Modifications of Functional Human Brain Networks by Transcutaneous Auricular Vagus Nerve Stimulation: Impact of Time of Day
Abstract
:1. Introduction
- modifications of the networks’ global (topology, stability, and robustness) and local characteristics (importance of network constituents) depend on the time of day the stimulation was performed; and
- a taVNS-related neuromodulatory effect on functional brain networks (i.e., a repeated stimulation amplifies network modifications induced by the previous stimulation) can be identified using short-term stimulations performed twice a day
2. Materials and Methods
2.1. Subjects
2.2. Transcutaneous Auricular Vagus Nerve Stimulation and Examination Schedule
2.3. EEG Recording and Data Pre-Processing
2.4. Characterising Evolving Functional Brain Networks on Global and Local Scale
2.5. Evaluating the Possible Influence of Biological Rhythms on Time-Dependent Network Characteristics
2.6. Classification of Stimulation Effects
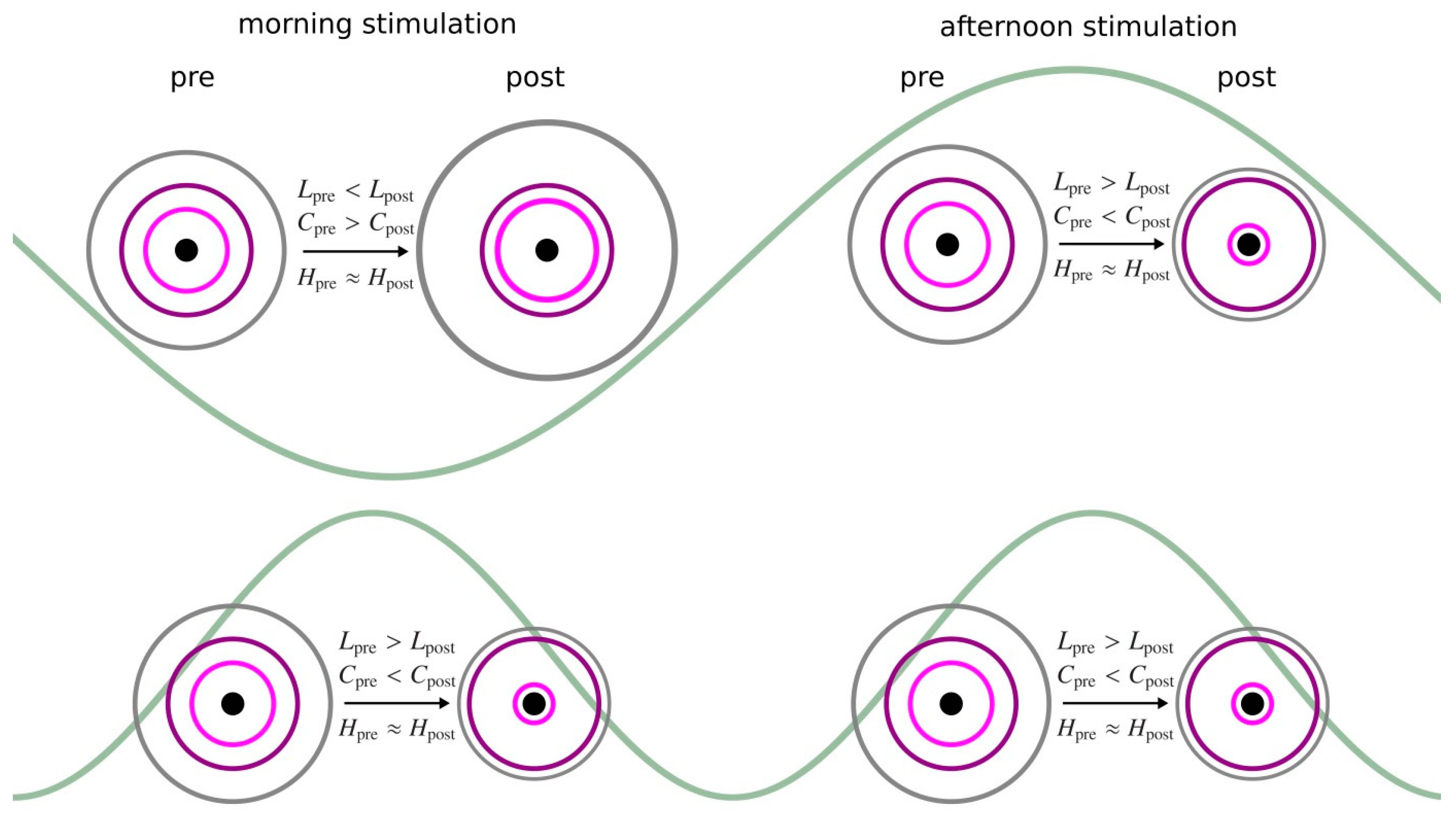
- immediate stimulation effect: network characteristics during the pre-stimulation phase and during the stimulation phase (pre → stim) differ significantly (either in the morning or in the afternoon);
- enduring stimulation effect: an immediate stimulation effect can be observed and network characteristics during the pre-stimulation phase and during the post-stimulation phase (pre → post) differ significantly (either in the morning or in the afternoon);
- prolonged stimulation effect: an immediate stimulation effect of the morning stimulation can be observed and network characteristics during the pre-stimulation phase 1 and during the pre-stimulation phase 2 (pre 1 → pre 2) differ significantly;
- longer-lasting stimulation effect: an immediate stimulation effect of the morning stimulation can be observed and network characteristics during the pre-stimulation phase 1 and during the post-stimulation phase 2 (pre 1 → post 2) differ significantly. If immediate effects can be observed for both stimulations (pre 1 → stim 1 and pre 2 → stim 2), we consider the long-lasting effect to be accumulating.
2.7. Statistical Analyses
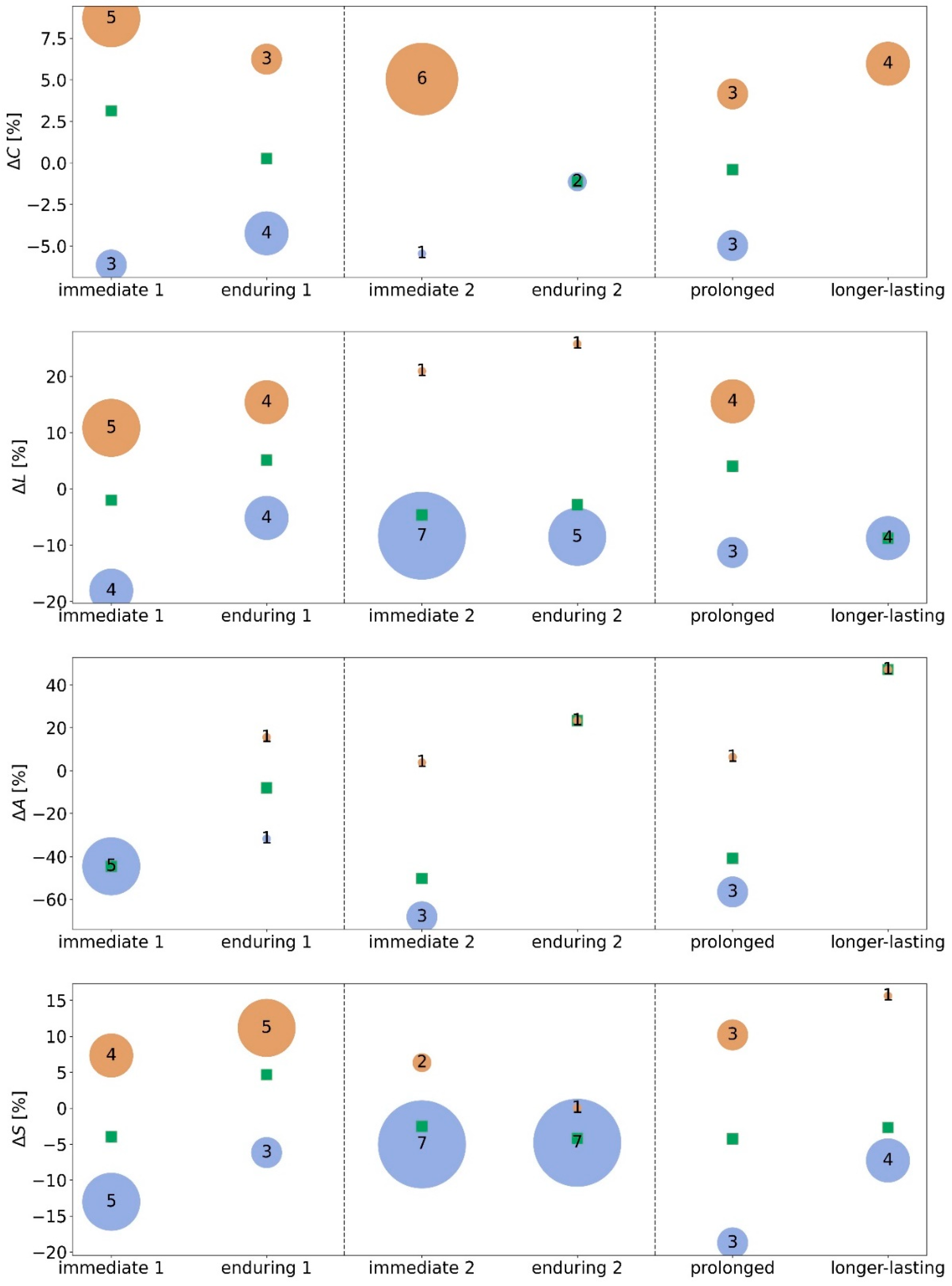
3. Results
3.1. Morning taVNS-Induced Immediate and Enduring Network Modifications on the Global and Local Scale
3.2. Afternoon taVNS-Induced Immediate and Enduring Network Modifications on the Global and Local Scale
