Abstract
Electric and magnetic stimulation of the human brain can be used to excite or inhibit neurons. Numerous methods have been designed over the years for this purpose with various advantages and disadvantages that are the topic of this review. Deep brain stimulation (DBS) is the most direct and focal application of electric impulses to brain tissue. Electrodes are placed in the brain in order to modulate neural activity and to correct parameters of pathological oscillation in brain circuits such as their amplitude or frequency. Transcranial magnetic stimulation (TMS) is a non-invasive alternative with the stimulator generating a magnetic field in a coil over the scalp that induces an electric field in the brain which, in turn, interacts with ongoing brain activity. Depending upon stimulation parameters, excitation and inhibition can be achieved. Transcranial electric stimulation (tES) applies electric fields to the scalp that spread along the skull in order to reach the brain, thus, limiting current strength to avoid skin sensations and cranial muscle pain. Therefore, tES can only modulate brain activity and is considered subthreshold, i.e., it does not directly elicit neuronal action potentials. In this review, we collect hints for neuroplastic changes such as modulation of behavior, the electric activity of the brain, or the evolution of clinical signs and symptoms in response to stimulation. Possible mechanisms are discussed, and future paradigms are suggested.
1. Introduction
Neuroplasticity of the nervous system covers a large variety of phenomena in order to describe changes in the brain on different levels as a reaction to dynamic physiological or pathological conditions. Neuroplasticity can be the result of neuronal reorganization on a molecular, synaptic, and morphometric neuronal level [1]. It can also refer to changes in neural circuits to adapt to changes in the environment (external stimuli) or changes in brain functioning resulting from diseases of the nervous system itself (internal changes) [2]. Several environmental changes induce neuroplasticity such as learning [3,4], sleep [5], aging [6], external stimuli which are accessible to sensory perception [7], and even changes in lifestyle [8]. In addition, a broad range of therapies, ranging from non-pharmacological behavioral therapies [9,10] to pharmacological therapies, induce electrophysiological and neuroplastic changes in the nervous system [11,12]. There is increasing evidence that brain stimulation techniques are beneficial in treating diseases of the brain. Some of these stimulation techniques have been approved by the Food and Drug Administration (FDA), such as transcranial magnetic stimulation (TMS) to treat depression or deep brain stimulation (DBS) to treat advanced Parkinson’s disease (PD).
In this review, we specifically focus on three modalities of brain stimulation techniques, namely TMS, DBS, and transcranial electric stimulation (tES). Although these methods have been investigated for a long time, to the best of our knowledge, their neuroplastic capacity has never been compared in a narrative review. Given the clinical application of these techniques, we focus on studies in humans and refer only briefly to animal models, wherever needed.
For TMS, neuroplasticity is commonly indexed by the change in cortical excitability before and after application of a course of repetitive TMS, which is measured by the amplitude of peripherally recorded motor evoked potentials (MEPs). In order to demonstrate plasticity in brain areas that do not elicit a behavioral reaction, TMS-evoked potentials (TEPs) and neuroimaging techniques can be used. Hence, the neuroplastic capacity of TMS will be subdivided according to the modalities used to reveal neuroplastic changes. Lasting after-effects of TMS have been described as resembling mechanisms of neuroplasticity and as being biologically similar to processes such as long-term potentiation and depression (LTP/LTD) [13,14].
While TMS is a hybrid method that is applied both in the clinic and in experimental settings, DBS in humans is an exclusively therapeutic application. Thus, for DBS, we will focus on clinical signs of plasticity and review DBS-induced neuroplastic changes in three selected disorders, for which DBS has proven to be an effective treatment. On a clinical level, we assume a neuroplastic process to be driven by an effect of neurostimulation, whenever signs or symptoms of a disease (i) improve over a longer course of ongoing stimulation (e.g., weeks or months after stimulation begin) and are stable in the stimulation parameters such as amperage or stimulation frequency, (ii) show a clinically stable course despite stopping the stimulation, and (iii) clinical side effects of neurostimulation occur after a longer stimulation period and are unrelated to disease progression (malplasticity). These considerations can also be transferred to stimulation-related neurobehavioral effects in healthy volunteers when changes in behavior occur over the course of an ongoing stimulation and clearly outlast the period of neurostimulation.
Finally, tES is mainly used in basic and applied research with preliminary clinical applications to date. Transcranial electric stimulation (tES) is an umbrella term and comprises several different techniques, including transcranial direct current stimulation (tDCS), alternating current stimulation (tACS), and random noise stimulation (tRNS). While these techniques are similar in terms of their setup, their effects on neuronal mechanisms and behavioral outcomes differ, and thus, will be discussed separately. To evaluate neuroplasticity, we consider tES-induced after-effects on physiological measurements such as EEG, MEG, EMG, and its effects on observable behavior. Neuroplasticity can occur at different timescales. Short-term plasticity refers to phenomena in the range of milliseconds to seconds which are probably due to neurotransmitter depletion or changes in neurotransmitter influx that modulate the firing rate of neurons. Long-term plasticity operates in the range of minutes to hours and effects can last as long as days, months, or years. For long-term plasticity to occur, changes in NMDA receptor activity, gene expression, and morphology of the synapse are assumed [15]. While brain stimulation also results in short-term plasticity, we focus on effects due to long-term plasticity for the purpose of this review.
A caveat of our selected definition to infer neuroplasticity is that we cannot directly test and evaluate assumptions about the cellular mechanisms of action in the human brain. We assume the cellular basis of these effects to be synaptic changes, including the involvement of AMPA and NMDA receptors, which, in turn, have secondary effects on neurons, networks, and behavior [16]. Hence, we have included studies that investigated the involvement of neurotransmitters known to be relevant for neuroplasticity and that showcased not only neurophysiological and behavioral signs of neuroplasticity but referred also to morphometric changes as a consequence of neurostimulation, whenever available. The reviewed neurostimulation methods are used in different contexts upon which the kind of evidence for neuroplasticity may depend. For example, probing neuroplasticity evaluated by administration of NMDA antagonist is a perfectly feasible approach in healthy young individuals but may be ethically questionable in a sample of diseased patients with chronic DBS. Due to such limitations, finding a general structure that allows us to identify evidence of neuroplasticity for all methods equally well was deemed impossible. Hence, we decided to review the methods in the context in which they are most commonly applied, as described above.
2. Transcranial Magnetic Stimulation (TMS)
2.1. Overview of TMS Methods
Transcranial magnetic stimulation (TMS) is a non-invasive technique for stimulation of distinct brain regions [13,14,17]. After placing a magnetic coil over a subject’s head, a brief, high-intensity magnetic field pulse can be generated, which, in turn, induces electric currents of sufficient magnitude to depolarize neurons [14]. A single pulse onto the primary motor cortex (M1) can activate the corticospinal tract, thus, inducing a contraction in the targeted contralateral muscle. Using electromyography (EMG), these contractions may be recorded as motor-evoked potentials (MEPs) [18]. As such, single-pulse TMS can be used to map functional cortical representations of muscles or speech functions, an FDA-approved technique known as cortical mapping [19], or can be used as a diagnostic tool, for example, to measure the central motor conduction time in multiple sclerosis [20]. Stimulation of non-motor cortical regions has no directly observable effect, but cortical reactivity to the stimulation can be recorded using EEG, observed in behavior, or subjectively experienced. For example, when applying single pulses to the visual cortex, subjects may report experiencing phosphenes or scotomas [21]. To overcome this issue, coregistration of TMS and EEG has become a successful method of investigating the neural reactivity of brain regions that do not provide an observable behavioral correlate [22]. With the advent of neuronavigation, it is possible to precisely modulate regions across the entire cortex in an individualized manner. Targets localized by functional and structural neuroimaging are becoming widely used and even real-time targeting of the brain’s fiber tracts through tractography-based TMS neuronavigation is currently being developed with promising results [23,24].
In addition to single pulses, TMS can be applied in trains of repeated TMS pulses (rTMS) at various stimulation frequencies to modulate neural activity. Repeated pulses have a more prolonged effect on the brain that outlasts the effects of the stimulation itself by minutes or even hours [25,26,27]. Importantly, rTMS exerts not only local but also distant effects through connectivity between regions, which can be revealed behaviorally, psychophysiologically, or by combining TMS with neuroimaging [28,29,30,31]. These lasting after-effects of rTMS may underlie its successful applications as therapeutic interventions. When rTMS sessions are applied daily over a period of days or weeks, they can produce significant clinical improvement in a variety of neurological and psychiatric disorders [32]. Regarding psychiatric disorders, the majority of evidence stems from antidepressant effectiveness in treatment-refractory major depressive disorder (MDD) [33,34]. TMS further received FDA approval for the treatment of migraine headache with aura, obsessive-compulsive disorder, smoking cessation, and anxiety comorbid with MDD [19] (for a complete overview of current guidelines on the therapeutic efficacy of rTMS, see [32]).
2.2. Neuroplasticity
2.2.1. After-Effects of TMS: Changes in MEPs
Because of the lack of an objective and reliable index of cortical excitability outside the M1, early attempts to evaluate TMS-induced neuroplasticity have been largely restricted to M1. Thus, we begin by reviewing neuroplastic evidence after TMS from M1. It should also be noted here that TMS is not only used to induce LTP-like plasticity but also to indirectly probe LTP-like plasticity in the human motor cortex in health and disease and to test the induction of motor cortical plasticity induced by other interventions, for instance, motor training or tDCS [35,36,37,38].
The nature of rTMS after-effects is complex and influenced by many parameters such as the frequency, intensity, and duration of the stimulation. Chen et al. [39] demonstrated that low-frequency (0.9 Hz) rTMS for 15 min (810 pulses, at a stimulation intensity of 115% of MEP threshold) resulted in a significant depression of MEP amplitude for at least 15 min after the rTMS protocol. By contrast, rTMS at 5 Hz, given in separated short trains, has been shown to facilitate motor cortical excitability for at least 30 min [27,40,41,42]. This led to the general assumption that low-frequency (1 Hz or less) rTMS decreases cortical excitability, whereas high-frequency (5 Hz or greater) rTMS increases cortical excitability [43]. However, this assumption has been challenged by a finding that suggests that the intertrain-interval used in high-frequency rTMS protocols is an additional factor, as a continuous stimulation at 5 Hz was found to induce inhibition instead of facilitation [44]. The duration of after-effects is thought to be dependent on the number of pulses given in a protocol, i.e., a higher number of pulses tends to produce greater and longer-lasting effects [42,45]. Nevertheless, it should be mentioned that succeeding studies did not consistently support these results [46] and the direction of effects can even be reversed with varying pulse numbers [47,48,49]. Stimulation intensity is often set as a certain percentage of an individual’s motor threshold (MT), which is defined as the minimum stimulus strength that produces a small MEP (usually 50–100 μV) in the target muscle, in about 50% of 10–20 consecutive trials [50]. Further, it can be distinguished between the motor threshold at rest (resting motor threshold (RMT)) and the motor threshold during slight activation of the muscle (active motor threshold (AMT)). Cortical excitability generally increases as a function of intensity, i.e., intensities less than MT tend to decrease cortical excitability, whereas intensities greater than MT increase cortical excitability [51,52].
More recently, new rTMS protocols that use “patterned” forms of rTMS have been developed. Theta burst stimulation (TBS) is the most commonly used form and consists of bursts of three pulses at 50 Hz, repeated at intervals of 200 ms [53]. This protocol is based on the naturally occurring theta rhythm (5 Hz) of the hippocampus and has been shown to induce synaptic plasticity in animal experiments [54]. TBS can be delivered as a single train of bursts lasting 20–40 s (continuous TBS (cTBS)) which has a primarily inhibitory effect on cortical excitability, for instance, 40 s of continuous TBS (cTBS) reduces the amplitude of MEPs for nearly 60 min [53]. As opposed to that, the burst train can be split up into twenty 2 s sequences repeated every 10 s (intermittent TBS (iTBS)), which has mainly excitatory effects (Figure 1). A total of 190 s of iTBS increases MEPs for at least 15 min [53]. TBS has gained popularity as it induces longer-lasting effects with shorter application time and lower stimulation intensity than conventional rTMS paradigms [55], and has drastically increased time efficiency in its clinical application [56].
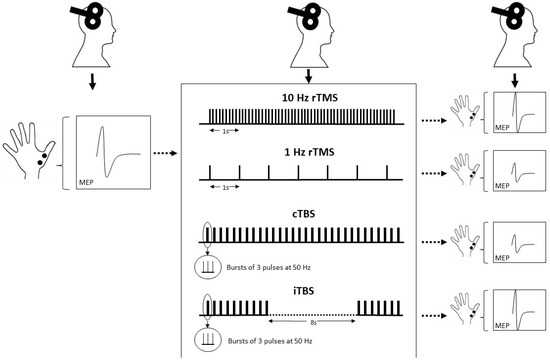
Figure 1.
Test amplitudes are elicited by single-pulse TMS before and after application of rTMS protocol and can be used as a measure of cortical excitability. The effect depends, among other factors, on stimulation frequency and pattern. rTMS at high frequencies (e.g., 10 Hz) increases cortical excitability, while low-frequency rTMS (e.g., 1 Hz) decreases cortical excitability. TBS is a patterned form of rTMS and decreases cortical excitability when applied as a continuous train and increases cortical excitability when applied intermittently, i.e., 2 s repeated every 10 s.
Paired associative stimulation (PAS) is another TMS protocol that combines TMS of the motor cortex with peripheral nerve stimulation (PNS) at the wrist [57]. When repeatedly paired with an interstimulus interval (ISI) of 25 ms, it allows for the synchronous arrival of electromagnetic stimulation and afferent (i.e., peripheral) stimulation in the brain, and facilitates cortical excitability. The application of 90 pairs of stimuli (rate 0.05 Hz) led to PAS-induced LTP-like plasticity, which was seen in a long-term increase (<30 min) of the MEP in the target muscle [57,58,59]. By contrast, a shorter interval between the TMS pulse and the PNS pulse (e.g., ISI of 10 ms) led to PAS-induced LTD-like plasticity and a decrease in cortical excitability [59]. Due to the shorter interval, the afferent pulse from the median nerve stimulus arrives shortly after the TMS pulse. Thus, PAS protocols demonstrate some of the characteristics of spike-timing dependent plasticity. In this concept, the precise temporal interval between presynaptic and postsynaptic spikes modulates LTP-like or LTD-like synaptic plasticity [60]. Instead of pairing a magnetic pulse with a peripheral stimulus, another variant is cortico-cortical PAS, which pairs two connected cortex areas with each other by using two TMS coils [61,62]. Moreover, TMS pulses can be paired with subcortical stimulation using implanted deep electrodes in patients with DBS [63]. One very promising novel clinical approach using PAS is the combination of high-frequency TMS of M1 with high-frequency PNS of the contralateral limb as a means of spinal cord rehabilitation. This approach aims to safely induce an LTP-like effect at corticomotoneuronal synapses of the spinal cord leading to improved corticospinal conduction in patients with incomplete spinal cord injury (SCI) [64].
