Electrophysiological Correlates of Romantic Love: A Review of EEG and ERP Studies with Beloved-Related Stimuli
Abstract
:1. Introduction
1.1. Neural Basis of Romantic Love
1.2. Valence and Familiarity
2. Electrophysiological Studies on Romantic Love
2.1. Electroencephalogram (EEG)
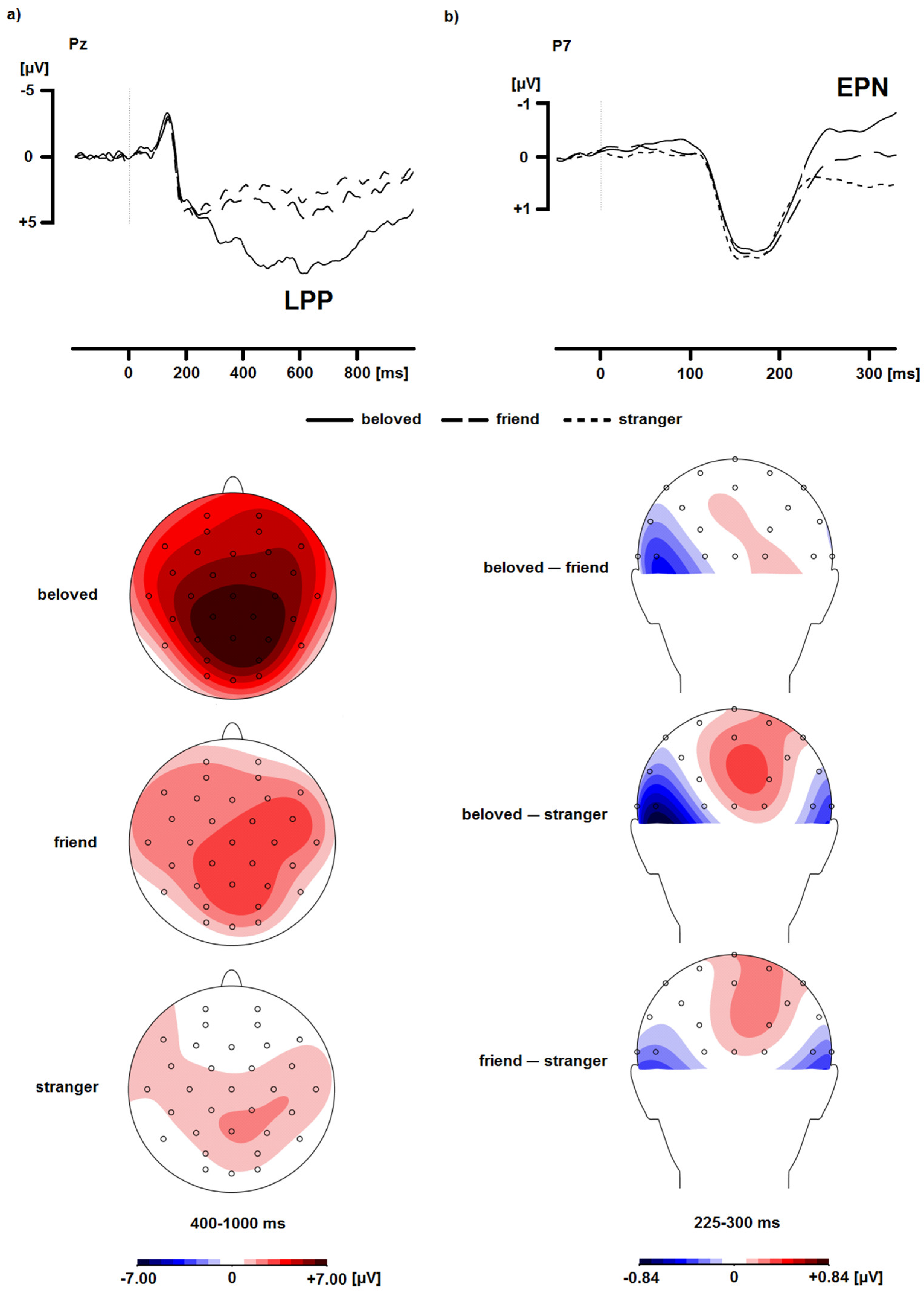
2.2. Event-Related Potentials (ERPs)
2.2.1. Late Positive Potential (LPP)
Passive Viewing Paradigms
Task Paradigms
2.2.2. Early Posterior Negativity (EPN)
2.2.3. Earlier ERP Components
3. Conclusions
3.1. EEG Correlates of Romantic Love
3.2. ERP Correlates of Romantic Love
3.3. Other Recommendations for Future Research
Funding
Conflicts of Interest
References
- Jankowiak, W.R.; Fischer, E.F. A cross-cultural perspective on romantic love. Ethnology 1992, 31, 149–155. [Google Scholar] [CrossRef]
- Carver, K.; Joyner, K.; Udry, J.R. National estimates of adolescent romantic relationships. In Adolescent Romantic Relations and Sexual Behavior: Theory, Research, and Practical Implications; Florsheim, P., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2003; pp. 23–56. [Google Scholar]
- Aron, E.N.; Aron, A. Extremities of love: The sudden sacrifice of career, family, dignity. J. Soc. Clin. Psychol. 1997, 16, 200–212. [Google Scholar] [CrossRef]
- Kim, H.K.; McKenry, P.C. The relationship between marriage and psychological well-being. J. Fam. Issues 2002, 23, 885–911. [Google Scholar] [CrossRef]
- Fisher, H.E.; Aron, A.; Mashek, D.; Li, H.; Brown, L.L. Defining the brain systems of lust, romantic attraction, and attachment. Arch. Sex. Behav. 2002, 31, 413–419. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P. Jealousy in couple relationships: Nature, assessment, and therapy. Behav. Res. Ther. 1997, 35, 973–985. [Google Scholar] [CrossRef]
- Rosenzweig, A.; Prigerson, H.; Miller, M.D.; Reynolds III, C.F. Breavement and late-life depression: Grief and its complications in the elderly. Annu. Rev. Med. 1997, 48, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.R.; Koch, E.J.; Hechenbleikner, N.R. Emotional responses to interpersonal rejection. In Interpersonal Rejection; Leary, M., Ed.; Oxford University Press: Oxford, UK, 2001; pp. 145–166. [Google Scholar]
- Amato, P.R. The consequences of divorce for adults and children. J. Marriage Fam. 2004, 62, 1269–1287. [Google Scholar] [CrossRef]
- Proulx, C.M.; Helms, H.M.; Buehler, C. Marital quality and personal well-being: A meta-analysis. J. Marriage Fam. 2007, 69, 576–593. [Google Scholar] [CrossRef]
- Monroe, S.; Rohde, P.; Seeley, J.; Lewinsohn, P. Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. J. Abnorm. Psychol. 1999, 108, 606–614. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Canetto, S.S.; Lester, D. Love and achievement motives in women’s and men’s suicide notes. J. Psychol. Interdiscip. Appl. 2002, 136, 573–576. [Google Scholar] [CrossRef]
- Garcia-Moreno, C.; Jansen, H.A.F.M.; Ellsberg, M.; Heise, L.; Watts, C.H. Prevalence of intimate partner violence: Findings from the WHO multi-country study on women’s health and domestic violence. Lancet 2006, 368, 1260–1269. [Google Scholar] [CrossRef]
