 (±4.51) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 6 Data analyzed: 6 |
| Correa et al., 2020 [39] Quasi-experimental study (2 gr.) | LTC institutions (patients with dementia/probable dementia) | IGPM: 85.1 (±8.7) CGCM: 85.3 (±7.6) | Probable dementia; (delirium symptom of advancing dementia) | Enrolled: n = 33 Data analyzed: 33 |
| McCaffrey and Locsin, 2004 [37] RCT (2 gr.) | Postoperative orthopedic unit (hip/knee patients) | Total: 73.3 (±4.8) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 66 Data analyzed: 66 |
| Cheong et al., 2016 [40] One-sample, within-subject | ACU (patients with delirium and dementia) | Total: 86.5 (±5.7) | Dementia with or without delirium | Enrolled: n = 25 Data analyzed: 25 (8 had delirium) |
| Sharda et al., 2019 [38] Pre-experimental (2 static gr.) | POSH clinic (postsurgical inpatients) | POSH: 75.0 CALM:74.6 (SD not reported) | Delirium risk (not diagnosed at enrolment) | Enrolled: n = 109 Data analyzed: 45 |
| Helmes and Wiancko, 2006 [41] One-sample, within-subject (multiple case study) | ACU (geriatric assessment ward and family medicine ward patients) | Total: 82.7 (±7.4) | Diagnosis of dementia and delirium | Enrolled: n = 9, (2 had delirium) Data analyzed: 7 (including 2 with delirium) |
| Study 1 | Intervention 2,3 and Control | Primary Aims (Delirium-Related) | Dose and Delivery |
|---|---|---|---|
| Khan et al., 2020 [31] | EC: Personalized music listening (n = 17) EC: Pre-selected slow tempo music listening (n = 17) CC: Audiobook/attention control (n = 18) | Prevention and treatment (To impact the incidence and severity of delirium in ICU patients) | 2 × 60 min p/day; 7 days (the same dose for all interventions) |
| Giovagnoli et al., 2018 [32] | EC: Active music therapy and Memantine (AMT) (n = 23) CC: Memantine (M) added to AchEI (n = 22) | Treatment (To affect language, global cognitive functioning, psycho-behavioral and social aspects, and daily activities of LTC patients) | AMT: 2 × 40 min p/week; 24 weeks. M: 20 mg per day |
| McCaffrey and Locsin, 2006 [36] | EC: Pre-selected music listening (n = 62) CC: Usual care (n = 62) | Prevention and treatment (To affect pain, cognition/acute confusion, the ability to ambulate and general satisfaction in postsurgical hip/knee patients) | Min. 1–4 × p/day, unreported duration; from awakening from anesthesia until discharge |
| McCaffrey 2009 [35] | EC: Pre-selected music listening (n = 11) CC: Usual care (n = 11) | Prevention and treatment (To affect cognitive function and acute confusion in postsurgical hip/knee patients) | Min. 4 × 60 min p/day; from awakening from anesthesia until discharge |
| Kim et al., 2020 [33] | EC: Interactive music therapy (IMT) (n = 44) EC: Passive, pre-selected, music listening (PML) (n = 44) CC: Usual care (n = 45) | Prevention (To prevent delirium through affecting sleep quality, melatonin/cortisol levels and pain in postsurgical ICU patients) | IMT: daytime (15–20 min), night-time (30 min). PML: night-time (30 min); from awakening until discharge |
| Johnson et al., 2018 [34] | EC: Pre-selected music listening (n = 20) CC: Usual care (n = 20) | Prevention and treatment (To affect delirium through decreasing physiologic variables in postsurgical patients) | 2 × 60 min, p/day; 3 days (at 2 p.m. and 8 p.m.) |
| Browning et al., 2020 [42] | EC: Personalized music listening (n = 3) CC: Usual care (n = 3) | Prevention and treatment (To impact incidence and severity of delirium in ICU patients) | 2 × 60 min p/day; 2 weeks |
| Correa et al., 2020 [39] | CC: Pre-selected Classical Music listening (n = 14) EC: Popular, Brazilian, personalized music listening (n = 19) | Treatment (To affect physiological, behavioral, and expressive outcomes in LTC patients with dementia/delirium) | 4 × 20 min p/week (same dose for both interventions) |
| McCaffrey and Locsin, 2004 [37] | EC: Pre-selected music listening (n = 33) CC: Usual care (n = 33) | Prevention and treatment (To reduce delirium episodes in postsurgical hip/knee patients) | Max. 3 × 60 min p/day, (or at any time desired); from awakening until discharge |
| Cheong et al., 2016 [40] | EC: Creative Music Therapy (CMT) CC: The usual care (n = 25; 8 had delirium) | Treatment (To impact mood and engagement in AC patients with delirium/dementia) | CMT: 1 × 30 min p/day; 2 days |
