Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting
2.2. Participants
2.2.1. Patients
2.2.2. Inclusion and Exclusion Criteria
2.3. Outcome Measures
2.3.1. Cognitive Function Research
2.3.2. Assessment of Affective Disorders and Fatigue
2.3.3. The Hachinski Ischemic Scale
2.3.4. Neuroimaging
2.3.5. Neurophysiological Testing
2.4. Statistical Analysis
3. Results
3.1. Types of Ischemic Stroke, Comorbidities and Neuroimaging Parameters
3.2. Cognitive and Psychoemotional Disorders
3.3. Study of Cognitive Evoked Potentials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.G.; Thayabaranathan, T.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.L.; Norrving, B.; Donnan, G.A.; Cadilhac, D.A. Global stroke statistics. Int. J. Stroke 2017, 12, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.M.; Valente, I.; Frisullo, G.; Morosetti, R.; Genovese, D.; Bartolo, A.; Gigli, R.; Rollo, C.; Scarcia, L.; Carosi, F.; et al. Mechanical thrombectomy in patients with stroke due to large vessel occlusion in the anterior circulation and low baseline NIHSS score. J. Integr. Neurosci. 2021, 20, 645–650. [Google Scholar] [CrossRef]
- Haussen, D.C.; Lima, F.O.; Bouslama, M.; Grossberg, J.A.; Silva, G.S.; Lev, M.H.; Furie, K.; Koroshetz, W.; Frankel, M.R.; Nogueira, R.G. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: An analysis from STOPStroke and GESTOR cohorts. J. Neurointerv. Surg. 2018, 10, 325–329. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.; Savitz, S.I.; Grotta, J.C.; Cai, C.; Parsha, K.N.; Farrell, C.M.; Imam, B.; Sitton, C.W.; Reddy, S.T.; et al. Endovascular Thrombectomy for Mild Strokes: How Low Should We Go? Stroke 2018, 49, 2398–2405. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Awad, L.N.; Lewek, M.D.; Kesar, T.M.; Franz, J.R.; Bowden, M.G. These legs were made for propulsion: Advancing the diagnosis and treatment of post-stroke propulsion deficits. J. Neuroeng. Rehabil. 2020, 17, 136. [Google Scholar] [CrossRef]
- Tater, P.; Pandey, S. Post-stroke Movement Disorders: Clinical Spectrum, Pathogenesis, and Management. Neurol. India 2021, 69, 272–283. [Google Scholar] [CrossRef]
- Mijajlović, M.D.; Pavlović, A.; Brainin, M.; Heiss, W.D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A.; et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017, 15, 11. [Google Scholar] [CrossRef]
- Barbay, M.; Diouf, M.; Roussel, M.; Godefroy, O. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement. Geriatr. Cogn. Disord. 2018, 46, 322–334. [Google Scholar] [CrossRef]