- Wilt, S.; Olson, S. Prevalence of domestic violence in the United States. J. Am. Women's Assoc. 1996, 51, 77–82. [Google Scholar]
- Meloy, J.R.; Fisher, H. Some thoughts on the neurobiology of stalking. J. Forensic Sci. 2005, 50, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Daly, M. Spousal homicide risk and estrangement. Violence Vict. 1993, 8, 3–16. [Google Scholar] [CrossRef]
- Carter, C.S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 1998, 23, 779–818. [Google Scholar] [CrossRef]
- Langeslag, S.J.E. Is the serotonergic system altered in romantic love? A literature review and research suggestions. In Psychology of Relationships; Cuyler, E., Ackhart, M., Eds.; Nova Publishers: Hauppage, NY, USA, 2009; pp. 213–218. [Google Scholar]
- Langeslag, S.J.E.; Van der Veen, F.M.; Fekkes, D. Blood levels of serotonin are differentially affected by romantic love in men and women. J. Psychophysiol. 2012, 26, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Mizuno, K.; Sasaki, A.T.; Wada, Y.; Tanaka, M.; Ishii, A.; Tajima, K.; Tsuyuguchi, N.; Watanabe, K.; Zeki, S.; et al. Imaging the passionate stage of romantic love by dopamine dynamics. Front. Hum. Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marazziti, D.; Canale, D. Hormonal changes when falling in love. Psychoneuroendocrinology 2004, 29, 931–936. [Google Scholar] [CrossRef]
- Langeslag, S.J.E.; Van der Veen, F.M.; Röder, C.H. Attention modulates the dorsal striatum response to love stimuli. Hum. Brain Mapp. 2014, 35, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.; Zeki, S. The neural basis of romantic love. Neuroreport 2000, 11, 3829–3834. [Google Scholar] [CrossRef]
- Aron, A.; Fisher, H.; Mashek, D.; Strong, G.; Li, H.; Brown, L. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005, 94, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, B.P.; Aron, A.; Fisher, H.E.; Brown, L.L. Neural correlates of long-term intense romantic love. Soc. Cogn. Affect. Neurosci. 2012, 7, 145–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoessel, C.; Stiller, J.; Bleich, S.; Bönsch, D.; Doerfler, A.; Garcia, M.; Richter-Schmidinger, T.; Kornhuber, J.; Forster, C. Differences and similarities on neuronal activities of people being happily and unhappily in love: A functional magnetic resonance imaging study. Neuropsychobiology 2011, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Aron, A.; Brown, L.; Cao, G.; Feng, T.; Weng, X. Reward and motivation systems: A brain mapping study of early-stage intense romantic love in Chinese participants. Hum. Brain Mapp. 2011, 32, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Younger, J.; Aron, A.; Parke, S.; Chatterjee, N.; Mackey, S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS ONE 2010, 5, e13309. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yu, J.; Song, M.; Yu, C.; Wang, F.; Sun, P.; Wang, D.; Zhang, D. EEG correlates of ten positive emotions. Front. Hum. Neurosci. 2017, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, Q.; Chen, Y.; Zhong, R. The effect of sweet taste on romantic semantic processing: An ERP study. Front. Psychol. 2019, 10, 1573. [Google Scholar] [CrossRef]