2.2.2. Combining TMS with EEG
Plasticity-like after-effects induced by rTMS were traditionally revealed in the motor cortex in an indirect manner by measuring the change in the MEP amplitude. The combination of TMS with EEG offers an alternative and more direct demonstration of neuroplasticity induced by TMS in humans [65]. Similar to the principles of MEPs, changes in amplitude and latency of the TMS-evoked potential (TEP) can be obtained from the EEG signal across the entire scalp and used to measure changes in cortical excitability [22]. TEPs have proven to be a sensitive and reliable measure of cortical excitability and are comparable to MEP amplitude recordings [66,67,68]. Motor cortex stimulation-induced TEPs are well characterized and differential effects of rTMS protocols on TEPs are largely consistent with those seen in MEPs. Traditional inhibitory protocols seem to produce a decrease in cortical excitability [69,70,71], whereas traditional excitatory protocols seem to increase cortical excitability [65,72]. Measuring TEPs with the combined use of TMS-EEG has several additional advantages over MEPs. First, it has been suggested that it is a more sensitive tool to assess cortical excitability than MEPs, as they are measurable at intensities that are significantly below the motor threshold [70,73,74]. Second, it is also possible to track the spread of activity from the stimulated site to neighboring areas and distant, functionally connected areas, as responses can be recorded from virtually any electrode on the scalp [21,74]. A TMS pulse on M1 in one hemisphere, for instance, spreads ipsilaterally via association fibers but also to the contralateral hemisphere via transcallosal fibers [22,65,75,76]. Third, while MEP paradigms are largely restricted to M1, the combined use of TMS-EEG allows studying after-effects of rTMS on practically any cortical area that is accessible to TMS. For example, TEPs are well defined when TMS is applied to the dorsolateral prefrontal cortex (DLPFC) and plasticity-like after-effects have been demonstrated within this region [67,68,77,78]. Applying TMS to areas other than the primary motor cortex has provided important insights into the generalizability of effects of intensity and duration of rTMS [79]. In addition to these advantages, a limitation of combining TMS with EEG is the risk of various TMS-evoked artifacts in the EEG signal, including TMS-induced muscle, decay, auditory, and blink startle artifacts [78]. However, extensive efforts have been made to minimize these artifacts in TMS-EEG recordings. For a complete overview and challenges of the TMS-EEG methodology, see [79].
In addition to TEPs, rTMS also produces changes in other EEG metrics, such as changes in TMS-evoked oscillatory brain activity and connectivity measures [80,81,82]. Interestingly, ongoing EEG features are now used to provide feedback to determine, for instance, the exact timing of rTMS pulses. Known as closed-loop stimulation, these approaches aim to enhance the neuroplastic capacity of rTMS by coupling the TMS parameters to real-time EEG biomarkers [83,84].
2.2.3. After-Effects Revealed by PET and MR Imaging
Similarly, other neuroimaging studies have demonstrated that rTMS not only induced changes in the area directly under the coil but also in more distant regions of the brain. For instance, rTMS can exert long-distance modulatory effects on subcortical brain regions, including activation of the striatal reward system (e.g., [85]). The magnitude of dopamine (DA) release in response to single rTMS has been shown to be comparable to the administration of d-amphetamine at a dose of 0.3 mg/kg, a compound known to increase synaptic dopamine signaling [86]. In addition to dopaminergic transmission, both the serotonergic system and the cholinergic system also seem to be involved in promoting the after-effects of rTMS [87].
Positron emission tomography (PET) studies have provided additional evidence for neuroplastic changes after rTMS, as it was shown that rTMS of the left M1 influenced the resting activity of the motor system beyond the duration of the stimulation [88,89]. Beyond that, metabolic changes were also evoked in brain regions interconnected with the stimulation site. These studies also demonstrated an acute reorganization of activity to other areas, as movement-related activity in the premotor cortex of the non-stimulated hemisphere increased after inhibitory rTMS, which was interpreted as a compensatory reaction to the inhibitory effect of 1 Hz rTMS [88]. This reorganization of activity probably resembles that in patients after recovery from stroke [90]. A rapid reorganization in functional brain networks induced by rTMS can also be seen using functional MRI. After 1 Hz rTMS to the left dorsal premotor cortex (PMd), a short-term reorganization was seen in the right PMd [91]. Yet, another fMRI study could show that both supra- and subthreshold rTMS over the left M1/S1 influenced the BOLD signal outside of the stimulated area (i.e., supplementary motor area, contralateral M1/S1), while only supra-threshold rTMS increased BOLD signal in the stimulated area [92]. A recent systematic review that presented 33 rTMS studies with pre- and post-rTMS measures of fMRI resting-state functional connectivity (RSFC) demonstrated reliable changes in RSFC after rTMS [93]. Interestingly, the direction of change was not always consistent with the direction traditionally observed in the stimulated brain area. More specifically, conventionally inhibitory stimulation protocols (e.g., 1 Hz) tended to increase RSFC, while the direction of changes after excitatory stimulation protocols was mixed. Moreover, rTMS-induced changes were not confined to the stimulated functional network, but a majority of changes were found in other brain networks. Hence, rTMS effects tend to spread across networks (ibid.). The importance of understanding these relationships can be of particular value, as there is growing interest in attempting to indirectly target distant brain areas through their connections with more accessible cortical areas. To this end, Wang et al. [31] targeted cortical-hippocampal networks by stimulating a subject-specific parietal region that showed high functional connectivity with the hippocampus. Crucially, they were able to demonstrate that increased functional connectivity in these networks positively correlated with associative memory performance after multi-session stimulation. These alterations likely represent neuroplasticity, as the effect persisted for 24 h after stimulation. Similarly, Mielacher et al. [94] augmented iTBS of the DLPFC for MDD treatment by adding daily sessions of stimulation over individualized parietal targets that were functionally connected to the hippocampus and found increased connectivity between hippocampus and DLPFC that lasted days after stimulation.
In addition to functional changes, TMS-induced neuroplasticity has also been demonstrated through structural changes in the human brain, beneath the site of stimulation, as well as in more distal brain regions [95]. Specifically, after a course of standard rTMS treatment for MDD, patients showed increased gray matter density brain volume in the left anterior cingulate cortex which correlated with the clinical response to treatment [96], a finding also shown by measuring MDD patients’ cortical thicknesses in the same region [97]. In a different study, several brain regions were shown to have increased in volume after treatment but this did not correlate to treatment response (left anterior cingulate cortex, the left insula, the left superior temporal gyrus, and the right angular gyrus) [96] as well as an increase in hippocampus volume [98]. Despite the absent relation to treatment response, a corresponding study pointed to another important aspect when considering plastic changes after prolonged rTMS treatment. Noda et al. [99] reported enhanced theta-gamma coupling at the C3 EEG-electrode site to be significantly correlated with hippocampal volumetric change, suggesting a potential structure-function relationship by the rTMS-induced plasticity. However, it should be cautioned that the physical basis of these morphological imaging methods remains poorly defined and seems to reflect tissue characteristics as well as the abundance and distribution of specific cell types (including neurons, glia, vasculature, but also subcellular components such as dendrites and spines) [100].
2.2.4. Pharmacological Evidence
Lasting after-effects of rTMS seem to implicate synaptic changes and are commonly explained by processes that are similar to LTP/LTD plasticity. Probably, the most direct evidence for this assumption stems from an in vitro model of repetitive magnetic stimulation using mouse organotypic entorhino-hippocampal slice cultures. It was found that 10 Hz stimulation not only led to a long-lasting increase in glutamatergic synaptic strength but also increased GluA1 levels as well as enlarged dendritic spines [101]. Since direct evidence is difficult to obtain in human subjects, pharmacological studies can provide essential information about the underlying mechanism of rTMS-induced after-effects by using drugs that act on receptors involved in neuroplasticity. One such study by Huang et al. [102] showed that the use of selective NMDA receptor antagonists interrupted the suppressive effect of cTBS and the facilitatory effect of iTBS. A similar effect of NMDA receptor antagonists was also found on PAS-induced after-effects [58,59] and 1 Hz rTMS [103]. Conversely, the use of d-cycloserine, a partial NMDA agonist, has been shown to further potentiate motor excitability after 10 Hz rTMS [104]. Moreover, it also modulates the effects of TBS-induced plasticity, although here it seems to reverse after-effects of iTBS from facilitation to inhibition [105,106]. A possible explanation for this might be the simultaneous inhibitory and excitatory effects with differing time course. Additionally, PAS- and TBS-induced plasticity were demonstrated to be modulated by calcium channel antagonists [107,108]. Taken together, it appears that the after-effects of rTMS rely on NMDA receptor-mediated glutamatergic function, suggesting that LTP/LTD mechanisms are involved. Ziemann et al. [109] used a temporary ischaemic block of the hand, which produced a reduction in GABAA inhibition in the contralateral motor cortex, to facilitate the induction of plasticity by a low-frequency rTMS paradigm. Comparable to in vitro studies, this finding provides further evidence that the effects of rTMS are due to an LTP-like mechanism.
Even though these studies show unanimously that TMS-induced potentiation and inhibition rely on NMDA receptors, there is mounting evidence that other processes such as neurotrophic, neuroinflammatory, and neuroendocrine factors, or even the neuro-glia network play a role in the observable after-effects (for an overview see [87]).
One exemplary line of evidence comes from studies investigating the brain-derived neurotrophic factor (BDNF) gene. A single nucleotide polymorphism (SNP) on the BDNF gene—BDNF Val66Met—is associated with hippocampal volume, episodic memory, as well as decreased experience-dependent plasticity in the motor cortex in the normal population [110]. Cheeran et al. [111] showed that Val66Met carriers responded differently to cTBS, iTBS, and PAS protocols as compared with Val66Val individuals, suggesting an influence of BDNF on the induction of rTMS after-effects. This, in turn, supports the notion that rTMS truly affects neuroplasticity.
2.2.5. Behavioral and Therapeutic Evidence
The literature reviewed above clearly demonstrates that rTMS elicits after-effects on the brain that outlast the period of stimulation and that these seem to indicate neuroplasticity. However, the exact nature of the after-effects is further complicated by the fact that they interact with voluntary muscle activity and behavioral learning [25] and depend on the history of synaptic activity in the stimulated region, in a manner that is compatible with a concept that is referred to as “metaplasticity” [112]. Metaplasticity is a higher-order form of synaptic plasticity and refers to neuronal activity that primes the subsequent induction of LTP or LTD [113]. A theoretical model for homeostatic metaplasticity is the Bienenstock–Cooper–Munro theory [114], which postulates that the threshold for inducing LTP and LTD is adjusted in response to previous time-averaged levels of postsynaptic activity. Importantly, rTMS plasticity paradigms seem to be consistent with the rules of metaplasticity, as shown in studies using priming stimulation [112,113,114,115]. More specifically, a prior history of increased activity (i.e., induced by another TMS protocol) enhances the effectiveness of inhibitory rTMS protocols, whereas a prior history of reduced activity enhances the effect of facilitatory rTMS [115,116,117,118]. Additionally, motor learning also seems to interact with rTMS after-effects homeostatically [25,119,120]. Such homeostatic interactions are in agreement with the notion that rTMS induces synaptic plasticity.
Ultimately, after-effects seem to also exert influences on behavior and cognition, including cognitive enhancement both in healthy volunteers [121] and in patients suffering from psychiatric/neurological diseases [122].
Neuroplastic changes after rTMS may also underlie the therapeutic benefits of therapy with TMS. The largest body of evidence of clinical effects can be found for treatment-refractory depression, for which most commonly an excitatory stimulation protocol is applied to the left DLPFC [32,123]. Recent evidence favors the use of iTBS protocols, as they have been shown to be clinically non-inferior to conventionally used high-frequency stimulation while allowing for a much shorter application time [56]. Moreover, high-dose (90,000 pulses administered over 50 sessions in five days (10 sessions/day)) intermittent TBS protocols with functional-connectivity-guided targeting demonstrate rapid-acting antidepressant effects even in patients with highly refractory depression [124].
3. Deep Brain Stimulation (DBS)
Deep brain stimulation (DBS) was introduced as a treatment for movement disorders in 1987, when A. Benabid implanted deep brain electrodes in the ventral intermediate nucleus of the thalamus (VIM) to treat severe tremor in essential tremor (ET) or Parkinson’s disease (PD) [125,126]. To date, DBS has proven to be effective and reached FDA approval for several indications, such as advanced PD, ET, medication refractory epilepsy, and has gained FDA humanitarian device exemptions for idiopathic dystonia syndromes (iDS) and obsessive-compulsive disorders. The neuroanatomic target structures include the subthalamic nucleus (STN), VIM, the internal part of the globus pallidum (GPi), the thalamic anterior nuclei, and the crus anterior of the internal capsule. A schematic illustration of STN-DBS is shown in Figure 2.
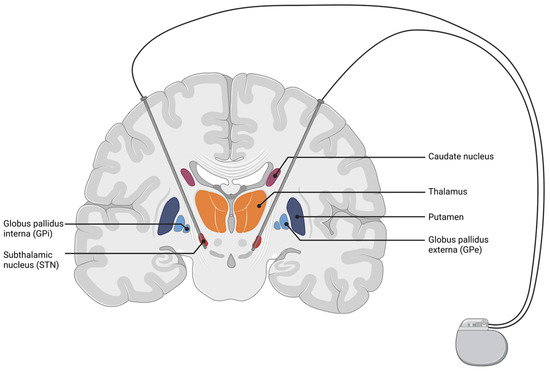
Figure 2.
Coronal cut of the brain, highlighting structures of the basal ganglia (BG) with an exemplary depiction of a DBS setup targeting the subthalamic nucleus. Electrical current is delivered from the implanted pulse generator to the targeted brain structure. The figure was created with biorender.com (accessed 1 February 2022).
3.1. Overview of DBS Methods
The results of the first series of investigations suggest that effective neurostimulation via deep brain electrodes acts like a lesion effect. In his seminal observation of the first patient treated with VIM DBS, Benabid reported that low-frequency stimulation of the VIM in the range of 30 to 50 Hz did not improve tremor but evoked sensory (paresthesias) and motor symptoms (contractions), whereas a stimulation frequency above 100 Hz dramatically improved tremor [125,126]. The VIM was the first target, because previous therapies such as electric or thermic coagulation of the VIM improved contralateral tremor [127], but were irreversible, tissue destructive and often included severe side effects such as sensory loss, paresthesia, or dysarthria. Systemic application of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP) and local injection of neurotoxin 6-hydroxydopamine (6-OHDA) create a Parkinson’s disease model in animals [128,129], which is the basis for studying the effects of DBS. Electrophysiological studies from animal models of PD and in PD patients demonstrate STN overactivity particularly in the beta frequency [130,131]. An additional chemical lesion in the STN has been shown to lead to an improvement in Parkinson’s symptoms [132]. Translating these findings into clinical research, high-frequency stimulation of the STN in patients with PD reduced signs and symptoms of PD and mimiced the effect of an STN lesion seen in animal studies. Thus, the mechanism of action of DBS was initially believed to be a local inhibition effect (“inhibition hypothesis”). Neuronal inhibition can be explained by a depolarization block in the vicinity of the stimulation, inactivating voltage-gated currents and activating inhibitory afferents, which might be specifically important for GPi stimulation in dystonia [133,134]. In PD, DBS also modifies the firing pattern within the BG, reducing abnormal firing patterns, such as bursts and oscillations in the beta frequency [135,136]. Decreasing the beta frequency within the BG is associated with clinical improvement in akinesia, rigidity, and albeit comparatively weaker, tremor [137]. DBS also excites afferent axons antidromically influencing the motor cortex probably via the hyperdirect pathway [138,139]. A more detailed analysis of cortical stimulation demonstrated a triphasic response pattern within the BG circuits (early excitation, inhibition, and late excitation) [140], DBS of the GPi, and STN inhibit cortical-evoked responses suggesting that it blocks information flow through the GPi (“disruption hypothesis”) [134]. In summary, the mechanisms of action of DBS are not fully understood, may depend on the composition of neuronal elements in the stimulated nucleus, and may depend on the underlying disease-specific pathophysiological conditions. However, these stimulation-induced changes have a network effect, demonstrated by an antidromic effect to the stimulation target afferent fibers, a filtering effect of patterns of oscillation within the BG circuits and downstream effects of efferent fibers influencing the next relay station throughout connectivity. Hence, neuroplastic changes induced by DBS within the nervous system are likely reflected in and observable as network effects.