- Weaver, N.A.; Kuijf, H.J.; Aben, H.P.; Abrigo, J.; Bae, H.J.; Barbay, M.; Best, J.G.; Bordet, R.; Chappell, F.M.; Chen, C.P.L.H.; et al. Strategic infarct locations for post-stroke cognitive impairment: A pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol. 2021, 20, 448–459. [Google Scholar] [CrossRef]
- Debette, S.; Schilling, S.; Duperron, M.G.; Larsson, S.C.; Markus, H.S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef]
- Gupta, A.; Giambrone, A.E.; Gialdini, G.; Finn, C.; Delgado, D.; Gutierrez, J.; Wright, C.; Beiser, A.S.; Seshadri, S.; Pandya, A.; et al. Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 719–725. [Google Scholar] [CrossRef]
- Parfenov, V.A. Vascular cognitive impairment and chronic cerebral ischemia (dyscirculatory encephalopathy). Neurol. Neuropsychiatry Psychosom. 2019, 11, 61–67. [Google Scholar] [CrossRef]
- Zirk, M.; Storm, V. Subjective Stroke Impact and Depressive Symptoms: Indications for a Moderating Role of Health-Related Locus of Control. Front. Psychiatry 2019, 10, 918. [Google Scholar] [CrossRef]
- Perna, R.; Harik, L. The role of rehabilitation psychology in stroke care described through case examples. NeuroRehabilitation 2020, 46, 195–204. [Google Scholar] [CrossRef]
- Brainin, M.; Tuomilehto, J.; Heiss, W.D.; Bornstein, N.M.; Bath, P.M.; Teuschl, Y.; Richard, E.; Guekht, A.; Quinn, T.; Post Stroke Cognition Study Group. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015, 22, 229-e16. [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Thommessen, B.; Wyller, T.B.; Engedal, K.; Fure, B. Risk factors for and incidence of subtypes of ischemic stroke. Funct. Neurol. 2012, 27, 35–40. [Google Scholar]
- Stiekema, A.P.M.; Nijsse, B.; de Kort, P.L.M.; Spikman, J.M.; Visser-Meily, J.M.A.; van Heugten, C.M. The relationship between social cognition and participation in the long term after stroke. Neuropsychol. Rehabil. 2021, 31, 278–292. [Google Scholar] [CrossRef]
- Halassa, M.M.; Kastner, S. Thalamic functions in distributed cognitive control. Nat. Neurosci. 2017, 20, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Todd, B.K. A comparative perspective on lateral biases and social behavior. In Cerebral Lateralization and Cognition: Evolutionary and Developmental Investigations of Behavioral Biases; Progress in Brain Research; Forrester, G.S., Hopkins, W.D., Hudry, K., Lindell, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 238, pp. 377–403. [Google Scholar] [CrossRef]