3.3. Prolonged and Longer-Lasting taVNS-Induced Modifications on the Global and Local Network Scale
4. Discussion
4.1. Time-of-Day-Dependence of taVNS-Mediated Network Modifications: From Global to Local
4.2. Prolonged and Longer-Lasting taVNS-Mediated Network Modifications
4.3. Can a Neuromodulatory Effect of Short-Term taVNS Be Identified?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lampros, M.; Vlachos, N.; Zigouris, A.; Voulgaris, S.; A Alexiou, G. Transcutaneous Vagus Nerve Stimulation (t-VNS) and epilepsy: A systematic review of the literature. Seizure 2021, 91, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Straube, A.; Ellrich, J.; Eren, O.; Blum, B.; Ruscheweyh, R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): A randomized, monocentric clinical trial. J. Headache Pain 2015, 16, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briand, M.-M.; Gosseries, O.; Staumont, B.; Laureys, S.; Thibaut, A. Transcutaneous Auricular Vagal Nerve Stimulation and Disorders of Consciousness: A Hypothesis for Mechanisms of Action. Front. Neurol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Colzato, L.; Beste, C. A literature review on the neurophysiological underpinnings and cognitive effects of transcutaneous vagus nerve stimulation: Challenges and future directions. J. Neurophysiol. 2020, 123, 1739–1755. [Google Scholar] [CrossRef]
- Carandina, A.; Rodrigues, G.D.; Di Francesco, P.; Filtz, A.; Bellocchi, C.; Furlan, L.; Carugo, S.; Montano, N.; Tobaldini, E. Effects of transcutaneous auricular vagus nerve stimulation on cardiovascular autonomic control in health and disease. Auton. Neurosci. 2021, 236, 102893. [Google Scholar] [CrossRef] [PubMed]
- van Beekum, C.J.; Willis, M.A.; von Websky, M.W.; Sommer, N.P.; Kalff, J.C.; Wehner, S.; Vilz, T.O. Electrical vagus nerve stimulation as a prophylaxis for SIRS and postoperative ileus. Auton. Neurosci. 2021, 235, 102857. [Google Scholar] [CrossRef]
- Kaniusas, E.; Kampusch, S.; Tittgemeyer, M.; Panetsos, F.; Gines, R.F.; Papa, M.; Kiss, A.; Podesser, B.; Cassara, A.M.; Tanghe, E.; et al. Current Directions in the Auricular Vagus Nerve Stimulation I–A Physiological Perspective. Front. Neurosci. 2019, 13, 854. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Guo, X.; Luo, M.; Li, S.; Liu, A.; Zhao, Y.; Zhao, B.; Wang, D.; Li, Z.; Zheng, X.; et al. Effect of Transcutaneous Vagus Nerve Stimulation at Auricular Concha for Insomnia: A Randomized Clinical Trial. Evidence-Based Complement. Altern. Med. 2020, 2020, 6049891. [Google Scholar] [CrossRef]