3.2. Studies Demonstrating Effects of Neuroplasticity
3.2.1. Evidence for Neuroplasticity in Essential Tremor
We define clinical evidence for neuroplasticity as improvement of clinical signs and symptoms over time in a constant neurostimulation setting. Constant neurostimulation, in this context, implies that neither volume of activated tissue nor amplitude or stimulation frequency were changed. Clinical signs of plasticity can also be assumed when side-effects of neurostimulation occur over time in a constant stimulation setting. Movement disorders are a suitable candidate to observe clinical effects of neuroplasticity over time. Symptoms are easily observable and allow for a complete and continuous observation of their evolution under chronic DBS. Other disorders, such as for example, epilepsy, where the target symptom of DBS is a reduction in seizure frequency, are much harder to monitor for clinically observable effects of DBS-induced neuroplasticity. Therefore, the disorders ET, PD, and iDS were chosen as examples of DBS-induced neuroplasticity.
The clinical effect of VIM-DBS in ET is two-fold. Initially, it starts as a lesion effect that often substantially improves upper limb tremor in the first days after DBS surgery. When the tremor reoccurs, DBS is initiated, demonstrating an immediate reduction of about 90% in tremor amplitude [141]. This effect is a direct consequence of the disruption of information flow throughout the volume of activated tissue within the VIM and the dentato-thalamic tract, respectively [142]. Most cases have shown a gradual worsening of tremor amplitude over a time frame of years [143,144,145]. The long-term loss of VIM-DBS efficacy may be the result of disease progression and habituation to neurostimulation. Habituation can be interpreted as a neuroplastic effect that diminishes the stimulation effect post electrode implantation and stimulation initiation; both processes are difficult to disentangle. While, ideally, the difference in tremor severity in a stimulation-ON setting between two time points would reflect disease progression alone, habituation effects are likely to contribute as well. Controlling for the rebound effect of the tremor, seen in a third of patients, Paschen and colleagues disentangled the loss of stimulation efficacy over time in a sample of 20 ET patients; 13% of overall worsening in the stimulation-ON condition was attributable to habituation, whereas 87% of worsening in tremor severity could be explained by disease progression [146]. While it is convenient to explain the decrease in clinical efficacy solely in terms of disease progression and habituation, other uncontrolled factors such as prolonged rebound effects or emotional stress during tremor recordings might also have affected the study results. Nonetheless, even if only to a small degree, habituation effects play a role in the time course of treatment by VIM-DBS for severe essential tremor. Patel and colleagues further reported habituation of VIM-DBS in patients suffering from medical refractory tremor in the course of a demyelinating sensorimotor peripheral neuropathy [147]. This observation shows that habituation of VIM-DBS effects is likely not disease specific. While the mechanisms of habituation are presently unknown, they are of interest to prolong the VIM-DBS effect on tremor suppression.
In conclusion, there is a need to understand habituation effects of DBS to identify risk factors associated with habituation and to characterize neuroanatomic structures within the volume of activated tissue. A better understanding of habituation may help to find better stimulation protocols, sweet spots in the area of the VIM, or to develop pharmacological strategies to stop or to delay malplasticity driving habituation. However, to date, there is insufficient data to answer the question if lesion-associated habituation and stimulation-associated habituation share the same mechanisms. This question is of relevance in the treatment of MR-guided focused ultrasound thalamotomy in the context of ET.
3.2.2. Evidence for Neuroplasticity in Parkinson’s Disease
Comparable to ET, STN-DBS in PD patients leads to an immediate improvement in motor functions [148,149]. Tremor, rigidity, and akinesia improve within seconds to minutes. Several studies have documented positive long-lasting effects of STN-DBS in PD even when the neurostimulation is switched off. After medication and stimulation wash-out phase of three to five days, Benabid et al. [150] reported a slight improvement in motor functions six months after surgery as compared with preoperative scores. This observation has been confirmed by other studies [151,152]. Larger clinical follow-up studies have reported equal, or even a slight, improvement in motor function present one to four years follow-up [153,154,155].
These findings are rather unexpected in a progressive disorder. Two studies compared the clinical motor status before electrode implantation six months and four years after electrode implantation and assessed cerebral blood flow (CBF) SPECT. As compared with preoperative baseline and six months after electrode implantation, CBF SPECT four years after surgery demonstrated increased rCBF in the supplement motor area (SMA) in conditions medication-OFF/stimulation-ON [156] and medication-OFF/stimulation-OFF [157]. Changes in rCBF correlated with clinical improvements.
Evidence that the observed rCBF differences are indicative of STN-DBS related neuroplasticity comes from a postmortem study [158] which identified no, or minimal, tissue damage in the vicinity of the electrode tips. This, in conjunction with the observed significant increase in rCBF in the pre-SMA from six months to four years after surgery, would argue against otherwise alternatively hypothesized progressive lesion effects to restore motor functions in a chronic STN-DBS setting.
It also seems unlikely that factors associated with the duration of medication withdrawal or duration of stimulation holidays explain the lack of clinical progression in the medication-OFF/stimulation-OFF condition. Typically, the motor status in PD patients reaches a plateau three hours after switching OFF STN-DBS [159]. Medication and stimulation withdrawal phase vary among studies, often ranging between 10 and 12 h in a majority of studies, in line with the reported studies. The hypothesis of a STN-DBS related neuroprotective effect on DA could also not be confirmed. [123I]FP-CIT SPECT measuring the level of dopaminergic neurons (DA) in vivo, showed a comparable decrease in binding of radioligand in STN-DBS and non-operated PD groups [160]. Another potentially confounding factor might be the patient’s level of physical activity. Stable motor performance significantly increases after surgery.
Investigations about the effect of improved motor performance on physical activity are currently lacking. This is relevant because physical activity interventions are known to be an effective strategy to improve motor symptoms in PD [161]. However, its long-term effect on rCBF in the pre-SMA has not been sufficiently investigated. In conclusion, the mechanisms of a slight beneficial effect of STN-DBS, as described on motor performance, are not known. One explanation beyond the training hypothesis is that STN-DBS induces neuroplasticity that restores pre-SMA function and, to a smaller amount, motor functions in PD.
Long-term STN-DBS is also associated with attenuation of STN resting-state beta band activity. PD is characterized by elevated resting-state beta band activity of local field potentials (LFPs) in the STN. STN-DBS effectiveness is indexed by a reduction in elevated beta band activity. Trager et al. [162] and Chen et al. [163] highlighted that long-term STN-DBS also attenuated beta band amplitudes at rest (stimulation-OFF). Attenuation was already evident three months post implantation and two months post high-frequency stimulation (HFS) start [163] and may be time limited, as no further adaption in the beta band was detected between six and twelve months by Trager et al. [162]. Moreover, it might initially occur broad brand (low- and high- beta band specific) and after two months attenuation might be limited to the high-beta band activity [163]. Lesioning effects, as an alternative explanation, appears unlikely, as attenuation occurred as compared with one month after implantation [163] and was only present in the stimulated STN, in a subset of bilaterally implanted, but unilaterally stimulated patients [162]. While beta band attenuation was associated with overall motor improvement in both studies, the exact relationship between STN resting-state beta amplitude and overall motor improvement is not clear.
Sensory motor integration has also been shown to improve after long-term STN-DBS in patients with PD. Short latency afferent inhibition (SAI) and long latency afferent inhibition (LAI) index different aspects of sensory motor integration, likely with different anatomical origins (see Turco et al. [164] for an overview); both refer to effects of cortical inhibition of sensory evoked potentials, and are impaired in PD [165]. Sailer et al. [165] found SAI to be impaired in PD only ON dopaminergic (MED-ON) medication as compared with HC, whereas LAI has been shown to be impaired regardless of medication status. Shukla et al. [166] assessed the long-term effects of STN-DBS on SAI and LAI, considering effects of DBS (ON/OFF) and dopaminergic medication (ON/OFF) over time. One month post implantation, the effects of STN-DBS were difficult to discern. However, six months post implantation, DBS-ON was able to offset the impairment on SAI caused by MED-ON. Furthermore, LAI normalized under DBS-ON in conjunction with MED-ON after six months. Proprioception deficits present under MED-ON improved in conjunction with DBS. However, the relationship between LAI and SAI improvements is not clear.
At present, long-term volumetric effects of long-term STN-DBS are lacking. To the best of our knowledge, only two studies have assessed volumetric changes after long-term STN-DBS retrospectively in a sample of PD patients undergoing staged bilateral implantation [167,168]. While both studies reported volumetric changes, they were in disagreement on whether long-term STN-DBS overall led to volume reductions or increases in the targeted brain structures. Sankar et al. [167] and Kern et al. [168] both found reductions in hippocampal volumes, although in different hemispheres (both hippocampi or only ipsilateral to the stimulated STN) and to different degrees (~14\% and ~3\%). The large decrease in hippocampal volume observed by Sankar et al. [167] was not accompanied by a decrease in neuropsychological measures. With regard to BG structure volumetric changes, both studies were in disagreement. Sankar et al. [167] reported increases in putamen volume contralateral to the stimulated STN, Kern et al. [168] found overall decreases in basal ganglia-thalamocortical circuits (includes caudate, putamen, pallidum, and thalamus), particularly ipsilateral to the stimulated STN. The disagreement in the results of both studies may partially be explained by variable stimulation durations, low imaging resolution (1.5T), and small sample sizes. While both studies differ in results and methodology, in conjunction, they illustrate that long-term STN-DBS might also affect brain volume
In terms of long-term neuroplastic functional changes, a longitudinal study by Ge et al. [169] assessed alternations of the PD-related metabolic covariance pattern (PDRP) expression using F-FDG PET in a group of healthy control participants, PD patients, and PD patients (N = 9), who underwent STN-DBS. DBS participants were scanned pre-operative, three, and twelve month post operation, with post-operation scans being performed OFF-Meds and OFF-DBS. The therapeutic decrease in UPDRS scores at three month post operation was associated with a reduction PDRP. Moreover, graph theoretical network analysis of the F-FDG PET images showed that the initally increased small-worldness coeffcient within the PDRP subspace (as compared was healthy controls) was normalized three month post operation. This illustrates the capacity of DBS to exert long-term effects on functional network organization.
Mechanisms by which neuroplastic/neuroprotective effects of STN-DBS in PD occur are presently only well investigated in animal models. Preclinical work suggests a prominent role for BDNF inducing neuroplastic changes, in the nigrostriatal system and the motor cortex (e.g., [170]). However, clinical confirmatory evidence is still not available. On the contrary, BDNF rs6265 polymorphism in Parkinson’s patients has been shown not to be predictive of clinical outcome two years post STN-DBS [171].
3.2.3. Evidence for Neuroplasticity in Dystonia
The GPi is the established stimulation area to treat dystonia. As compared with ET or PD, the evolution of the antidystonic effect after GPi-DBS differs. Whereas phasic movement patterns respond fast after switching on neurostimulation [172], tonic postures and patterns improve only over weeks or even months after stimulation onset. Most studies have demonstrated a plateau in the treatment effect after three months [173]. Therefore, it is plausible that a long-lasting neuroplastic effect occurs, which leads to an improvement in symptoms over time. Dystonic symptoms may recur rapidly when the stimulation is discontinued in the first years [174]. However, in some cases, it has also been observed that after cessation of long-term stimulation, the therapeutic effect remained sustained over time [175,176]. Among the potential explanations for these neurological benefits, it can be assumed that DBS therapy may have the capacity to induce plastic changes that lessen or obviats the need for further treatment [177].
Whereas in PD patients reduced neural plasticity is often observed [178], dystonia patients exhibit excess neural plasticity [179,180,181]. Ruge et al. [180] tested long-term effects of GPi-DBS on neural plasticity via paired associative stimulation (PAS) and short-latency intracortical inhibition (SICI). SICI was increased as compared with HC pre-DBS implantation (indexing reduced inhibition) and reached normal levels over the course of treatment (one-, two-, and three-months post implantation). Response to PAS was also increased as compared with HC prior—indexing increased plasticity. Whereas SICI measures reduced gradually over the course of treatment, responses to PAS dropped sharply below HC response before gradually returning to normal levels as compared with HC. Increased plasticity preoperative has also been shown to correlate with symptom severity and benefit of DBS three months post implantation [181].
Ni et al. [182] linked GPi-DBS and the normalization of neural plasticity directly. In dystonia patients who had received clinically effective DBS (at least six months prior), single pulse GPi-DBS (only at clinically effective contacts and dosages) resulted in two distinct evoked potentials (EP) in the motor cortex. A negative EP at ~10 ms and a positive EP at ~25 ms with specific facilitatory and inhibitory effects on motor evoked potentials (MEP). In combination with TMS, if the interstimulus interval was 10 ms, MEP amplitudes were relatively increased. In contrast, if the interstimulus interval was 25 ms, MEP amplitudes decreased. Single pulse GPi-DBS and PAS with interstimulus intervals at 10 ms or 25 ms lead to motor cortical facilitation during and 30 min post PAS. The effect was more pronounced at interstimulus intervals of 25 ms. While these effects occur presumably after normalization of initially observed hyperplasticity [180], Ni et al. [182] provided evidence for the causal relevance of GPi-DBS for cortical plasticity in dystonia.
4. Transcranial Electric Stimulation (tES)
4.1. Overview of tES Methods
Transcranial electric stimulation refers to a variety of methods where a small electric current is non-invasively applied to the brain via two or more electrodes on the scalp. The current flows through the skin, bone, and brain tissue from one electrode to another. Depending on the precise stimulation parameters (particularly the waveform), tES can be subdivided into different techniques among which transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS) are the most commonly used to date. As compared with TMS and DBS, tES methods are relatively young and no reliable stimulation protocols have yet been established for successful therapy of neurological or psychiatric conditions, although first trials are underway to move the methods towards clinical applications (for an overview of clinical research on tDCS, see Zhao et al. [183], for an overview of clinical research on tACS, see Elyamany et al. [184]).
Due to skin sensations in response to electric stimulation, the intensity of tES is limited to one or a few mA (milliamperes). This results in an important difference to DBS and TMS, which are considered to be super-threshold brain stimulation techniques, i.e., the electric field resulting from stimulation can directly excite or inhibit neurons to fire action potentials or suppress firing, respectively. In contrast, tES is considered to be a subthreshold brain stimulation technique, since the electric field inside the brain is comparably weak and will only modulate the likeliness of neuronal firing in case of tDCS or the spike timing in case of tACS. The spike rate is not directly manipulated.
It is important to know where to place the stimulation electrodes on the scalp in order to target a specific brain region. For this purpose, finite element models have been used to predict the pattern of current density resulting from electric stimulation. At first, T1- and T2-weighted images are acquired with magnetic resonance imaging (MRI). Then, the images are segmented into tissues of different conductivity. Lastly, a computer algorithm (e.g., the Roast toolbox in MATLAB or SIMNIBS) computes the pattern of current flow for the selected electrode montage (as shown in Figure 3). Recently, we were able to demonstrate that a high correlation of the pattern of current flow and the source localization of the brain activity that was intended to be modulated by tACS resulted in stronger effects of amplitude enhancement (Kasten et al. [185]).
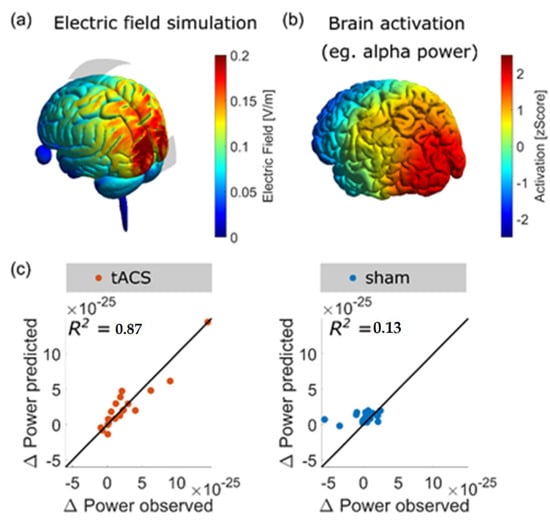
Figure 3.