- Hou, J.; Chen, X.; Liu, J.; Yao, F.; Huang, J.; Ndasauka, Y.; Ma, R.; Zhang, Y.; Lan, J.; Liu, L.; et al. How does adult attachment affect human recognition of love-related and sex-related stimuli: An ERP study. Front. Psychol. 2016, 7, 596. [Google Scholar] [CrossRef] [Green Version]
- Marosi, E.; Rodríguez, H.; Yañez, G.; Bernal, J.; Rodríguez, M.; Fernández, T.; Silva, J.; Reyes, A.; Guerrero, V. Broad band spectral measurements of EEG during emotional tasks. Int. J. Neurosci. 2001, 108, 251–279. [Google Scholar] [CrossRef]
- Marosi, E.; Bazán, O.; Yañez, G.; Bernal, J.; Fernández, T.; Rodríguez, M.; Silva, J.; Reyes, A. Narrow-band spectral measurements of EEG during emotional tasks. Int. J. Neurosci. 2002, 112, 871–891. [Google Scholar] [CrossRef]
- Cannas Aghedu, F.; Sarlo, M.; Zappasodi, F.; Acevedo, B.P.; Bisiacchi, P.S. Romantic love affects emotional processing of love-unrelated stimuli: An EEG/ERP study using a love induction task. Brain Cogn. 2021, 151, 105733. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.; Aron, A.; Fisher, H. Identifying, evoking, and measuring intense feelings of romantic love. Represent. Res. Soc. Psychol. 2000, 24, 48–55. [Google Scholar]
- Langeslag, S.J.E.; Surti, K. Increasing love feelings, marital satisfaction, and motivated attention to the spouse. J. Psychophysiol. 2022; in press. [Google Scholar] [CrossRef]
- Langeslag, S.J.E.; Van Strien, J.W. Regulation of romantic love feelings: Preconceptions, strategies and feasibility. PLoS ONE 2016, 11, e0161087. [Google Scholar] [CrossRef]
- Birbaumer, N.; Lutzenberger, W.; Elbert, H.; Flor, H.; Rockstroh, B. Imagery and brain processes. In The Structure of Emotion: Psychophysiological, Cognitive and Clinical Aspects; Birbaumer, N., Öhman, A., Eds.; Hogrefe & Huber Publishing: Seattle, WA, USA, 1993; pp. 122–138. [Google Scholar]
- Abbott, A. Prominent German neuroscientist committed misconduct in ‘brain-reading’ research. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Peterson, C.K. Electroencephalographic methods in social and personality psychology. In Methods in Social Neuroscience; Harmon-Jones, E., Beer, S., Eds.; Guilford Press: New York, NY, USA, 2009. [Google Scholar]
- Başar, E.; Schmiedt-Fehr, C.; Öniz, A.; Başar-Eroğlu, C. Brain oscillations evoked by the face of a loved person. Brain Res. 2008, 1214, 105–115. [Google Scholar] [CrossRef]
- Kelley, N.J.; Hortensius, R.; Schutter, D.J.L.G.; Harmon-Jones, E. The relationship of approach/avoidance motivation and asymmetric frontal cortical activity: A review of studies manipulating frontal asymmetry. Int. J. Psychophysiol. 2017, 119, 19–30. [Google Scholar] [CrossRef]
- Bartholow, B.D.; Amodio, D.M. Using event-related brain potentials in social psychological research: A brief review and tutorial. In Methods in Social Neuroscience; Harmon-Jones, E., Beer, S., Eds.; Guilford Press: New York, NY, USA, 2009. [Google Scholar]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; The MIT Press: Cambridge, UK, 2005. [Google Scholar]