- Kaniusas, E.; Szeles, J.C.; Kampusch, S.; Alfageme-Lopez, N.; Yucumá, D.; Li, X.; Mayol, J.; Neumayer, C.; Papa, M.; Panetsos, F. Non-invasive Auricular Vagus Nerve Stimulation as a Potential Treatment for Covid19-Originated Acute Respiratory Distress Syndrome. Front. Physiol. 2020, 11, 890. [Google Scholar] [CrossRef]
- Azabou, E.; Bao, G.; Bounab, R.; Heming, N.; Annane, D. Vagus Nerve Stimulation: A Potential Adjunct Therapy for COVID-19. Front. Med. 2021, 8, 625836. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Straube, A.; Eren, O. tVNS in the management of headache and pain. Auton. Neurosci. 2021, 236, 102875. [Google Scholar] [CrossRef] [PubMed]
- von Wrede, R.; Surges, R. Transcutaneous vagus nerve stimulation in the treatment of drug-resistant epilepsy. Auton. Neurosci. 2021, 235, 102840. [Google Scholar] [CrossRef] [PubMed]
- Sabers, A.; Aumüller-Wagner, S.; Christensen, L.R.; Henning, O.; Kostov, K.; Lossius, M.; Majoie, M.; Mertens, A.; Nielsen, L.; Vonck, K.; et al. Feasibility of transcutaneous auricular vagus nerve stimulation in treatment of drug resistant epilepsy: A multicenter prospective study. Epilepsy Res. 2021, 177, 106776. [Google Scholar] [CrossRef]
- von Wrede, R.; Rings, T.; Schach, S.; Helmstaedter, C.; Lehnertz, K. Transcutaneous auricular vagus nerve stimulation induces stabilizing modifications in large-scale functional brain networks: Towards understanding the effects of taVNS in subjects with epilepsy. Sci. Rep. 2021, 11, 7906. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Kessler, K.M.; Terracall, E.; Castellanos, A.; Linnaluoto, M.K.; Myerburg, R.J. Reproducibility and circadian rhythm of heart rate variability in healthy subjects. Am. J. Cardiol. 1990, 65, 391–393. [Google Scholar] [CrossRef]
- Van Dijk, N.; Boer, M.C.; De Santo, T.; Grovale, N.; Aerts, A.J.J.; Boersma, L.; Wieling, W. Daily, weekly, monthly, and seasonal patterns in the occurrence of vasovagal syncope in an older population. Europace 2007, 9, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Jarczok, M.N.; Aguilar-Raab, C.; Koenig, J.; Kaess, M.; Borniger, J.C.; Nelson, R.J.; Hall, M.; Ditzen, B.; Thayer, J.F.; Fischer, J.E. The Heart´s rhythm ‘n’ blues: Sex differences in circadian variation patterns of vagal activity vary by depressive symptoms in predominantly healthy employees. Chronobiol. Int. 2018, 35, 896–909. [Google Scholar] [CrossRef]