(a) Visualization of current density pattern for a montage with stimulation electrodes at EEG locations Cz and Oz; (b) source localization of the human alpha generator from a MEG experiment; (c) it was recently shown that properties of the electric field (i.e., the similarity of the electric field and the activation map and the strength of the field) can be used to model the expected effect of an alpha-tACS experiment aiming to increase the amplitude of the alpha activity. Only after active (but not sham) stimulation the model predicted changes in alpha amplitude. Adapted from Ref. [185].
4.2. Assumed Neuronal Mechanisms
4.2.1. tDCS
During tDCS, a static electric field is applied between two or more electrodes for a duration commonly ranging between 5 and 20 min. The static field induces a subtle shift in a neuron’s resting membrane potential. Depending on its polarity, neurons exposed to the electric field are slightly de- or hyperpolarized, increasing or decreasing the likelihood of firing an action potential in response to incoming post-synaptic potentials [186,187,188].
In humans, the effects of tDCS were first investigated by assessing the size of motor evoked potentials (MEPs), which occur in response to single-pulse TMS over the motor cortex. Generally, it has been observed that the size of MEPs increased after anodal stimulation of the motor cortex, whilst decreasing after cathodal stimulation [35,189]. This has led to the notion that tDCS increases cortical excitability below the anode, while decreasing excitability below the cathode. However, more recent modeling work has pointed out that the exact effects on the neuronal level are highly dependent on the neuron’s orientation relative to the applied electric field, which is strongly determined by cortical folding [190]. In addition to acute effects during stimulation, it has often been observed that physiological effects persist for several tens of minutes after tDCS is switched off [35,189]. The duration of these effects depends on the duration of tDCS application. While stimulation durations of 5 to 7 min induce only short-lived after-effects in the range of 1–5 min, durations of 9–13 min can induce long-lasting alterations of MEPs for 30 or even up to 90 min [189]. In addition to these initial benefits of increasing tDCS dosage by longer stimulation durations, several studies have report an overall nonlinear relationship between tDCS dose and after-effects when stimulation amplitudes and/or durations are further increased. For example, the excitatory effect of anodal tDCS has been observed to revert after prolonged application for 26 min [191]. Similarly, the inhibitory effect of cathodal tDCS was reversed after 20 min of application when stimulation intensity was increased from 1 mA to 2 mA [192]. A more recent systematic comparison of stimulation amplitudes of anodal and cathodal tDCS, additionally, found no evidence for a correlation of stimulation current and the strength of tDCS after-effects on MEPs [193]. Interestingly, after-effects of tDCS do seem to accumulate in protocols that utilize temporally spaced, repeated sessions of stimulation [194,195], suggesting a possible involvement of late-stage LTP-like plasticity [191].
Although tDCS effects are most widely studied with respect to their influence on MEPs, tDCS has been shown to affect more direct measures of human brain activity such as eliciting lasting changes in EEG activity. It has been suggested that, due to its effect on cortical excitability, tDCS modulates EEG oscillations which sources are located in the stimulated target regions in the brain. However, which frequency band in the EEG is affected by stimulation as well as the direction of these effects seem rather inconsistent, difficult to predict, and may vary depending on the background task. For example, Miller et al. [196] reported a reduction in frontal-midline theta band power following anodal tDCS during a sustained attention task, while Zaehle et al. [197] reported a decrease in theta band power after cathodal tDCS, accompanied by similar spectral changes in the alpha band during a working memory task. When targeting regions in the motor cortex, Ardolino et al. [198] reported increased power in the delta and theta band following cathodal tDCS, while Matsumotu et al. [199] found that anodal tDCS increased event-related desynchronization of motor cortical mu-oscillations, while cathodal tDCS reduced it. Again, the two studies differ in terms of the underlying background tasks (rest vs. motor imagery). When targeting posterior brain regions during rest, Spitoni et al. [200] found a positive effect of anodal tDCS on spectral power which was limited to the alpha band, but no effect of cathodal stimulation. A similar effect was later reported by Mangia et al. [201], who, however, also reported a significant effect of anodal tDCS on power in the beta band. Interestingly, EEG changes elicited by tDCS are commonly only observed for a few minutes after stimulation, which is in contrast to the partly hour long effects observed on MEPs.
Another line of evidence comes from the investigation of changes in brain connectivity as a consequence of tDCS (for a review, see [202]). For example, resting-state data have been recorded with functional magnetic resonance imaging (fMRI) before and after tDCS stimulation. Participants who received stimulation revealed significant changes in regional brain connectivity in the default mode network and in fronto-parietal networks as compared with participants who received sham stimulation [203].
TDCS has also been applied as a therapeutic tool in multiple diseases. For example, tDCS has been demonstrated to suppress the symptoms of depression (for review, see [204]). An evidence-based analysis reported that tDCS was probably also effective to treat symptoms of fibromyalgia and addiction/craving [205].
While acute effects of tDCS during stimulation have been linked to shifts in membrane polarization, offline effects of tDCS are usually explained by processes of LTP- and LTD-like synaptic plasticity (for an overview see Stagg and Nitsche [206]). In particular, it has been shown that selective NMDA receptor antagonists reduce or completely abolish after-effects of anodal and cathodal tDCS on motor cortical excitability in vivo and in vitro [207,208]. Evidence from in vitro stimulation of slice preparations of mice further suggested an involvement of the brain-derived neurotrophic factor (BDNF) [208,209], which was involved in all stages of NMDA receptor-dependent LTP, whereas its precursor peptide (pro-BDNF) has been associated with LTD [210,211]. More recently, it was observed that a frequent polymorphism in the BDNF gene (nonconservative amino acid substitution from valine (Val) to methionine (Met) on codon 66) modulates the size of after-effects of anodal (but not cathodal) tDCS [212]. In that study, participants with the Val66Met polymorphism showed stronger enhancement of MEPs after tDCS as compared with participants with Val66Val after about 20 min post stimulation. Interestingly, this finding is contrary to the effect of the polymorphism on after-effects in other stimulation methods, where participants carrying the Val66Met polymorphism tended to show reduced or even abolished responses to the stimulation protocol (e.g., iTBS [212] and tACS [213]). Delivering an NMDA agonist prior to anodal tDCS of the human motor cortex increased the duration of enhanced MEPs from one hour to one day [214]. Long-lasting after-effects of tDCS on motion perception have even been found to persist over a time period of 28 days [215].
4.2.2. tACS
The effects that tACS has on ongoing brain oscillations during stimulation are believed to rely on neural entrainment, while the after-effects that outlast the end of stimulation are assumed to be implemented by neural plasticity [216]. By definition, entrainment itself does not outlast the stimulation period. Nevertheless, the effect of entrainment does not vanish instantly. For a few cycles after stimulation offset, the internal phase of the oscillation is still coupled to the external force, as has been reported for rTMS [217] and tACS [218]. After-effects indicate that tACS interferes with cortical neurons and demonstrate the efficacy of the method with potential for therapeutic applications.
It has been demonstrated that 10 min of tACS at an individual’s EEG alpha frequency (IAF) resulted in enhanced EEG alpha amplitudes in a time window of three minutes after the end of stimulation [219]. In order to investigate the duration of this after-effect, another study recorded 30 min of EEG after 20 min of tACS at IAF and observed elevated alpha amplitudes for the whole time interval after stimulation had ended [220]. Interestingly, the effect could only be observed when participants had their eyes open and started out with low alpha amplitudes, but not when they had their eyes closed and started out with high alpha amplitudes. Since that after-effect was still observable even at the end of the recording interval, yet another study recorded EEG for an even longer time period after stimulation, i.e., 90 min and demonstrated that 20 min of tACS achieved an after-effect on EEG alpha oscillations for 70 min [221].
Importantly, the after-effect of enhanced EEG alpha amplitudes also modulates cognitive processing after the end of stimulation. For example, improved mental rotation ability has been observed for about one hour after the end of tACS at individual alpha frequency over the visual cortex [222]. Along the same lines, tACS in the alpha frequency range improved multiple other visual processing abilities [223,224]. If other brain regions are stimulated, alpha-tACS can also achieve after-effects on other cognitive functions such as word processing in the prefrontal cortex [225] or motor behavior over the precentral cortex [226].
Notably, after-effects of tACS were not always detected [227], indicating that the phenomenon possibly depends upon stimulation intensity and/or duration. In line with that finding, it has been demonstrated that one second of tACS was not sufficient to achieve any after-effects suggesting a dose-response relationship [228]. This is in line with animal experiments that stimulated for a few seconds and were able to demonstrate entrainment but no after-effects. Another form of dose-response relationship has been demonstrated more recently by studies that were able to relate the strength of tACS-induced neurophysiological and behavioral after-effects to individual differences in the applied electric field, which could vary substantially due to anatomical differences [185,229].
It should be noted that after-effects of tACS on brain oscillations are not only observed on EEG/MEG amplitudes but have also been demonstrated for other parameters such as, for example, phase coherence between hemispheres probably reflecting changes in functional connectivity [230,231].
Note in addition that entrainment and plasticity are not mutually exclusive and may rely on each other [227]. It is plausible to assume that a successful entrainment during stimulation might be a necessary requirement for the generation of neuroplasticity reflecting enduring after-effects. The first evidence for the assumed interaction of online entrainment and after-effect was reported by [232]. These authors were able to demonstrate that the strength of an increased alpha amplitude after the end of stimulation correlated positively with the power during stimulation. These findings suggest a relationship between entrainment and plasticity, in which stronger entrainment predicts stronger after-effects.
However, Vossen et al. [227] showed that entrainment may not be required to produce tACS after-effects. They applied short durations of tACS at individual alpha frequency with short breaks of an equal duration. The experiment was composed of four conditions: short/phase continuous (i.e., no phase shifts between trains of stimulation) with three seconds of stimulation and three seconds of break; long/phase continuous with eight seconds of stimulation and eight seconds of break; long/phase discontinuous with eight seconds of stimulation and break, and phase shifts of 0, 90, 180, or 270 between trains of stimulation, as well as a sham condition with only one train of stimulation at the start of the experiment. The authors compared pre- versus post-stimulation EEG periods and reported a significant increase in alpha power for the long stimulation trains as compared with short stimulation trains and sham. The increased after-effect was observed irrespective of the continuity of phase, suggesting that entrainment was not required for after-effects.
Clinical studies using tACS have revealed that stimulation in the gamma frequency range can improve memory scores in Alzheimer’s disease [233]. In patients suffering from schizophrenia, tACS in the alpha frequency range was successful to decrease auditory hallucinations [234,235]. For reviews on further long-term effects of tACS in clinical populations, see [184,236].
Animal experiments can investigate synaptic plasticity by stimulating the pre-synaptic neuron and recording from the post-synaptic neuron. Such experiments have revealed that synaptic weights change depending upon the relative timing of pre- and post-synaptic spikes, a rule that is referred to as spike-timing dependent plasticity ((STDP) see Figure 4a). A simulation using artificial neural networks has tested whether this synaptic mechanism was susceptible to repetitive stimulation and could be responsible for the after-effects of tACS [219]. As shown in Figure 4b, neurons were interconnected with axons of different axonal delay times representing different resonance properties of the established neuronal loops. When this network was stimulated with a spike train of 10 Hz and synapses were updated according to the STDP rule, the synapses were strengthened that were incorporated in loops with resonance frequencies at the stimulation frequency and slightly above (Figure 4c).
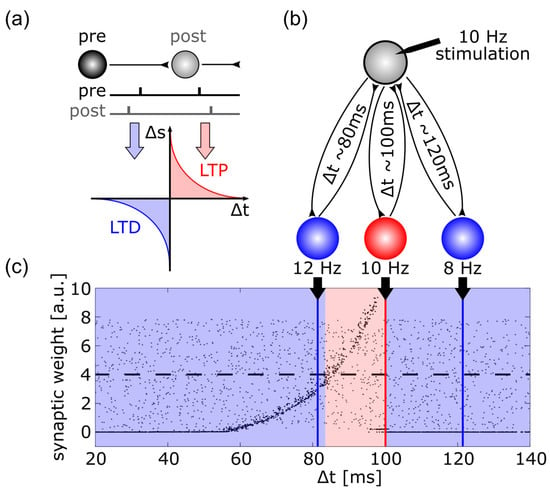
Figure 4.
This figure illustrates the mechanism of spike-timing dependent plasticity (STDP) which might explain after-effects of transcranial brain stimulation. (a) Synaptic weights are increased if a post-synaptic potential follows a pre-synaptic spike, i.e., long-term potentiation (LTP) occurs, if, however, a post-synaptic potential occurs prior to a pre-synaptic spike, long-term depression (LTD) is the result; (b) schematic illustration of a network simulation: A driving neuron (gray) established a recurrent loop with each neuron of a hidden layer, the total synaptic delay (i.e., the sum of both delays of the loop) varied between 20 and 140 ms (only three such loops are shown here), the driving neuron was stimulated with a spike train of 10 Hz; (c) synaptic weights of the back projection as a function of the total synaptic delay of the recurrent loops: grey dots display synaptic weights at the start of the simulation ranging from 0 to 8, black dots represent synaptic weights after the end of simulation. External stimulation of the driving neuron at 10 Hz resulted in synaptic weights above the initial average of 4 (dashed line) for recurrent loops with a total delay between 82 and 100 ms, indicating LTP (region shaded in red). For all other delays, synaptic weights were reduced, indicating LTD (region shaded in blue).
All of the above evidence is, of course, only indirect evidence for synaptic plasticity. It would be desirable to see that neurotransmitters known to be involved in plasticity play a role in the observed after-effects. A recent study demonstrated that when an NMDA receptor antagonist was given to participants, the after-effect of 20 Hz tACS on motor cortex excitability and EEG beta oscillations was abolished [237]. Another finding that pointed in the same direction investigated the effect of a genetic polymorphism of the brain-derived neurotrophic factor (BDNF) [213]. The authors were able to demonstrate that the observed increase in the EEG alpha amplitude after stimulation with alpha-tACS as compared with a control group was a function of the Val66-Met polymorphism of the BDNF gene.
4.2.3. tRNS
tRNS uses band-limited white noise as a signal for electrical stimulation. The effect of tRNS is believed to be due to modulation of ion channels and/or the noise raising the peaks of subthreshold neural oscillations above the threshold for firing, a mechanism referred to as stochastic resonance [238]. It has been demonstrated that 10 min of tRNS of the motor cortex led to enhanced TMS-evoked motor potentials for up to 60 min after the end of stimulation [239]. The effect also seems to be dose dependent, i.e., five and six minutes of tRNS result in after-effects, while 4 min of stimulation are not sufficient [240]. In contrast to the after-effects of tDCS and tACS, the after-effect of tRNS is not modulated by NMDA receptor agonists or antagonists but is suppressed by the GABA agonist lorazepam [241]. Interestingly, the BDNF polymorphism (Val66Val/Val66Met) that has been suggested to modulate the induction of LTP-like plasticity in other brain stimulation methods such as iTBS, anodal tDCS, and tACS, has not been found to influence tRNS after-effects on MEPs [212].
The fact that tRNS achieves after-effects on oscillatory EEG activity [242], despite its inability to entrain brain oscillations due to its non-rhythmic pattern [238], supports the abovementioned notion that entrainment may not be required for synaptic plasticity effects of tES.