- Blake, M.L.; Tompkins, C.A.; Scharp, V.L.; Meigh, K.M.; Wambaugh, J. Contextual Constraint Treatment for coarse coding deficit in adults with right hemisphere brain damage: Generalisation to narrative discourse comprehension. Neuropsychol. Rehabil. 2015, 25, 15–52. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Zietemann, V.; Georgakis, M.K.; Dondaine, T.; Müller, C.; Mendyk, A.M.; Kopczak, A.; Hénon, H.; Bombois, S.; Wollenweber, F.A.; Bordet, R.; et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology 2018, 91, e1838–e1850. [Google Scholar] [CrossRef]
- Lim, K.B.; Kim, J.; Lee, H.J.; Yoo, J.H.; You, E.C.; Kang, J. Correlation between montreal cognitive assessment and functional outcome in subacute stroke patients with cognitive dysfunction. Ann. Rehabil. Med. 2018, 42, 26–34. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, S.W.; Kim, C.; Kim, G.H.; Noh, H.J.; Kim, S.T.; Kwak, K.C.; Yoon, U.; Lee, J.M.; Lee, J.W.; et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann. Neurol. 2013, 73, 584–593. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, B.R.; Han, E.Y. Association between Evoked Potentials and Balance Recovery in Subacute Hemiparetic Stroke Patients. Ann. Rehabil. Med. 2015, 39, 451–461. [Google Scholar] [CrossRef]
- Dejanović, M.; Ivetić, V.; Nestorović, V.; Erić, M.; Stanojević, Z.; Leštarević, S. The role of P300 event-related potentials in the cognitive recovery after the stroke. Acta Neurol. Belg. 2015, 115, 589–595. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, 46–110. [Google Scholar] [CrossRef]
- Hong, J.S.; Lee, J.H.; Yoon, Y.H.; Choi, J.H.; Shin, J.E.; Kim, S.M.; Park, Y.G. The assessment of reliability of cognitive evoked potential in normal person. Ann. Rehabil. Med. 2013, 37, 263–268. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Publishing: Arlington, VA, USA, 2013; 992p. [Google Scholar]
- Tang, E.; Price, C.I.; Robinson, L.; Exley, C.; Desmond, D.W.; Köhler, S.; Staals, J.; Yin Ka Lam, B.; Wong, A.; STROKOG Collaboration; et al. Assessing the Predictive Validity of Simple Dementia Risk Models in Harmonized Stroke Cohorts. Stroke 2020, 51, 2095–2102. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Vakhnina, N.V. Stroke and cognitive disorders. Neurol. Neuropsychiatry Psychosom. 2011, 3, 8–16. (In Russian) [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Thommessen, B.; Wyller, T.B.; Engedal, K.; Øksengård, A.R.; Stenset, V.; Løken, K.; Aaberg, M.; Fure, B. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement. Geriatr. Cogn. Disord. 2011, 32, 401–407. [Google Scholar] [CrossRef]