- Chow, E.; Bernjak, A.; Williams, S.; Fawdry, R.A.; Hibbert, S.; Freeman, J.; Sheridan, P.J.; Heller, S.R. Risk of Cardiac Arrhythmias During Hypoglycemia in Patients With Type 2 Diabetes and Cardiovascular Risk. Diabetes 2014, 63, 1738–1747. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.J.; Yang, J.N.; Scheer, F.A. The impact of the circadian timing system on cardiovascular and metabolic function. Prog. Brain Res. 2012, 199, 337–358. [Google Scholar] [CrossRef] [Green Version]
- Rings, T.; von Wrede, R.; Bröhl, T.; Schach, S.; Helmstaedter, C.; Lehnertz, K. Impact of Transcutaneous Auricular Vagus Nerve Stimulation on Large-Scale Functional Brain Networks: From Local to Global. Front. Physiol. 2021, 12, 1328. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Lehnertz, K.; Ansmann, G.; Bialonski, S.; Dickten, H.; Geier, C.; Porz, S. Evolving networks in the human epileptic brain. Phys. D Nonlinear Phenom. 2013, 267, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Lehnertz, K.; Rings, T.; Bröhl, T. Time in Brain: How Biological Rhythms Impact on EEG Signals and on EEG-Derived Brain Networks. Front. Netw. Physiol. 2021, 1, 755016. [Google Scholar] [CrossRef]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M. Networks, 2nd ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Newman, M.E.J. Assortative Mixing in Networks. Phys. Rev. Lett. 2002, 89, 208701. [Google Scholar] [CrossRef] [Green Version]
- Bialonski, S.; Lehnertz, K. Assortative mixing in functional brain networks during epileptic seizures. Chaos Interdiscip. J. Nonlinear Sci. 2013, 23, 33139. [Google Scholar] [CrossRef] [Green Version]
- Bröhl, T.; Lehnertz, K. Centrality-based identification of important edges in complex networks. Chaos Interdiscip. J. Nonlinear Sci. 2019, 29, 033115. [Google Scholar] [CrossRef] [Green Version]
- Fruengel, R.; Bröhl, T.; Rings, T.; Lehnertz, K. Reconfiguration of human evolving large-scale epileptic brain networks prior to seizures: An evaluation with node centralities. Sci. Rep. 2020, 10, 21921. [Google Scholar] [CrossRef]
- Geier, C.; Lehnertz, K. Long-term variability of importance of brain regions in evolving epileptic brain networks. Chaos Interdiscip. J. Nonlinear Sci. 2017, 27, 043112. [Google Scholar] [CrossRef]
- Kuhnert, M.-T.; Geier, C.; Elger, C.E.; Lehnertz, K. Identifying important nodes in weighted functional brain networks: A comparison of different centrality approaches. Chaos Interdiscip. J. Nonlinear Sci. 2012, 22, 023142. [Google Scholar] [CrossRef] [PubMed]