5. Comparison of Methods Regarding Neuronal Plasticity
In this narrative review, we present three stimulation methods, i.e., TMS, DBS, and tES, and summarize the evidence suggesting a neuroplastic capacity of these neurostimulation techniques.
For TMS, evidence suggesting that after-effects are produced through neuroplastic mechanisms comes from three types of results: (i) rTMS protocols induce changes in cortical excitability, as seen in MEP amplitudes, which outlast the period of stimulation; (ii) lasting after-effects on brain activity after rTMS protocols can also be revealed using neuroimaging; (iii) the effects of rTMS are altered by drugs that act on receptors involved in neuroplasticity, for esample, NMDA receptor antagonists. As a restriction, it must be stated that patients in clinical brain stimulation studies are often also treated pharmacologically (e.g., pharmacological treatment kept stable), hence, reported long-lasting effects might be favored by metaplastic phenomena induced by chronic pharmacological treatment [243].
For DBS, the time course of the evolution of symptom relief after switching on GPi stimulation for dystonia is compatible with the assumption of a neuroplastic effect. Especially tonic patterns of a dystonic syndrome improved after weeks or months of active stimulation. In some patients, it has been observed that during stimulation cessation, after long-term stimulation, the therapeutic effect is sustained over time. In VIM-DBS for ET, habituation may reflect neuroplasticity, whereas in STN-DBS, hints toward evidence for neuroplasticity come from electrophysiological studies reporting after-effects of stimulation in the beta band range of oscillatory activity.
For transcranial electric stimulation, the evidence for after-effects comes mainly from three types of results: (i) TMS-induced MEPs reveal modulations of cortical excitation during electrical stimulation of the motor cortex, these changes outlast the end of the stimulation period; (ii) parameters of EEG and MEG oscillations such as amplitude and phase coherence have been observed to be elevated after the end of stimulation lasting for up to an hour; (iii) behavioral changes such as reaction times or error rates in cognitive experiments induced by tES that outlast the end of stimulation as well as reduced symptoms in neuropsychiatric diseases. At least the first two types of results are also modulated by neurotransmitters known to be involved in synaptic plasticity.
All three brain stimulation methods reviewed here reveal indirect signs of neuroplasticity, i.e., after-effects of elevated EEG/MEG amplitudes as well as behavioral or clinical after-effects. The three stimulation techniques differ in terms of the volume of tissue activated. We hypothesize that the neuroplastic effects are mediated by different mechanisms, i.e., how these stimulation techniques influence brain networks. DBS stimulates a focal circumscribed volume of tissue, acting as a hub in a neural network. Small brain nuclei such as STN and VIM circumscribe fiber tracts, such as the ansa lenticularis, are powerful interfaces within the motor network. Network effects, therefore, arise from a focal manipulation of network hubs within a deregulated neuronal system; tES and rTMS are less focal neurostimulation techniques. However, their potency to change EEG oscillations argues for their impact to influence brain network functions. Taken together, all three stimulation techniques have a capacity to interfere with brain networks and modify neuronal network functions.
Importantly, it has also been demonstrated that genetic polymorphisms and drugs that affect the function of neurotransmitters responsible for synaptic plasticity result in modulations of those after-effects. This makes it plausible to assume that the observed after-effects are, in fact, due to synaptic plasticity. Due to the large number of participants/patients that are required for studies on genetic polymorphisms, the evidence is sparse in DBS, which requires the implantation of electrodes in patients, as compared with TMS/tES.
The three methods of brain stimulation reviewed here operate at different time scales. The duration of rTMS, especially at high stimulation frequencies, is limited to the order of several minutes due to the relatively high amount of energy that is delivered to the brain; tES can be applied for up to about 30 min continuously due to its reduced energy as compared with TMS; DBS is typically applied chronically over years. Nevertheless, it would be interesting for future studies to directly compare the three described methods with each other regarding their effects upon synaptic plasticity.
The three methods also differ with regard to their focality. DBS is the most focal method with stimulation electrodes directly inserted into brain tissue, thereby, directly stimulating neurons in their vicinity. rTMS is a little less focal, since the magnetic field generated by the coil has to penetrate the skull before inducing an electric field inside the brain tissue. This electric field is strongest in superficial brain areas and decreases in intensity in deeper brain areas. tES is the least focal of the three methods. The electric field has to penetrate the skull and reaches all the way from one stimulation electrode to the other. For conventional, two-electrode montages, the maximum of the resulting electric field inside the brain occurs in the area between the two electrodes. For more advanced montages using a smaller area for current injection and a larger area to return the current, the field can be focused in the proximity of the injecting electrode. If the electrodes are placed too close to each other, the electric current is shunted by the scalp and only little current reaches brain tissue. While tES methods allow for stimulating superficial brain regions, targeting deep brain regions is not possible without strong co-stimulation of the overlaying cortex. A relatively new method, transcranial temporal interference stimulation (tTIS), has been developed in an animal model and aims to avoid this disadvantage of tES [244]. For tTIS, two pairs of electrodes are placed on the scalp, each introducing a banana-shaped region of current density inside the brain. Sine waves of slightly different frequencies are fed into the brain via each pair, for example, 1000 Hz and 1010 Hz, both frequencies being outside the frequency range relevant for brain activity, i.e., above 1000 Hz. In these brain areas where the two regions of current flow overlap, the two frequencies interact and a beat frequency can be seen at the difference frequency, i.e., 10 Hz. Simulations of the electric fields during tTIS suggest that this approach can target deep brain regions, whilst substantially reducing co-stimulation of the overlaying brain regions [245]. In addition, simulations of computational neural network models suggest that such beat frequencies are, in principle, capable of engaging neural oscillations [246,247], albeit at much higher stimulation intensities as compared with conventional tES methods. Notably, a first study has recently demonstrated that this method is able to modulate human brain activity [248]. Future studies should evaluate whether plasticity can be induced by this method.
Limitations
The literature that we cited revealed partially conflicting results. This is most likely due to small sample sizes of the studies. Sample size becomes particularly an issue when studies attempt to relate neuroimaging results of a few subjects to heterogeneous clinical scores which can vary widely even within the same subject. In that case, we focused on reporting imaging and electrophysiological evidence. In addition, we decided not to review literature on animal research that investigated plasticity directly at the synaptic level. Instead, we focused on human studies applying neurostimulation. This decision limits our conclusions since only animal studies can unequivocally demonstrate synaptic changes. Human studies on neurostimulation can only observe indirect effects of neuroplasticity. In contrast to animal models of brain stimulation, brain stimulation in general, and specifically in DBS, works over a period of years to decades [249,250], which cannot be recreated in an animal study. It has to be noted that after-effects observed after neurostimulation could also result from other mechanisms than neuroplasticity. Potentially, neurostimulation could result in other effects such as up- or downregulating the secretion of neurotransmitters such that altered levels of these neurotransmitters outlast the end of stimulation. Especially in the case of altered behavior, indirect effects of neurostimulation are conceivable. For example, if PD patients experience improved motor function during neurostimulation, it can be assumed that they, in turn, move more after neurostimulation. In that case, the observed after-effects could also be due to increased mobility.
In order to demonstrate more unequivocally that the abovementioned after-effects of brain stimulation are, in fact, due to neuroplasticity, future studies should focus on the involvement of relevant neurotransmitters, receptors, genes, etc. [251]. For example, positron emission tomography (PET) is feasible in parallel to all three brain stimulation methods described in this review (TMS [85,89], DBS [252,253], and tES [254,255]). In the past, however, PET was mainly used to investigate how brain activity changes in response to brain stimulation, i.e., regional cerebral blood flow was assessed [256]. It is, however, also possible to investigate how brain stimulation changes the binding of very specialized ligands to certain neurotransmitters and their receptors [257]. Crucially, a recent study used PET imaging to visualize AMPA receptors in humans [258]. A combination of PET imaging and brain stimulation would further our understanding of the interplay between brain stimulation and neuroplasticity.
Author Contributions
Conceptualization, J.K., M.K., K.G., F.H.K., C.S.H., K.W. and R.H.; data curation, K.G.; writing—original draft, J.K., M.K., K.G., F.H.K., C.S.H., K.W. and R.H.; writing—review and editing, J.K., M.K., K.G., F.H.K., C.S.H., K.W. and R.H.; visualization, J.K., K.G. and F.H.K.; project administration, M.K.; funding acquisition, C.S.H., K.W. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy, EXC 2177/1, Project ID 390895286 (grant to C.S.H) and DFG Graduate School 2783 (Principal investigators C.S.H and K.W). The APC was covered by the Open-Access Fund of the University of Oldenburg.
Conflicts of Interest
C.S.H. holds a patent on brain stimulation and cooperates in scientific projects with Neuroconn, a manufacturer of tES systems. K.W. received speaker honoraria and travel grants from Boston Scientific, a manufacturer of DBS systems. R.H. received an expert honoraria from Atheneum Consultations.
References
- Griesbach, G.S.; Hovda, D.A. Cellular and Molecular Neuronal Plasticity. Handb. Clin. Neurol. 2015, 128, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Buchtel, H.A. Neuronal Plasticity: Historical Roots and Evolution of Meaning. Exp. Brain Res. 2009, 192, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Gaser, C.; Kempermann, G.; Kuhn, H.G.; Winkler, J.; Büchel, C.; May, A. Temporal and Spatial Dynamics of Brain Structure Changes during Extensive Learning. J. Neurosci. 2006, 26, 6314–6317. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.J.G.D.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and Persistent Dendritic Spines in the Neocortex in Vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef]
- Lanza, G.; DelRosso, L.M.; Ferri, R. Sleep and Homeostatic Control of Plasticity. Handb. Clin. Neurol. 2022, 184, 53–72. [Google Scholar] [CrossRef]
- Arcos-Burgos, M.; Lopera, F.; Sepulveda-Falla, D.; Mastronardi, C. Neural Plasticity during Aging. Neural Plast. 2019, 2019, 6042132. [Google Scholar] [CrossRef]
- Timmermans, W.; Xiong, H.; Hoogenraad, C.C.; Krugers, H.J. Stress and Excitatory Synapses: From Health to Disease. Neuroscience 2013, 248, 626–636. [Google Scholar] [CrossRef]
- Mendez Colmenares, A.; Voss, M.W.; Fanning, J.; Salerno, E.A.; Gothe, N.P.; Thomas, M.L.; McAuley, E.; Kramer, A.F.; Burzynska, A.Z. White Matter Plasticity in Healthy Older Adults: The Effects of Aerobic Exercise. Neuroimage 2021, 239, 118305. [Google Scholar] [CrossRef]
- Mitterová, K.; Klobušiaková, P.; Šejnoha Minsterová, A.; Kropáčová, S.; Balážová, Z.; Točík, J.; Vaculíková, P.; Skotáková, A.; Grmela, R.; Rektorová, I. Impact of Cognitive Reserve on Dance Intervention-Induced Changes in Brain Plasticity. Sci. Rep. 2021, 11, 18527. [Google Scholar] [CrossRef]
- Shigihara, Y.; Hoshi, H.; Shinada, K.; Okada, T.; Kamada, H. Non-Pharmacological Treatment Changes Brain Activity in Patients with Dementia. Sci. Rep. 2020, 10, 6744. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Leuchter, A.F. The Effect of Psychotropic Drugs on Cortical Excitability and Plasticity Measured with Transcranial Magnetic Stimulation: Implications for Psychiatric Treatment. J. Affect. Disord. 2019, 253, 126–140. [Google Scholar] [CrossRef]
- Zhuang, X.; Mazzoni, P.; Kang, U.J. The Role of Neuroplasticity in Dopaminergic Therapy for Parkinson Disease. Nat. Rev. Neurol. 2013, 9, 248–256. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Tsodyks, M.; Wu, S. Short-Term Synaptic Plasticity. Scholarpedia 2013, 8, 3153. [Google Scholar] [CrossRef]
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation Technol. Neural Interface 2022, in press. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive Human Brain Stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef]
- Rothwell, J.C.; Hallett, M.; Berardelli, A.; Eisen, A.; Rossini, P.; Paulus, W. Magnetic Stimulation: Motor Evoked Potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 97–103. [Google Scholar]
- Cohen, S.L.; Bikson, M.; Badran, B.W.; George, M.S. A Visual and Narrative Timeline of US FDA Milestones for Transcranial Magnetic Stimulation (TMS) Devices. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2022, 15, 73–75. [Google Scholar] [CrossRef]