- Pelton, T.A.; Wing, A.M.; Fraser, D.; van Vliet, P. Differential Effects of Parietal and Cerebellar Stroke in Response to Object Location Perturbation. Front. Hum. Neurosci. 2015, 9, 293. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Jia, Y. Cortical infarction of the right parietal lobe and neurogenic heart disease: A report of three cases. Neural Regen. Res. 2012, 7, 943–947. [Google Scholar] [CrossRef]
- Thompson, H.E.; Henshall, L.; Jefferies, E. The role of the right hemisphere in semantic control: A case-series comparison of right and left hemisphere stroke. Neuropsychologia 2016, 85, 44–61. [Google Scholar] [CrossRef]
- Wang, J.; Conder, J.A.; Blitzer, D.N.; Shinkareva, S.V. Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2010, 31, 1459–1468. [Google Scholar] [CrossRef]
- Desai, R.H.; Reilly, M.; van Dam, W. The multifaceted abstract brain. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170122. [Google Scholar] [CrossRef]
- Molad, J.; Hallevi, H.; Korczyn, A.D.; Kliper, E.; Auriel, E.; Bornstein, N.M.; Ben Assayag, E. Vascular and Neurodegenerative Markers for the Prediction of Post-Stroke Cognitive Impairment: Results from the TABASCO Study. J. Alzheimers Dis. 2019, 70, 889–898. [Google Scholar] [CrossRef]
- Jacquin, A.; Binquet, C.; Rouaud, O.; Graule-Petot, A.; Daubail, B.; Osseby, G.V.; Bonithon-Kopp, C.; Giroud, M.; Béjot, Y. Post-stroke cognitive impairment: High prevalence and determining factors in a cohort of mild stroke. J. Alzheimers Dis. 2014, 40, 1029–1038. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Kim, J.S. Post-stroke Mood and Emotional Disturbances: Pharmacological Therapy Based on Mechanisms. J. Stroke 2016, 18, 244–255. [Google Scholar] [CrossRef]
- Terroni, L.; Sobreiro, M.; Conforto, A.B.; Adda, C.C.; Guajardo, V.D.; de Lucia, M.; Fráguas, R. Association among depression, cognitive impairment and executive dysfunction after stroke. Dement. Neuropsychol. 2012, 6, 152–157. [Google Scholar] [CrossRef]