- Hajcak, G.; MacNamara, A.; Olvet, D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol. 2010, 35, 129–155. [Google Scholar] [CrossRef]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.M.; Birbaumer, N.; Lang, P.J. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Schupp, H.T.; Flaisch, T.; Stockburger, J.; Junghöfer, M. Emotion and attention: Event-related brain potential studies. Prog. Brain Res. 2006, 156, 31–51. [Google Scholar]
- Burdwood, E.N.; Simons, R.F. Pay attention to me! Late ERPs reveal gender differences in attention allocated to romantic partners. Psychophysiology 2016, 53, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Langeslag, S.J.E.; Van Strien, J.W. Romantic love and attention: Early and late event-related potentials. Biol. Psychol. 2019, 146, 107737. [Google Scholar] [CrossRef] [PubMed]
- Langeslag, S.J.E.; Jansma, B.M.; Franken, I.H.; Van Strien, J.W. Event-related potential responses to love-related facial stimuli. Biol. Psychol. 2007, 76, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, L.; Dai, J.; Yang, S.; Wang, N.; Luo, Y.-J. Event-related potential responses to beloved and familiar faces in different marriage styles: Evidence from Mosuo subjects. Front. Psychol. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langeslag, S.J.E.; Olivier, J.R.; Köhlen, M.E.; Nijs, I.M.; Van Strien, J.W. Increased attention and memory for beloved-related information during infatuation: Behavioral and electrophysiological data. Soc. Cogn. Affect. Neurosci. 2015, 10, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Rafaela, R.C.; Vico, C.; Volchan, E.; Anllo-Vento, L.; Vila, J. Filial versus romantic love: Contributions from peripheral and central electrophysiology. Biol. Psychololgy 2011, 88, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Langeslag, S.J.E.; Franken, I.H.; Van Strien, J.W. Dissociating love-related attention from task-related attention: An event-related potential oddball study. Neurosci. Lett. 2008, 431, 236–240. [Google Scholar] [CrossRef]
- Langeslag, S.J.E.; Van Strien, J.W. Preferential processing of task-irrelevant beloved-related information and task performance: Two event-related potential studies. Neuropsychologia 2020, 145, 106497. [Google Scholar] [CrossRef]
- Hajcak, G.; Weinberg, A.; MacNamara, A.; Foti, D. ERPs and the study of emotion. In The Oxford Handbook of Event-Related Potential Components; Luck, S.J., Kappenman, E.S., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 441–472. [Google Scholar]
- Langeslag, S.J.E.; Van Strien, J.W. Early visual processing of snakes and angry faces: An ERP study. Brain Res. 2018, 1678, 297–303. [Google Scholar] [CrossRef]
- Junghöfer, M.; Bradley, M.M.; Elbert, T.R.; Lang, P.J. Fleeting images: A new look at early emotion discrimination. Psychophysiology 2001, 38, 175–178. [Google Scholar] [CrossRef]
- Schupp, H.T.; Stockburger, J.; Codispoti, M.; Junghöfer, M.; Weike, A.I.; Hamm, A.O. Selective visual attention to emotion. J. Neurosci. 2007, 27, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.E. Lust, attraction, and attachment in mammalian reproduction. Hum. Nat. 1998, 9, 23–52. [Google Scholar] [CrossRef] [PubMed]