- Snow, N.J.; Wadden, K.P.; Chaves, A.R.; Ploughman, M. Transcranial Magnetic Stimulation as a Potential Biomarker in Multiple Sclerosis: A Systematic Review with Recommendations for Future Research. Neural Plast. 2019, 2019, e6430596. [Google Scholar] [CrossRef]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J.; Cracco, J.B.; Rudell, A.P.; Eberle, L. Transcranial Magnetic Stimulation in Study of the Visual Pathway. J. Clin. Neurophysiol. 1998, 15, 288–304. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal Responses to Magnetic Stimulation Reveal Cortical Reactivity and Connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef]
- Modak, A.; Fitzgerald, P.B. Personalising Transcranial Magnetic Stimulation for Depression Using Neuroimaging: A Systematic Review. World J. Biol. Psychiatry 2021, 22, 647–669. [Google Scholar] [CrossRef]
- Aydogan, D.B.; Souza, V.H.; Lioumis, P.; Ilmoniemi, R.J. Towards Real-Time Tractography-Based TMS Neuronavigation. Brain Stimul. 2021, 14, 1609. [Google Scholar] [CrossRef]
- Ziemann, U. TMS Induced Plasticity in Human Cortex. Rev. Neurosci. 2004, 15, 253–266. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor Cortex Plasticity Protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Valls-Solé, J.; Wassermann, E.M.; Hallett, M. Responses to Rapid-Rate Transcranial Magnetic Stimulation of the Human Motor Cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef]
- Eldaief, M.C.; Halko, M.A.; Buckner, R.L.; Pascual-Leone, A. Transcranial Magnetic Stimulation Modulates the Brain’s Intrinsic Activity in a Frequency-Dependent Manner. Proc. Natl. Acad. Sci. USA 2011, 108, 21229–21234. [Google Scholar] [CrossRef]
- Raij, T.; Nummenmaa, A.; Marin, M.-F.; Porter, D.; Furtak, S.; Setsompop, K.; Milad, M.R. Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biol. Psychiatry 2018, 84, 129–137. [Google Scholar] [CrossRef]
- Siebner, H.R.; Bergmann, T.O.; Bestmann, S.; Massimini, M.; Johansen-Berg, H.; Mochizuki, H.; Bohning, D.E.; Boorman, E.D.; Groppa, S.; Miniussi, C.; et al. Consensus Paper: Combining Transcranial Stimulation with Neuroimaging. Brain Stimul. 2009, 2, 58–80. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Kiebs, M.; Hurlemann, R.; Mutz, J. Repetitive Transcranial Magnetic Stimulation in Non-Treatment-Resistant Depression. Br. J. Psychiatry 2019, 215, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative Efficacy and Acceptability of Non-Surgical Brain Stimulation for the Acute Treatment of Major Depressive Episodes in Adults: Systematic Review and Network Meta-Analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Freitas, C.; Farzan, F.; Pascual-Leone, A. Assessing Brain Plasticity across the Lifespan with Transcranial Magnetic Stimulation: Why, How, and What Is the Ultimate Goal? Front. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Turco, C.V.; Nelson, A.J. Transcranial Magnetic Stimulation to Assess Exercise-Induced Neuroplasticity. Front. Neuroergonomics 2021, 2, 17. [Google Scholar] [CrossRef]
- Chen, R.; Classen, J.; Gerloff, C.; Celnik, P.; Wassermann, E.M.; Hallett, M.; Cohen, L.G. Depression of Motor Cortex Excitability by Low-Frequency Transcranial Magnetic Stimulation. Neurology 1997, 48, 1398–1403. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Rothwell, J.C.; Romeo, S.; Currà, A.; Gilio, F.; Modugno, N.; Manfredi, M. Facilitation of Muscle Evoked Responses after Repetitive Cortical Stimulation in Man. Exp. Brain Res. 1998, 122, 79–84. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Modulation of Corticospinal Excitability by Repetitive Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2000, 111, 800–805. [Google Scholar] [CrossRef]
- Peinemann, A.; Reimer, B.; Löer, C.; Quartarone, A.; Münchau, A.; Conrad, B.; Siebner, H.R. Long-Lasting Increase in Corticospinal Excitability after 1800 Pulses of Subthreshold 5 Hz Repetitive TMS to the Primary Motor Cortex. Clin. Neurophysiol. 2004, 115, 1519–1526. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A Comprehensive Review of the Effects of RTMS on Motor Cortical Excitability and Inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Rothkegel, H.; Sommer, M.; Paulus, W. Breaks during 5Hz RTMS Are Essential for Facilitatory after Effects. Clin. Neurophysiol. 2010, 121, 426–430. [Google Scholar] [CrossRef]
- Touge, T.; Gerschlager, W.; Brown, P.; Rothwell, J.C. Are the After-Effects of Low-Frequency RTMS on Motor Cortex Excitability Due to Changes in the Efficacy of Cortical Synapses? Clin. Neurophysiol. 2001, 112, 2138–2145. [Google Scholar] [CrossRef]
- Tang, Z.; Xuan, C.; Li, X.; Dou, Z.; Lan, Y.; Wen, H. Effect of Different Pulse Numbers of Transcranial Magnetic Stimulation on Motor Cortex Excitability: Single-blind, Randomized Cross-over Design. CNS Neurosci. Ther. 2019, 25, 1277–1281. [Google Scholar] [CrossRef]
- McCalley, D.M.; Lench, D.H.; Doolittle, J.D.; Imperatore, J.P.; Hoffman, M.; Hanlon, C.A. Determining the Optimal Pulse Number for Theta Burst Induced Change in Cortical Excitability. Sci. Rep. 2021, 11, 8726. [Google Scholar] [CrossRef]
- Gamboa, O.L.; Antal, A.; Moliadze, V.; Paulus, W. Simply Longer Is Not Better: Reversal of Theta Burst after-Effect with Prolonged Stimulation. Exp. Brain Res. 2010, 204, 181–187. [Google Scholar] [CrossRef]
- Jung, S.H.; Shin, J.E.; Jeong, Y.-S.; Shin, H.-I. Changes in Motor Cortical Excitability Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation of Different Stimulation Durations. Clin. Neurophysiol. 2008, 119, 71–79. [Google Scholar] [CrossRef]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord and Roots: Basic Principles and Procedures for Routine Clinical Application. Report of an IFCN Committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Brown, T.L.; Daskalakis, Z.J.; Chen, R.; Kulkarni, J. Intensity-Dependent Effects of 1 Hz RTMS on Human Corticospinal Excitability. Clin. Neurophysiol. 2002, 113, 1136–1141. [Google Scholar] [CrossRef]
- Modugno, N.; Nakamura, Y.; MacKinnon, C.; Filipovic, S.; Bestmann, S.; Berardelli, A.; Rothwell, J. Motor Cortex Excitability Following Short Trains of Repetitive Magnetic Stimuli. Exp. Brain Res. 2001, 140, 453–459. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Larson, J.; Wong, D.; Lynch, G. Patterned Stimulation at the Theta Frequency Is Optimal for the Induction of Hippocampal Long-Term Potentiation. Brain Res. 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Cárdenas-Morales, L.; Nowak, D.A.; Kammer, T.; Wolf, R.C.; Schönfeldt-Lecuona, C. Mechanisms and Applications of Theta-Burst RTMS on the Human Motor Cortex. Brain Topogr. 2010, 22, 294–306. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of Theta Burst versus High-Frequency Repetitive Transcranial Magnetic Stimulation in Patients with Depression (THREE-D): A Randomised Non-Inferiority Trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of Plasticity in the Human Motor Cortex by Paired Associative Stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Benecke, R.; Cohen, L.G.; Classen, J. Mechanisms of Enhancement of Human Motor Cortex Excitability Induced by Interventional Paired Associative Stimulation. J. Physiol. 2002, 543, 699–708. [Google Scholar] [CrossRef]
- Wolters, A.; Sandbrink, F.; Schlottmann, A.; Kunesch, E.; Stefan, K.; Cohen, L.G.; Benecke, R.; Classen, J. A Temporally Asymmetric Hebbian Rule Governing Plasticity in the Human Motor Cortex. J. Neurophysiol. 2003, 89, 2339–2345. [Google Scholar] [CrossRef]
- Feldman, D.E. The Spike-Timing Dependence of Plasticity. Neuron 2012, 75, 556–571. [Google Scholar] [CrossRef]
- Arai, N.; Müller-Dahlhaus, F.; Murakami, T.; Bliem, B.; Lu, M.-K.; Ugawa, Y.; Ziemann, U. State-Dependent and Timing-Dependent Bidirectional Associative Plasticity in the Human SMA-M1 Network. J. Neurosci. 2011, 31, 15376–15383. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.R.; Johnen, V.M.; Nelissen, N.; O’Shea, J.; Rushworth, M.F.S. Noninvasive Associative Plasticity Induction in a Corticocortical Pathway of the Human Brain. J. Neurosci. 2011, 31, 17669–17679. [Google Scholar] [CrossRef] [PubMed]
- Udupa, K.; Bahl, N.; Ni, Z.; Gunraj, C.; Mazzella, F.; Moro, E.; Hodaie, M.; Lozano, A.M.; Lang, A.E.; Chen, R. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson’s Disease. J. Neurosci. 2016, 36, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.; Lioumis, P.; Kirveskari, E.; Savolainen, S.; Mäkelä, J.P. A Novel Paired Associative Stimulation Protocol with a High-Frequency Peripheral Component: A Review on Results in Spinal Cord Injury Rehabilitation. Eur. J. Neurosci. 2021, 53, 3242–3257. [Google Scholar] [CrossRef]
- Esser, S.K.; Huber, R.; Massimini, M.; Peterson, M.J.; Ferrarelli, F.; Tononi, G. A Direct Demonstration of Cortical LTP in Humans: A Combined TMS/EEG Study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Casarotto, S.; Lauro, L.J.R.; Bellina, V.; Casali, A.G.; Rosanova, M.; Pigorini, A.; Defendi, S.; Mariotti, M.; Massimini, M. EEG Responses to TMS Are Sensitive to Changes in the Perturbation Parameters and Repeatable over Time. PLoS ONE 2010, 5, e10281. [Google Scholar] [CrossRef]
- Lioumis, P.; Kicić, D.; Savolainen, P.; Mäkelä, J.P.; Kähkönen, S. Reproducibility of TMS-Evoked EEG Responses. Hum. Brain Mapp. 2009, 30, 1387–1396. [Google Scholar] [CrossRef]
- Kerwin, L.J.; Keller, C.J.; Wu, W.; Narayan, M.; Etkin, A. Test-Retest Reliability of Transcranial Magnetic Stimulation EEG Evoked Potentials. Brain Stimul. 2018, 11, 536–544. [Google Scholar] [CrossRef]
- Casula, E.P.; Tarantino, V.; Basso, D.; Arcara, G.; Marino, G.; Toffolo, G.M.; Rothwell, J.C.; Bisiacchi, P.S. Low-Frequency RTMS Inhibitory Effects in the Primary Motor Cortex: Insights from TMS-Evoked Potentials. NeuroImage 2014, 98, 225–232. [Google Scholar] [CrossRef]
- Van Der Werf, Y.D.; Paus, T. The Neural Response to Transcranial Magnetic Stimulation of the Human Motor Cortex. I. Intracortical and Cortico-Cortical Contributions. Exp. Brain Res. 2006, 175, 231–245. [Google Scholar] [CrossRef]
- Vernet, M.; Bashir, S.; Yoo, W.-K.; Perez, J.M.; Najib, U.; Pascual-Leone, A. Insights on the Neural Basis of Motor Plasticity Induced by Theta Burst Stimulation from TMS–EEG. Eur. J. Neurosci. 2013, 37, 598–606. [Google Scholar] [CrossRef]
- Veniero, D.; Maioli, C.; Miniussi, C. Potentiation of Short-Latency Cortical Responses by High-Frequency Repetitive Transcranial Magnetic Stimulation. J. Neurophysiol. 2010, 104, 1578–1588. [Google Scholar] [CrossRef]
- Komssi, S.; Kähkönen, S.; Ilmoniemi, R.J. The Effect of Stimulus Intensity on Brain Responses Evoked by Transcranial Magnetic Stimulation. Hum. Brain Mapp. 2004, 21, 154–164. [Google Scholar] [CrossRef]
- Komssi, S.; Kähkönen, S. The Novelty Value of the Combined Use of Electroencephalography and Transcranial Magnetic Stimulation for Neuroscience Research. Brain Res. Rev. 2006, 52, 183–192. [Google Scholar] [CrossRef]
- Komssi, S.; Aronen, H.J.; Huttunen, J.; Kesäniemi, M.; Soinne, L.; Nikouline, V.V.; Ollikainen, M.; Roine, R.O.; Karhu, J.; Savolainen, S.; et al. Ipsi- and Contralateral EEG Reactions to Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2002, 113, 175–184. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial Magnetic Stimulation and Cortical Evoked Potentials: A TMS/EEG Co-Registration Study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef]
- Chung, S.W.; Lewis, B.P.; Rogasch, N.C.; Saeki, T.; Thomson, R.H.; Hoy, K.E.; Bailey, N.W.; Fitzgerald, P.B. Demonstration of Short-Term Plasticity in the Dorsolateral Prefrontal Cortex with Theta Burst Stimulation: A TMS-EEG Study. Clin. Neurophysiol. 2017, 128, 1117–1126. [Google Scholar] [CrossRef]
- Lioumis, P.; Zomorrodi, R.; Hadas, I.; Daskalakis, Z.J.; Blumberger, D.M. Combined Transcranial Magnetic Stimulation and Electroencephalography of the Dorsolateral Prefrontal Cortex. J. Vis. Exp. 2018, 138, e57983. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical Utility and Prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Measuring Brain Stimulation Induced Changes in Cortical Properties Using TMS-EEG. Brain Stimul. 2015, 8, 1010–1020. [Google Scholar] [CrossRef]
- Qiu, S.; Yi, W.; Wang, S.; Zhang, C.; He, H. The Lasting Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation on Resting State EEG in Healthy Subjects. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Ponzo, V.; Stampanoni Bassi, M.; Veniero, D.; Caltagirone, C.; Koch, G. Cerebellar Theta Burst Stimulation Modulates the Neural Activity of Interconnected Parietal and Motor Areas. Sci. Rep. 2016, 6, 36191. [Google Scholar] [CrossRef] [PubMed]
- Tervo, A.E.; Nieminen, J.O.; Lioumis, P.; Metsomaa, J.; Souza, V.H.; Sinisalo, H.; Stenroos, M.; Sarvas, J.; Ilmoniemi, R.J. Closed-Loop Optimization of Transcranial Magnetic Stimulation with Electroencephalography Feedback. Brain Stimul. 2022, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, C.; Belardinelli, P.; Müller-Dahlhaus, F.; Ziemann, U. Closed-Loop Neuroscience and Non-Invasive Brain Stimulation: A Tale of Two Loops. Front. Cell. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Mulert, C.; Grossheinrich, N.; Rupprecht, R.; Möller, H.-J.; Hegerl, U.; et al. Acute Prefrontal RTMS Increases Striatal Dopamine to a Similar Degree as D-Amphetamine. Psychiatry Res. 2007, 156, 251–255. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Lee, L.; Siebner, H.R.; Rowe, J.B.; Rizzo, V.; Rothwell, J.C.; Frackowiak, R.S.J.; Friston, K.J. Acute Remapping within the Motor System Induced by Low-Frequency Repetitive Transcranial Magnetic Stimulation. J. Neurosci. 2003, 23, 5308–5318. [Google Scholar] [CrossRef]
- Rounis, E.; Lee, L.; Siebner, H.R.; Rowe, J.B.; Friston, K.J.; Rothwell, J.C.; Frackowiak, R.S.J. Frequency Specific Changes in Regional Cerebral Blood Flow and Motor System Connectivity Following RTMS to the Primary Motor Cortex. NeuroImage 2005, 26, 164–176. [Google Scholar] [CrossRef]
- Ridding, M.C.; Rothwell, J.C. Is There a Future for Therapeutic Use of Transcranial Magnetic Stimulation? Nat. Rev. Neurosci. 2007, 8, 559–567. [Google Scholar] [CrossRef]