- Douiri, A.; Rudd, A.G.; Wolfe, C.D. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 2013, 44, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, H.; Kalska, H.; Ylikoski, R.; Madureira, S.; Verdelho, A.; van der Flier, W.M.; Scheltens, P.; Barkhof, F.; Visser, M.C.; Fazekas, F.; et al. Longitudinal cognitive decline in subcortical ischemic vascular disease: The LADIS Study. Cerebrovasc. Dis. 2009, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Radman, N.; Staub, F.; Aboulafia-Brakha, T.; Berney, A.; Bogousslavsky, J.; Annoni, J.-M. Poststroke fatigue following minor infarcts: A prospective study. Neurology 2012, 79, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Cushing, B.; Rohlfing, G.; Edwards, M.; Davenloo, H.; D’Agostino, D.; Hall, J.R.; O’Bryant, S.E. The Hachinski ischemic scale and cognition: The influence of ethnicity. Age Ageing 2014, 43, 364–369. [Google Scholar] [CrossRef]
- Braverman, E.R.; Blum, K.; Damle, U.J.; Kerner, M.; Dushaj, K.; Oscar-Berman, M. Evoked potentials, and neuropsychological tests validate Positron Emission Topography (PET) brain metabolism in cognitively impaired patients. PLoS ONE 2013, 8, e55398. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Mishra, N.; Tripathi, R.K.; Gurnani, K.C. P300 latency as an indicator of severity in major depressive disorder. Ind. Psychiatry J. 2015, 24, 163–167. [Google Scholar] [CrossRef]
- Campanella, S. Use of cognitive event-related potentials in the management of psychiatric disorders: Towards an individual follow-up and multi-component clinical approach. World J. Psychiatry 2021, 11, 153–168. [Google Scholar] [CrossRef]
| Type of Ischemic Stroke | Group 1, n = 40 | Group 2, n = 40 | p Value |
|---|---|---|---|
| Cardioembolic | 8 (20%) | 7 (17.5%) | 0.82 |
| Lacunar | 12 (30%) | 10 (25%) | 0.617 |
| Atherothrombotic | 16 (40%) | 20 (50%) | 0.369 |
| Other | 4 (10%) | 3 (7.5%) | 0.755 |
| Neuroimaging (localization of the focus) | |||
| Frontal cortex | 1 (2.5%) | 0% | 0.314 |
| Basal ganglia | 6 (15%) | 14 (35%) * | 0.039 |
| Fronto-temporal cortex | 4 (10%) | 4 (10%) | 1.0 |
| Temporal cortex | 1 (2.5%) | 0% | 0.314 |
| Parietal cortex | 12 (30%) * | 4 (10%) | 0.025 |
| Parieto-temporal cortex | 2 (5%) | 5 (12.5%) | 0.211 |
| Parieto-occipital cortex | 3 (7.5%) | 6 (15%) | 0.327 |
| ASPECTS score (points) | 9.0 ± 2.7 | 9.1 ± 2.8 | 1.0 |
| Neuroimaging (preexisting conditions) | |||
| Cortical atrophy | 11 (27.5%) * | 4 (10%) | 0.045 |
| Leukoaraiosis | 2 (5%) | 18 (45%) * | 0.00001 |
| Hydrocephalus | 20 (50%) | 20 (50%) | 1.0 |
| CT evidence of recurrent stroke | 12 (30%) * | 4 (10%) | 0.025 |