- Bentin, S.; Allison, T.; Puce, A.; Perze, E.; McCarthy, G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996, 8, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Joyce, C.; Rossion, B. The face-sensitive N170 and VPP components manifest the same brain processes: The effect of reference electrode site. Clin. Neurophysiol. 2005, 116, 2613–2631. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef] [Green Version]
- Vico, C.; Guerra, P.; Robles, H.; Vila, J.; Anllo-Vento, L. Affective processing of loved faces: Contributions from peripheral and central electrophysiology. Neuropsychologia 2010, 48, 2894–2902. [Google Scholar] [CrossRef]
- Compton, R.J. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behav. Cogn. Neurosci. Rev. 2003, 2, 115–129. [Google Scholar] [CrossRef]
- Nakamura, K.; Arai, S.; Kawabata, H. Prioritized identification of attractive and romantic partner faces in rapid serial visual presentation. Arch. Sex. Behav. 2017, 46, 2327–2338. [Google Scholar] [CrossRef]
- Musa, C.Z.; Lépine, J.P. Cognitive aspects of social phobia: A review of theories and experimental research. Eur. Psychiatry 2000, 15, 59–66. [Google Scholar] [CrossRef]
- Everaert, J.; Koster, E.H.; Derakshan, N. The combined cognitive bias hypothesis in depression. Clin. Psychol. Rev. 2012, 32, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Langeslag, S.J.E.; van Steenbergen, H. Cognitive control in romantic love: The roles of infatuation and attachment in interference and adaptive cognitive control. Cogn. Emot. 2020, 34, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Steenbergen, H.; Langeslag, S.J.E.; Band, G.P.H.; Hommel, B. Reduced cognitive control in passionate lovers. Motiv. Emot. 2014, 38, 444–450. [Google Scholar] [CrossRef]
- Rugg, M.D.; Allan, K. Memory retrieval: An electrophysiological perspective. In The New Cognitive Neurosciences; Gazzaniga, M.S., Ed.; The MIT Press: Cambridge, UK, 2000; pp. 805–816. [Google Scholar]
- Schweinberger, S.R.; Burton, A.M. Covert recognition and the neural system for face processing. Cortex 2003, 39, 9–30. [Google Scholar] [CrossRef]
- Krigolson, O.E. Event-related brain potentials and the study of reward processing: Methodological considerations. Int. J. Psychophysiol. 2018, 132, 175–183. [Google Scholar] [CrossRef]
- Larson, M.J.; Clayson, P.E.; Clawson, A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014, 93, 283–297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langeslag, S.J.E. Electrophysiological Correlates of Romantic Love: A Review of EEG and ERP Studies with Beloved-Related Stimuli. Brain Sci. 2022, 12, 551. https://doi.org/10.3390/brainsci12050551
Langeslag SJE. Electrophysiological Correlates of Romantic Love: A Review of EEG and ERP Studies with Beloved-Related Stimuli. Brain Sciences. 2022; 12(5):551. https://doi.org/10.3390/brainsci12050551
Chicago/Turabian StyleLangeslag, Sandra J. E. 2022. "Electrophysiological Correlates of Romantic Love: A Review of EEG and ERP Studies with Beloved-Related Stimuli" Brain Sciences 12, no. 5: 551. https://doi.org/10.3390/brainsci12050551
APA StyleLangeslag, S. J. E. (2022). Electrophysiological Correlates of Romantic Love: A Review of EEG and ERP Studies with Beloved-Related Stimuli. Brain Sciences, 12(5), 551. https://doi.org/10.3390/brainsci12050551