- O’Shea, J.; Johansen-Berg, H.; Trief, D.; Göbel, S.; Rushworth, M.F.S. Functionally Specific Reorganization in Human Premotor Cortex. Neuron 2007, 54, 479–490. [Google Scholar] [CrossRef]
- Bestmann, S.; Baudewig, J.; Siebner, H.R.; Rothwell, J.C.; Frahm, J. Subthreshold High-Frequency TMS of Human Primary Motor Cortex Modulates Interconnected Frontal Motor Areas as Detected by Interleaved FMRI-TMS. Neuroimage 2003, 20, 1685–1696. [Google Scholar] [CrossRef]
- Beynel, L.; Powers, J.P.; Appelbaum, L.G. Effects of Repetitive Transcranial Magnetic Stimulation on Resting-State Connectivity: A Systematic Review. NeuroImage 2020, 211, 116596. [Google Scholar] [CrossRef]
- Mielacher, C.; Schultz, J.; Kiebs, M.; Dellert, T.; Metzner, A.; Graute, L.; Högenauer, H.; Maier, W.; Lamm, C.; Hurlemann, R. Individualized Theta-Burst Stimulation Modulates Hippocampal Activity and Connectivity in Patients with Major Depressive Disorder. Pers. Med. Psychiatry 2020, 23–24, 100066. [Google Scholar] [CrossRef]
- May, A.; Hajak, G.; Gänßbauer, S.; Steffens, T.; Langguth, B.; Kleinjung, T.; Eichhammer, P. Structural Brain Alterations following 5 Days of Intervention: Dynamic Aspects of Neuroplasticity. Cereb. Cortex 2007, 17, 205–210. [Google Scholar] [CrossRef]
- Lan, M.J.; Chhetry, B.T.; Liston, C.; Mann, J.J.; Dubin, M. Transcranial Magnetic Stimulation of Left Dorsolateral Prefrontal Cortex Induces Brain Morphological Changes in Regions Associated with a Treatment Resistant Major Depressive Episode: An Exploratory Analysis. Brain Stimul. 2016, 9, 577–583. [Google Scholar] [CrossRef]
- Boes, A.D.; Uitermarkt, B.D.; Albazron, F.M.; Lan, M.J.; Liston, C.; Pascual-Leone, A.; Dubin, M.J.; Fox, M.D. Rostral Anterior Cingulate Cortex Is a Structural Correlate of Repetitive TMS Treatment Response in Depression. Brain Stimul. 2018, 11, 575–581. [Google Scholar] [CrossRef]
- Hayasaka, S.; Nakamura, M.; Noda, Y.; Izuno, T.; Saeki, T.; Iwanari, H.; Hirayasu, Y. Lateralized Hippocampal Volume Increase Following High-Frequency Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients with Major Depression. Psychiatry Clin. Neurosci. 2017, 71, 747–758. [Google Scholar] [CrossRef]
- Noda, Y.; Zomorrodi, R.; Daskalakis, Z.J.; Blumberger, D.M.; Nakamura, M. Enhanced Theta-Gamma Coupling Associated with Hippocampal Volume Increase Following High-Frequency Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients with Major Depression. Int. J. Psychophysiol. 2018, 133, 169–174. [Google Scholar] [CrossRef]
- Asan, L.; Falfán-Melgoza, C.; Beretta, C.A.; Sack, M.; Zheng, L.; Weber-Fahr, W.; Kuner, T.; Knabbe, J. Cellular Correlates of Gray Matter Volume Changes in Magnetic Resonance Morphometry Identified by Two-Photon Microscopy. Sci. Rep. 2021, 11, 4234. [Google Scholar] [CrossRef]
- Vlachos, A.; Müller-Dahlhaus, F.; Rosskopp, J.; Lenz, M.; Ziemann, U.; Deller, T. Repetitive Magnetic Stimulation Induces Functional and Structural Plasticity of Excitatory Postsynapses in Mouse Organotypic Hippocampal Slice Cultures. J. Neurosci. 2012, 32, 17514–17523. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Chen, R.-S.; Rothwell, J.C.; Wen, H.-Y. The After-Effect of Human Theta Burst Stimulation Is NMDA Receptor Dependent. Clin. Neurophysiol. 2007, 118, 1028–1032. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Benitez, J.; Oxley, T.; Daskalakis, J.Z.; de Castella, A.R.; Kulkarni, J. A Study of the Effects of Lorazepam and Dextromethorphan on the Response to Cortical 1 Hz Repetitive Transcranial Magnetic Stimulation. Neuroreport 2005, 16, 1525–1528. [Google Scholar] [CrossRef]
- Brown, J.C.; DeVries, W.H.; Korte, J.E.; Sahlem, G.L.; Bonilha, L.; Short, E.B.; George, M.S. NMDA Receptor Partial Agonist, d-Cycloserine, Enhances 10 Hz RTMS-Induced Motor Plasticity, Suggesting Long-Term Potentiation (LTP) as Underlying Mechanism. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2020, 13, 530–532. [Google Scholar] [CrossRef]
- Selby, B.; MacMaster, F.P.; Kirton, A.; McGirr, A. D-Cycloserine Blunts Motor Cortex Facilitation after Intermittent Theta Burst Transcranial Magnetic Stimulation: A Double-Blind Randomized Placebo-Controlled Crossover Study. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, 1063–1065. [Google Scholar] [CrossRef]
- Teo, J.T.H.; Swayne, O.B.; Rothwell, J.C. Further Evidence for NMDA-Dependence of the after-Effects of Human Theta Burst Stimulation. Clin. Neurophysiol. 2007, 118, 1649–1651. [Google Scholar] [CrossRef]
- Wankerl, K.; Weise, D.; Gentner, R.; Rumpf, J.-J.; Classen, J. L-Type Voltage-Gated Ca2+ Channels: A Single Molecular Switch for Long-Term Potentiation/Long-Term Depression-like Plasticity and Activity-Dependent Metaplasticity in Humans. J. Neurosci. 2010, 30, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Mann, J.; Rumpf, J.-J.; Hallermann, S.; Classen, J. Differential Regulation of Human Paired Associative Stimulation-Induced and Theta-Burst Stimulation-Induced Plasticity by L-Type and T-Type Ca2+ Channels. Cereb. Cortex 2017, 27, 4010–4021. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Hallett, M.; Cohen, L.G. Mechanisms of Deafferentation-Induced Plasticity in Human Motor Cortex. J. Neurosci. 1998, 18, 7000–7007. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Chan, S.; Pringle, E.; Schallert, K.; Procaccio, V.; Jimenez, R.; Cramer, S.C. BDNF Val66met Polymorphism Is Associated with Modified Experience-Dependent Plasticity in Human Motor Cortex. Nat. Neurosci. 2006, 9, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, B.; Talelli, P.; Mori, F.; Koch, G.; Suppa, A.; Edwards, M.; Houlden, H.; Bhatia, K.; Greenwood, R.; Rothwell, J.C. A Common Polymorphism in the Brain-Derived Neurotrophic Factor Gene (BDNF) Modulates Human Cortical Plasticity and the Response to RTMS. J. Physiol. 2008, 586, 5717–5725. [Google Scholar] [CrossRef]
- Abraham, W.C. Metaplasticity: Tuning Synapses and Networks for Plasticity. Nat. Rev. Neurosci. 2008, 9, 387. [Google Scholar] [CrossRef]
- Karabanov, A.; Ziemann, U.; Hamada, M.; George, M.S.; Quartarone, A.; Classen, J.; Massimini, M.; Rothwell, J.; Siebner, H.R. Consensus Paper: Probing Homeostatic Plasticity of Human Cortex with Non-Invasive Transcranial Brain Stimulation. Brain Stimul. 2015, 8, 993–1006. [Google Scholar] [CrossRef]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the Development of Neuron Selectivity: Orientation Specificity and Binocular Interaction in Visual Cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef]
- Müller, J.F.M.; Orekhov, Y.; Liu, Y.; Ziemann, U. Homeostatic Plasticity in Human Motor Cortex Demonstrated by Two Consecutive Sessions of Paired Associative Stimulation. Eur. J. Neurosci. 2007, 25, 3461–3468. [Google Scholar] [CrossRef]
- Murakami, T.; Müller-Dahlhaus, F.; Lu, M.-K.; Ziemann, U. Homeostatic Metaplasticity of Corticospinal Excitatory and Intracortical Inhibitory Neural Circuits in Human Motor Cortex. J. Physiol. 2012, 590, 5765–5781. [Google Scholar] [CrossRef]
- Ni, Z.; Gunraj, C.; Kailey, P.; Cash, R.F.H.; Chen, R. Heterosynaptic Modulation of Motor Cortical Plasticity in Human. J. Neurosci. 2014, 34, 7314–7321. [Google Scholar] [CrossRef]
- Todd, G.; Flavel, S.C.; Ridding, M.C. Priming Theta-Burst Repetitive Transcranial Magnetic Stimulation with Low- and High-Frequency Stimulation. Exp. Brain Res. 2009, 195, 307–315. [Google Scholar] [CrossRef]
- Hamada, M.; Terao, Y.; Hanajima, R.; Shirota, Y.; Nakatani-Enomoto, S.; Furubayashi, T.; Matsumoto, H.; Ugawa, Y. Bidirectional Long-Term Motor Cortical Plasticity and Metaplasticity Induced by Quadripulse Transcranial Magnetic Stimulation. J. Physiol. 2008, 586, 3927–3947. [Google Scholar] [CrossRef]
- Stefan, K.; Wycislo, M.; Gentner, R.; Schramm, A.; Naumann, M.; Reiners, K.; Classen, J. Temporary Occlusion of Associative Motor Cortical Plasticity by Prior Dynamic Motor Training. Cereb. Cortex 2006, 16, 376–385. [Google Scholar] [CrossRef]
- Patel, R.; Silla, F.; Pierce, S.; Theule, J.; Girard, T.A. Cognitive Functioning before and after Repetitive Transcranial Magnetic Stimulation (RTMS): A Quantitative Meta-Analysis in Healthy Adults. Neuropsychologia 2020, 141, 107395. [Google Scholar] [CrossRef] [PubMed]
- Guse, B.; Falkai, P.; Wobrock, T. Cognitive Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation: A Systematic Review. J. Neural Transm. 2010, 117, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S.; Stimpson, K.H.; Nejad, R.; Barmak, F.; Veerapal, C.; Khan, N.; Cherian, K.; Felber, E.; et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef]
- Benabid, A.L. Lasker Award Winner Alim Louis Benabid. Nat. Med. 2014, 20, 1121–1123. [Google Scholar] [CrossRef]
- Benabid, A.L.; Pollak, P.; Louveau, A.; Henry, S.; de Rougemont, J. Combined (Thalamotomy and Stimulation) Stereotactic Surgery of the VIM Thalamic Nucleus for Bilateral Parkinson Disease. Appl. Neurophysiol. 1987, 50, 344–346. [Google Scholar] [CrossRef]
- Benabid, A.L.; Benazzouz, A.; Hoffmann, D.; Limousin, P.; Krack, P.; Pollak, P. Long-Term Electrical Inhibition of Deep Brain Targets in Movement Disorders. Mov. Disord. 1998, 13 (Suppl. 3), 119–125. [Google Scholar] [CrossRef]
- Eldridge, R.; Rocca, W.A. The Clinical Syndrome of Striatal Dopamine Deficiency: Parkinsonism Induced by MPTP. N. Engl. J. Med. 1985, 313, 1159–1160. [Google Scholar] [CrossRef]
- Kaakkola, S.; Teräväinen, H. Animal Models of Parkinsonism. Pharmacol. Toxicol. 1990, 67, 95–100. [Google Scholar] [CrossRef]
- Deffains, M.; Holland, P.; Moshel, S.; Ramirez de Noriega, F.; Bergman, H.; Israel, Z. Higher Neuronal Discharge Rate in the Motor Area of the Subthalamic Nucleus of Parkinsonian Patients. J. Neurophysiol. 2014, 112, 1409–1420. [Google Scholar] [CrossRef][Green Version]
- Kühn, A.A.; Trottenberg, T.; Kivi, A.; Kupsch, A.; Schneider, G.-H.; Brown, P. The Relationship between Local Field Potential and Neuronal Discharge in the Subthalamic Nucleus of Patients with Parkinson’s Disease. Exp. Neurol. 2005, 194, 212–220. [Google Scholar] [CrossRef]
- Bergman, H.; Wichmann, T.; DeLong, M.R. Reversal of Experimental Parkinsonism by Lesions of the Subthalamic Nucleus. Science 1990, 249, 1436–1438. [Google Scholar] [CrossRef]
- Chiken, S.; Nambu, A. High-Frequency Pallidal Stimulation Disrupts Information Flow through the Pallidum by GABAergic Inhibition. J. Neurosci. 2013, 33, 2268–2280. [Google Scholar] [CrossRef]
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Brown, P. Oscillatory Nature of Human Basal Ganglia Activity: Relationship to the Pathophysiology of Parkinson’s Disease. Mov. Disord. 2003, 18, 357–363. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological Synchronization in Parkinson’s Disease: Networks, Models and Treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef]
- Tinkhauser, G.; Pogosyan, A.; Tan, H.; Herz, D.M.; Kühn, A.A.; Brown, P. Beta Burst Dynamics in Parkinson’s Disease OFF and ON Dopaminergic Medication. Brain 2017, 140, 2968–2981. [Google Scholar] [CrossRef]
- Moran, A.; Stein, E.; Tischler, H.; Belelovsky, K.; Bar-Gad, I. Dynamic Stereotypic Responses of Basal Ganglia Neurons to Subthalamic Nucleus High-Frequency Stimulation in the Parkinsonian Primate. Front. Syst. Neurosci. 2011, 5, 21. [Google Scholar] [CrossRef]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Hamada, I.; Kita, H.; Imanishi, M.; Akazawa, T.; Ikeuchi, Y.; Hasegawa, N. Excitatory Cortical Inputs to Pallidal Neurons via the Subthalamic Nucleus in the Monkey. J. Neurophysiol. 2000, 84, 289–300. [Google Scholar] [CrossRef]
- Flora, E.D.; Perera, C.L.; Cameron, A.L.; Maddern, G.J. Deep Brain Stimulation for Essential Tremor: A Systematic Review. Mov. Disord. 2010, 25, 1550–1559. [Google Scholar] [CrossRef]
- Groppa, S.; Herzog, J.; Falk, D.; Riedel, C.; Deuschl, G.; Volkmann, J. Physiological and Anatomical Decomposition of Subthalamic Neurostimulation Effects in Essential Tremor. Brain 2014, 137, 109–121. [Google Scholar] [CrossRef]
- Barbe, M.T.; Liebhart, L.; Runge, M.; Pauls, K.A.M.; Wojtecki, L.; Schnitzler, A.; Allert, N.; Fink, G.R.; Sturm, V.; Maarouf, M.; et al. Deep Brain Stimulation in the Nucleus Ventralis Intermedius in Patients with Essential Tremor: Habituation of Tremor Suppression. J. Neurol. 2011, 258, 434–439. [Google Scholar] [CrossRef]
- Favilla, C.G.; Ullman, D.; Wagle Shukla, A.; Foote, K.D.; Jacobson, C.E.; Okun, M.S. Worsening Essential Tremor Following Deep Brain Stimulation: Disease Progression versus Tolerance. Brain 2012, 135, 1455–1462. [Google Scholar] [CrossRef]
- Putzke, J.D.; Whaley, N.R.; Baba, Y.; Wszolek, Z.K.; Uitti, R.J. Essential Tremor: Predictors of Disease Progression in a Clinical Cohort. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1235–1237. [Google Scholar] [CrossRef]
- Paschen, S.; Forstenpointner, J.; Becktepe, J.; Heinzel, S.; Hellriegel, H.; Witt, K.; Helmers, A.-K.; Deuschl, G. Long-Term Efficacy of Deep Brain Stimulation for Essential Tremor: An Observer-Blinded Study. Neurology 2019, 92, e1378–e1386. [Google Scholar] [CrossRef]
- Patel, N.; Ondo, W.; Jimenez-Shahed, J. Habituation and Rebound to Thalamic Deep Brain Stimulation in Long-Term Management of Tremor Associated with Demyelinating Neuropathy. Int. J. Neurosci. 2014, 124, 919–925. [Google Scholar] [CrossRef]
- Witt, K.; Kopper, F.; Deuschl, G.; Krack, P. Subthalamic Nucleus Influences Spatial Orientation in Extra-Personal Space. Mov. Disord. 2006, 21, 354–361. [Google Scholar] [CrossRef]
- Schmalbach, B.; Günther, V.; Raethjen, J.; Wailke, S.; Falk, D.; Deuschl, G.; Witt, K. The Subthalamic Nucleus Influences Visuospatial Attention in Humans. J. Cogn. Neurosci. 2014, 26, 543–550. [Google Scholar] [CrossRef]
- Benabid, A.L.; Koudsié, A.; Benazzouz, A.; Fraix, V.; Ashraf, A.; Le Bas, J.F.; Chabardes, S.; Pollak, P. Subthalamic Stimulation for Parkinson’s Disease. Arch. Med. Res. 2000, 31, 282–289. [Google Scholar] [CrossRef]