| Sharda et al., 2019 [38] | EC: Confusion Avoidance Led by personalized Music (CALM) (n = 45) CC: Usual care (157) | Prevention and treatment (By affecting pain and anxiety to prevent/treat delirium in postsurgical inpatients) | CALM: Min. 2 × 20 min p/day, or at any time desired |
| Helmes and Wiancko, 2006 [41] | EC: Pre-selected music listening (Baroque music) CC: No music (2 trials of each condition compared in n = 9 participants; 2 had delirium) | Treatment (To reduce the frequency of disruptive behaviors in AC patients) | Minimum 4 × 30 min per day—minimum 3 days |
| Study 1 | Delirium Outcomes and Tools | Other Outcomes and Tools 2 |
|---|---|---|
| Khan et al., 2020 [31] | OUTCOMES: Number of delirium-free/coma-free days and severity TOOLS: RASS; CAM-ICU; CAM-ICU-7 | Anxiety (Face Anxiety Scale—VAS) Pain (CPOT) Physiological stress (HR, BP, RR) Sleep (STOP-BANG-RCS-Q) Mobility (physical/occupational therapy notes) |
| Giovagnoli et al., 2018 [32] | OUTCOMES: NR but delirium measured as one of the neuropsychiatric symptoms of advancing dementia TOOLS: NPI-Q | Language (SIB-L) Social interactions, memory, orientation, attention, praxis, visual–spatial ability and orientation (SIB) Independence in daily activities, instrumental activities (ADL and IADL) Psychic and behavioral symptoms of dementia (NPI-Q) Neurocognitive functions (MMSE) Perceived social support (LSNS) |
| McCaffrey and Locsin, 2006 [36] | OUTCOMES: Number of episodes of delirium/acute confusion TOOLS: Nurses’ narrative notes in medical records | Pain (numeric rating scale; number of pain medications) Ambulation (medical records and notes from nurses and physical therapists) Patient satisfaction (self-rating-post-discharge phone call.) |
| McCaffrey 2009 [35] | OUTCOMES: Presence and severity of delirium/acute confusion TOOLS: NEECHAM | Cognitive function (MMSE) Physiological measurements (oxygen saturation, BP, RR) |
| Kim et al., 2020 [33] | OUTCOMES: Incidence of delirium TOOLS: CAM-ICU | Quality and the duration of sleep (RCS-Q) Pain (NRS) Recovery after anesthesia (QoR-40) Cortisol and melatonin levels (Salivette tube) |
| Johnson et al., 2018 [34] | OUTCOMES: Presence of delirium/acute confusion TOOLS: CAM-ICU | Physiological measurements (SBP, HR, RR) |
| Browning et al., 2020 [42] | OUTCOMES: Incidence and severity of delirium TOOLS: CAM-ICU; RASS | NR |
| Correa et al., 2020 [39] | OUTCOMES: NR, but delirium measured as one of the neuropsychiatric symptoms of advancing dementia TOOLS: NPI-Q | Severity of neuropsychiatric manifestation (NPI-Q). Cardiovascular biofeedback (Cardio emotion) Facial expressions (FACS) Body movements (reactions grouped into body parts) |
| McCaffrey and Locsin, 2004 [37] | OUTCOMES: Number of delirium episodes TOOLS: Nurses’ notes and checklists | Ambulation (physiotherapists’ notes) |
| Cheong et al., 2016 [40] | OUTCOMES: NR, but delirium is assessed at baseline TOOLS: CAM | Engagement regulation (MPES) Mood regulation (OERS) |
| Sharda et al., 2019 [38] | OUTCOME: Incidence of delirium TOOL: ICD codes | Length of hospital stay (hospital records) Pain and mood (patient survey) |
| Helmes and Wiancko, 2006 [41] | OUTCOMES: NR, but delirium is assessed at baseline TOOLS: NR | Frequency and incidence of repetitive vocalizations/shouting and banging objects (systematic observations) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubovic, J.; Neerland, B.E.; Aune, D.; Baker, F.A. Music Interventions and Delirium in Adults: A Systematic Literature Review and Meta-Analysis. Brain Sci. 2022, 12, 568. https://doi.org/10.3390/brainsci12050568
Golubovic J, Neerland BE, Aune D, Baker FA. Music Interventions and Delirium in Adults: A Systematic Literature Review and Meta-Analysis. Brain Sciences. 2022; 12(5):568. https://doi.org/10.3390/brainsci12050568
Chicago/Turabian StyleGolubovic, Jelena, Bjørn Erik Neerland, Dagfinn Aune, and Felicity A. Baker. 2022. "Music Interventions and Delirium in Adults: A Systematic Literature Review and Meta-Analysis" Brain Sciences 12, no. 5: 568. https://doi.org/10.3390/brainsci12050568
APA StyleGolubovic, J., Neerland, B. E., Aune, D., & Baker, F. A. (2022). Music Interventions and Delirium in Adults: A Systematic Literature Review and Meta-Analysis. Brain Sciences, 12(5), 568. https://doi.org/10.3390/brainsci12050568