| Electrode | Group 1, n = 40 | Group 2, n = 40 | p-Value | Electrode | Group 1, n = 40 | Group 2, n = 40 | p-Value |
|---|---|---|---|---|---|---|---|
| F3–A1 | F4–A2 | ||||||
| Lat P300 | 476.4 ± 8.7 * | 430.4 ± 7.8 | 0.00001 | Lat P300 | 466.2 ± 8.4 * | 436.3 ± 5.6 | 0.00001 |
| Amp P300 | 8.0 ± 3.4 | 6.7 ± 2.1 * | 0.043 | Amp P300 | 8.9 ± 3.3 | 7.6 ± 2.6 | 0.0539 |
| C3–A1 | C4–A2 | ||||||
| Lat P300 | 472.2 ± 8.3 * | 424.2 ± 7.9 | 0.00001 | Lat P300 | 485.8 ± 7.9 * | 413.4 ± 7.2 | 0.00001 |
| Amp P300 | 8.1 ± 2.4 | 6.8 ± 2.6 * | 0.0227 | Amp P300 | 9.0 ± 3.3 | 7.7 ± 1.9 * | 0.0339 |
| Fz–A1 | Cz–A2 | ||||||
| Lat P300 | 424.3 ± 8.4 * | 413.3 ± 7.7 | 0.00001 | Lat P300 | 455.8 ± 7.6 * | 427.7 ± 5.3 | 0.00001 |
| Amp P300 | 9.4 ± 3.7 | 6.3 ± 2.8 * | 0.001 | Amp P300 | 9.9 ± 3.9 | 8.9 ± 3.2 | 0.2137 |
| Parameter | Group 1, n = 40 | Group 2, n = 40 |
|---|---|---|
| Lesion localization | Parietal cortex | Subcortical structures |
| Neuroimaging markers | Atrophy, signs of recurrent ischemic stroke | Signs of chronic brain ischemia (leukoaraiosis) |
| Neurophysiological markers | P300 latency prolongation in all leads | Decreased amplitude of P300 in Fz–A1, C3, F3, C4 leads |
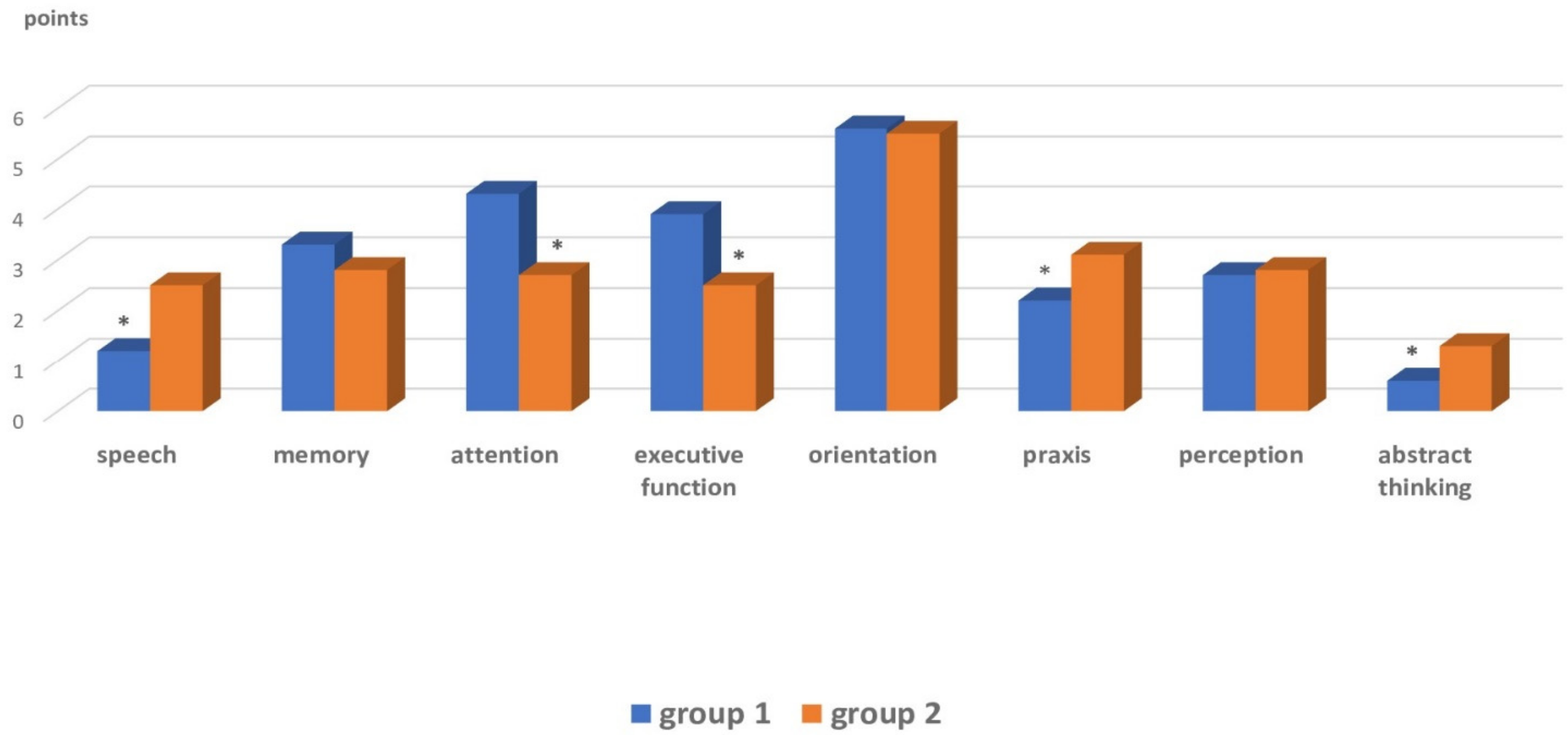
| Cognitive dysfunction | Praxis, speech, abstract thinking | Executive function, attention |
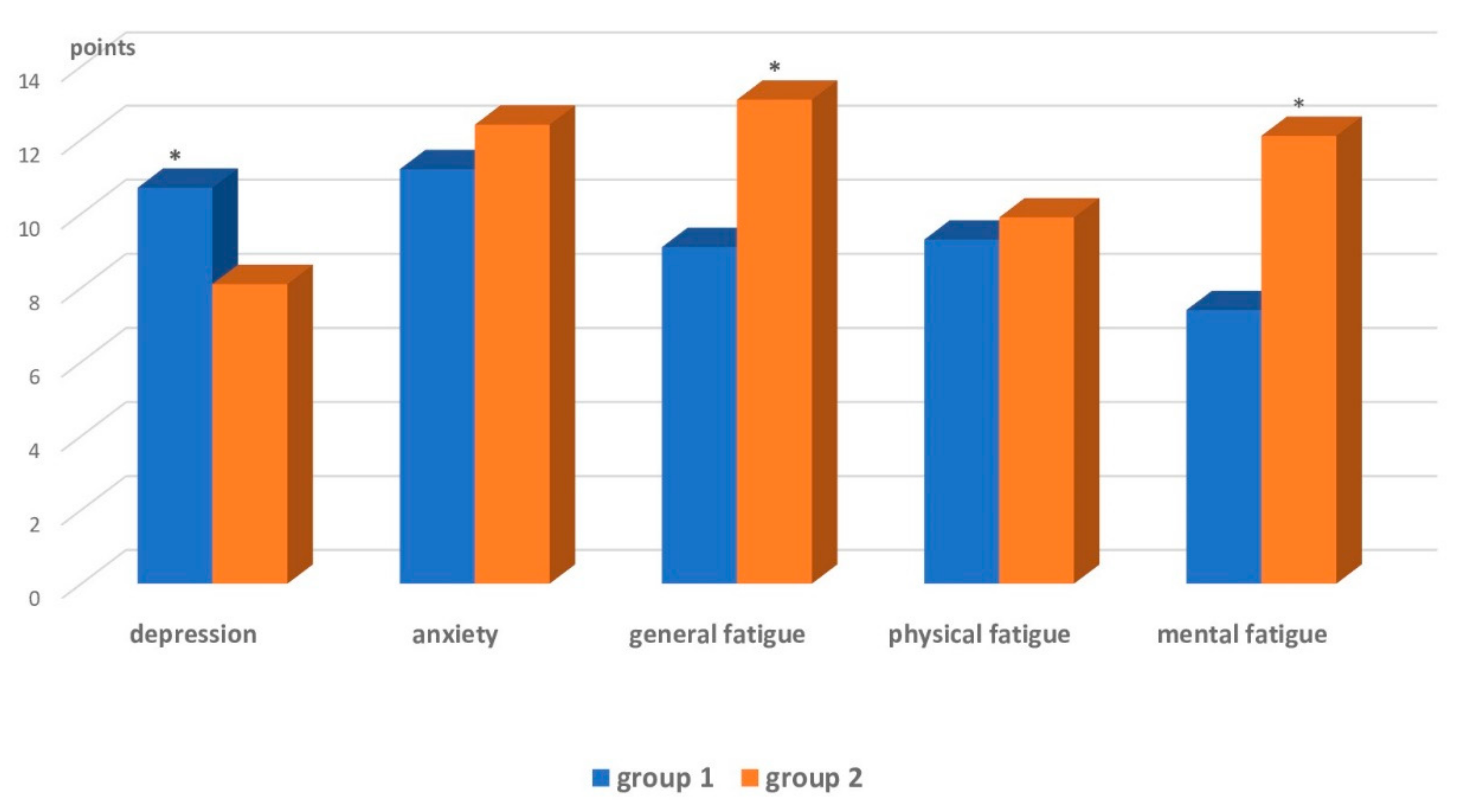
| Affective range and fatigue | Subclinical depression, clinical anxiety | Increased general and mental fatigue, clinical anxiety |
| Hachinsky’s scale | Mixed (vascular and atrophic) nature of dementia is likely | Likely vascular dementia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tynterova, A.; Perepelitsa, S.; Golubev, A. Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke. Brain Sci. 2022, 12, 554. https://doi.org/10.3390/brainsci12050554
Tynterova A, Perepelitsa S, Golubev A. Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke. Brain Sciences. 2022; 12(5):554. https://doi.org/10.3390/brainsci12050554
Chicago/Turabian StyleTynterova, Anastasia, Svetlana Perepelitsa, and Arкady Golubev. 2022. "Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke" Brain Sciences 12, no. 5: 554. https://doi.org/10.3390/brainsci12050554
APA StyleTynterova, A., Perepelitsa, S., & Golubev, A. (2022). Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke. Brain Sciences, 12(5), 554. https://doi.org/10.3390/brainsci12050554