- Bröhl, T.; Lehnertz, K. A straightforward edge centrality concept derived from generalizing degree and strength. Sci. Rep. 2022, 12, 4407. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.H.; Jeong, H.; Barabási, A.-L.; Tu, Y. Weighted Evolving Networks. Phys. Rev. Lett. 2001, 86, 5835–5838. [Google Scholar] [CrossRef] [Green Version]
- Barrat, A.; Barthelemy, M.; Pastor-Satorras, R.; Vespignani, A. The architecture of complex weighted networks. Proc. Natl. Acad. Sci. USA 2004, 101, 3747–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Press, W.H.; Rybicki, G.B. Fast Algorithm for Spectral Analysis of Unevenly Sampled Data. Astrophys. J. 1989, 338, 277–280. [Google Scholar] [CrossRef]
- Farmer, A.D.; Strzelczyk, A.; Finisguerra, A.; Gourine, A.V.; Gharabaghi, A.; Hasan, A.; Burger, A.M.; Jaramillo, A.M.; Mertens, A.; Majid, A.; et al. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front. Hum. Neurosci. 2021, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Beekwilder, J.P.; Beems, T. Overview of the Clinical Applications of Vagus Nerve Stimulation. J. Clin. Neurophysiol. 2010, 27, 130–138. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Wrede, R.; Bröhl, T.; Rings, T.; Pukropski, J.; Helmstaedter, C.; Lehnertz, K. Modifications of Functional Human Brain Networks by Transcutaneous Auricular Vagus Nerve Stimulation: Impact of Time of Day. Brain Sci. 2022, 12, 546. https://doi.org/10.3390/brainsci12050546
von Wrede R, Bröhl T, Rings T, Pukropski J, Helmstaedter C, Lehnertz K. Modifications of Functional Human Brain Networks by Transcutaneous Auricular Vagus Nerve Stimulation: Impact of Time of Day. Brain Sciences. 2022; 12(5):546. https://doi.org/10.3390/brainsci12050546
Chicago/Turabian Stylevon Wrede, Randi, Timo Bröhl, Thorsten Rings, Jan Pukropski, Christoph Helmstaedter, and Klaus Lehnertz. 2022. "Modifications of Functional Human Brain Networks by Transcutaneous Auricular Vagus Nerve Stimulation: Impact of Time of Day" Brain Sciences 12, no. 5: 546. https://doi.org/10.3390/brainsci12050546
APA Stylevon Wrede, R., Bröhl, T., Rings, T., Pukropski, J., Helmstaedter, C., & Lehnertz, K. (2022). Modifications of Functional Human Brain Networks by Transcutaneous Auricular Vagus Nerve Stimulation: Impact of Time of Day. Brain Sciences, 12(5), 546. https://doi.org/10.3390/brainsci12050546