- Kumar, R.; Lozano, A.M.; Kim, Y.J.; Hutchison, W.D.; Sime, E.; Halket, E.; Lang, A.E. Double-Blind Evaluation of Subthalamic Nucleus Deep Brain Stimulation in Advanced Parkinson’s Disease. Neurology 1998, 51, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Sestini, S.; Scotto di Luzio, A.; Ammannati, F.; De Cristofaro, M.T.R.; Passeri, A.; Martini, S.; Pupi, A. Changes in Regional Cerebral Blood Flow Caused by Deep-Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease. J. Nucl. Med. 2002, 43, 725–732. [Google Scholar] [PubMed]
- Østergaard, K.; Sunde, N.A. Evolution of Parkinson’s Disease during 4 Years of Bilateral Deep Brain Stimulation of the Subthalamic Nucleus. Mov. Disord. 2006, 21, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Gorospe, A.; Guridi, J.; Ramos, E.; Linazasoro, G.; Rodriguez-Palmero, M.; Obeso, J.A. Bilateral Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease. Neurology 2000, 55, S45–S51. [Google Scholar]
- Simuni, T.; Jaggi, J.L.; Mulholland, H.; Hurtig, H.I.; Colcher, A.; Siderowf, A.D.; Ravina, B.; Skolnick, B.E.; Goldstein, R.; Stern, M.B.; et al. Bilateral Stimulation of the Subthalamic Nucleus in Patients with Parkinson Disease: A Study of Efficacy and Safety. J. Neurosurg. 2002, 96, 666–672. [Google Scholar] [CrossRef]
- Sestini, S.; Ramat, S.; Formiconi, A.R.; Ammannati, F.; Sorbi, S.; Pupi, A. Brain Networks Underlying the Clinical Effects of Long-Term Subthalamic Stimulation for Parkinson’s Disease: A 4-Year Follow-up Study with RCBF SPECT. J. Nucl. Med. 2005, 46, 1444–1454. [Google Scholar]
- Sestini, S.; Pupi, A.; Ammannati, F.; Silvia, R.; Sorbi, S.; Castagnoli, A. Are There Adaptive Changes in the Human Brain of Patients with Parkinson’s Disease Treated with Long-Term Deep Brain Stimulation of the Subthalamic Nucleus? A 4-Year Follow-up Study with Regional Cerebral Blood Flow SPECT. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1646–1657. [Google Scholar] [CrossRef]
- Haberler, C.; Alesch, F.; Mazal, P.R.; Pilz, P.; Jellinger, K.; Pinter, M.M.; Hainfellner, J.A.; Budka, H. No Tissue Damage by Chronic Deep Brain Stimulation in Parkinson’s Disease. Ann. Neurol. 2000, 48, 372–376. [Google Scholar] [CrossRef]
- Temperli, P.; Ghika, J.; Villemure, J.-G.; Burkhard, P.R.; Bogousslavsky, J.; Vingerhoets, F.J.G. How Do Parkinsonian Signs Return after Discontinuation of Subthalamic DBS? Neurology 2003, 60, 78–81. [Google Scholar] [CrossRef]
- Lokkegaard, A.; Werdelin, L.M.; Regeur, L.; Karlsborg, M.; Jensen, S.R.; Brødsgaard, E.; Madsen, F.F.; Lonsdale, M.N.; Friberg, L. Dopamine Transporter Imaging and the Effects of Deep Brain Stimulation in Patients with Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 508–516. [Google Scholar] [CrossRef]
- Álvarez-Bueno, C.; Deeks, J.J.; Cavero-Redondo, I.; Jolly, K.; Torres-Costoso, A.I.; Price, M.; Fernandez-Rodriguez, R.; Martínez-Vizcaíno, V. Effect of Exercise on Motor Symptoms in Patients with Parkinson’s Disease: A Network Meta-Analysis. J. Geriatr. Phys. Ther. 2021. [Google Scholar] [CrossRef]
- Trager, M.H.; Koop, M.M.; Velisar, A.; Blumenfeld, Z.; Nikolau, J.S.; Quinn, E.J.; Martin, T.; Bronte-Stewart, H. Subthalamic Beta Oscillations Are Attenuated after Withdrawal of Chronic High Frequency Neurostimulation in Parkinson’s Disease. Neurobiol. Dis. 2016, 96, 22–30. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, C.; Tian, Y.; Orlov, N.; Zhang, J.; Guo, Y.; Xu, S.; Jiang, C.; Hao, H.; Neumann, W.-J.; et al. Neuromodulation Effects of Deep Brain Stimulation on Beta Rhythm: A Longitudinal Local Field Potential Study. Brain Stimul. 2020, 13, 1784–1792. [Google Scholar] [CrossRef]
- Turco, C.V.; El-Sayes, J.; Savoie, M.J.; Fassett, H.J.; Locke, M.B.; Nelson, A.J. Short- and Long-Latency Afferent Inhibition; Uses, Mechanisms and Influencing Factors. Brain Stimul. 2018, 11, 59–74. [Google Scholar] [CrossRef]
- Sailer, A.; Molnar, G.F.; Paradiso, G.; Gunraj, C.A.; Lang, A.E.; Chen, R. Short and Long Latency Afferent Inhibition in Parkinson’s Disease. Brain 2003, 126, 1883–1894. [Google Scholar] [CrossRef]
- Shukla, A.W.; Moro, E.; Gunraj, C.; Lozano, A.; Hodaie, M.; Lang, A.; Chen, R. Long-Term Subthalamic Nucleus Stimulation Improves Sensorimotor Integration and Proprioception. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1020–1028. [Google Scholar] [CrossRef]
- Sankar, T.; Li, S.X.; Obuchi, T.; Fasano, A.; Cohn, M.; Hodaie, M.; Chakravarty, M.M.; Lozano, A.M. Structural Brain Changes Following Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Mov. Disord. 2016, 31, 1423–1425. [Google Scholar] [CrossRef]
- Kern, D.S.; Uy, D.; Rhoades, R.; Ojemann, S.; Abosch, A.; Thompson, J.A. Discrete Changes in Brain Volume after Deep Brain Stimulation in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 928–937. [Google Scholar] [CrossRef]
- Ge, J.; Wang, M.; Lin, W.; Wu, P.; Guan, Y.; Zhang, H.; Huang, Z.; Yang, L.; Zuo, C.; Jiang, J.; et al. Metabolic Network as an Objective Biomarker in Monitoring Deep Brain Stimulation for Parkinson’s Disease: A Longitudinal Study. EJNMMI Res. 2020, 10, 131. [Google Scholar] [CrossRef]
- Spieles-Engemann, A.L.; Steece-Collier, K.; Behbehani, M.M.; Collier, T.J.; Wohlgenant, S.L.; Kemp, C.J.; Cole-Strauss, A.; Levine, N.D.; Gombash, S.E.; Thompson, V.B.; et al. Subthalamic Nucleus Stimulation Increases Brain Derived Neurotrophic Factor in the Nigrostriatal System and Primary Motor Cortex. J. Parkinsons Dis. 2011, 1, 123–136. [Google Scholar] [CrossRef]
- Sortwell, C.E.; Hacker, M.L.; Fischer, D.L.; Konrad, P.E.; Davis, T.L.; Neimat, J.S.; Wang, L.; Song, Y.; Mattingly, Z.R.; Cole-Strauss, A.; et al. BDNF Rs6265 Genotype Influences Outcomes of Pharmacotherapy and Subthalamic Nucleus Deep Brain Stimulation in Early-Stage Parkinson’s Disease. Neuromodulation 2021, in press. [Google Scholar] [CrossRef]
- Vidailhet, M.; Jutras, M.-F.; Roze, E.; Grabli, D. Deep Brain Stimulation for Dystonia. Handb. Clin. Neurol. 2013, 116, 167–187. [Google Scholar] [CrossRef]
- Volkmann, J.; Mueller, J.; Deuschl, G.; Kühn, A.A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal Neurostimulation in Patients with Medication-Refractory Cervical Dystonia: A Randomised, Sham-Controlled Trial. Lancet Neurol. 2014, 13, 875–884. [Google Scholar] [CrossRef]
- Grips, E.; Blahak, C.; Capelle, H.H.; Bäzner, H.; Weigel, R.; Sedlaczek, O.; Krauss, J.K.; Wöhrle, J.C. Patterns of Reoccurrence of Segmental Dystonia after Discontinuation of Deep Brain Stimulation. J. Neurol. Neurosurg. Psychiatry 2007, 78, 318–320. [Google Scholar] [CrossRef]
- Cheung, T.; Zhang, C.; Rudolph, J.; Alterman, R.L.; Tagliati, M. Sustained Relief of Generalized Dystonia despite Prolonged Interruption of Deep Brain Stimulation. Mov. Disord. 2013, 28, 1431–1434. [Google Scholar] [CrossRef]
- Goto, S.; Yamada, K. Long Term Continuous Bilateral Pallidal Stimulation Produces Stimulation Independent Relief of Cervical Dystonia. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1506–1507. [Google Scholar] [CrossRef][Green Version]
- Hebb, M.O.; Chiasson, P.; Lang, A.E.; Brownstone, R.M.; Mendez, I. Sustained Relief of Dystonia Following Cessation of Deep Brain Stimulation. Mov. Disord. 2007, 22, 1958–1962. [Google Scholar] [CrossRef]
- Udupa, K.; Chen, R. Motor Cortical Plasticity in Parkinson’s Disease. Front. Neurol. 2013, 4, 128. [Google Scholar] [CrossRef]
- Quartarone, A.; Hallett, M. Emerging Concepts in the Physiological Basis of Dystonia. Mov. Disord. 2013, 28, 958–967. [Google Scholar] [CrossRef]
- Ruge, D.; Tisch, S.; Hariz, M.I.; Zrinzo, L.; Bhatia, K.P.; Quinn, N.P.; Jahanshahi, M.; Limousin, P.; Rothwell, J.C. Deep Brain Stimulation Effects in Dystonia: Time Course of Electrophysiological Changes in Early Treatment. Mov. Disord. 2011, 26, 1913–1921. [Google Scholar] [CrossRef]
- Kroneberg, D.; Plettig, P.; Schneider, G.-H.; Kühn, A.A. Motor Cortical Plasticity Relates to Symptom Severity and Clinical Benefit From Deep Brain Stimulation in Cervical Dystonia. Neuromodulation Technol. Neural Interface 2018, 21, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kim, S.J.; Phielipp, N.; Ghosh, S.; Udupa, K.; Gunraj, C.A.; Saha, U.; Hodaie, M.; Kalia, S.K.; Lozano, A.M.; et al. Pallidal Deep Brain Stimulation Modulates Cortical Excitability and Plasticity. Ann. Neurol. 2018, 83, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (TDCS): Clinical Applications and Safety Concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, O.; Leicht, G.; Herrmann, C.S.; Mulert, C. Transcranial Alternating Current Stimulation (TACS): From Basic Mechanisms towards First Applications in Psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 135–156. [Google Scholar] [CrossRef]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating Electric Field Modeling and Neuroimaging to Explain Inter-Individual Variability of TACS Effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G.R. Effects of Uniform Extracellular DC Electric Fields on Excitability in Rat Hippocampal Slices in Vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef]
- Creutzfeldt, O.D.; Fromm, G.H.; Kapp, H. Influence of Transcortical D-c Currents on Cortical Neuronal Activity. Exp. Neurol. 1962, 5, 436–452. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Rahman, A.; Reato, D.; Arlotti, M.; Gasca, F.; Datta, A.; Parra, L.C.; Bikson, M. Cellular Effects of Acute Direct Current Stimulation: Somatic and Synaptic Terminal Effects. J. Physiol. 2013, 591, 2563–2578. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated Sessions of Noninvasive Brain DC Stimulation Is Associated with Motor Function Improvement in Stroke Patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Miller, J.; Berger, B.; Sauseng, P. Anodal Transcranial Direct Current Stimulation (TDCS) Increases Frontal-Midline Theta Activity in the Human EEG: A Preliminary Investigation of Non-Invasive Stimulation. Neurosci. Lett. 2015, 588, 114–119. [Google Scholar] [CrossRef]
- Zaehle, T.; Sandmann, P.; Thorne, J.D.; Jäncke, L.; Herrmann, C.S. Transcranial Direct Current Stimulation of the Prefrontal Cortex Modulates Working Memory Performance: Combined Behavioural and Electrophysiological Evidence. BMC Neurosci. 2011, 12, 2. [Google Scholar] [CrossRef]
- Ardolino, G.; Bossi, B.; Barbieri, S.; Priori, A. Non-Synaptic Mechanisms Underlie the after-Effects of Cathodal Transcutaneous Direct Current Stimulation of the Human Brain. J. Physiol. 2005, 568, 653–663. [Google Scholar] [CrossRef]
- Matsumoto, J.; Fujiwara, T.; Takahashi, O.; Liu, M.; Kimura, A.; Ushiba, J. Modulation of Mu Rhythm Desynchronization during Motor Imagery by Transcranial Direct Current Stimulation. J. Neuroeng. Rehabil. 2010, 7, 27. [Google Scholar] [CrossRef]
- Spitoni, G.F.; Cimmino, R.L.; Bozzacchi, C.; Pizzamiglio, L.; Di Russo, F. Modulation of Spontaneous Alpha Brain Rhythms Using Low-Intensity Transcranial Direct-Current Stimulation. Front. Hum. Neurosci. 2013, 7, 529. [Google Scholar] [CrossRef]
- Mangia, A.L.; Pirini, M.; Cappello, A. Transcranial Direct Current Stimulation and Power Spectral Parameters: A TDCS/EEG Co-Registration Study. Front. Hum. Neurosci. 2014, 8, 601. [Google Scholar] [CrossRef]
- Reed, T.; Cohen Kadosh, R. Transcranial Electrical Stimulation (TES) Mechanisms and Its Effects on Cortical Excitability and Connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during FMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. TDCS for the Treatment of Depression: A Comprehensive Review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Podda, M.V.; Cocco, S.; Mastrodonato, A.; Fusco, S.; Leone, L.; Barbati, S.A.; Colussi, C.; Ripoli, C.; Grassi, C. Anodal Transcranial Direct Current Stimulation Boosts Synaptic Plasticity and Memory in Mice via Epigenetic Regulation of Bdnf Expression. Sci. Rep. 2016, 6, 22180. [Google Scholar] [CrossRef]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of Synaptic Responses to High-Frequency Stimulation and LTP by Neurotrophins in the Hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef]
- Woo, N.H.; Teng, H.K.; Siao, C.-J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of P75NTR by ProBDNF Facilitates Hippocampal Long-Term Depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Chaieb, L.; Moliadze, V.; Monte-Silva, K.; Poreisz, C.; Thirugnanasambandam, N.; Nitsche, M.A.; Shoukier, M.; Ludwig, H.; Paulus, W. Brain-Derived Neurotrophic Factor (BDNF) Gene Polymorphisms Shape Cortical Plasticity in Humans. Brain Stimul. 2010, 3, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Riddle, J.; McPherson, T.; Atkins, A.K.; Walker, C.P.; Ahn, S.; Frohlich, F. Brain-Derived Neurotrophic Factor (BDNF) Polymorphism May Influence the Efficacy of TACS to Modulate Neural Oscillations. Brain Stimul. 2020, 13, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Jaussi, W.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Consolidation of Human Motor Cortical Neuroplasticity by D-Cycloserine. Neuropsychopharmacology 2004, 29, 1573–1578. [Google Scholar] [CrossRef]
- Olma, M.C.; Dargie, R.A.; Behrens, J.R.; Kraft, A.; Irlbacher, K.; Fahle, M.; Brandt, S.A. Long-Term Effects of Serial Anodal TDCS on Motion Perception in Subjects with Occipital Stroke Measured in the Unaffected Visual Hemifield. Front. Hum. Neurosci. 2013, 7, 314. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial Alternating Current Stimulation: A Review of the Underlying Mechanisms and Modulation of Cognitive Processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Matuschek, J.; Fellner, M.-C. Entrainment of Prefrontal Beta Oscillations Induces an Endogenous Echo and Impairs Memory Formation. Curr. Biol. 2014, 24, 904–909. [Google Scholar] [CrossRef]
- Marshall, L.; Helgadóttir, H.; Mölle, M.; Born, J. Boosting Slow Oscillations during Sleep Potentiates Memory. Nature 2006, 444, 610–613. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- Neuling, T.; Rach, S.; Herrmann, C.S. Orchestrating Neuronal Networks: Sustained after-Effects of Transcranial Alternating Current Stimulation Depend upon Brain States. Front. Hum. Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef]
- Kasten, F.H.; Dowsett, J.; Herrmann, C.S. Sustained Aftereffect of α-TACS Lasts Up to 70 min after Stimulation. Front. Hum. Neurosci. 2016, 10, 245. [Google Scholar] [CrossRef]
- Kasten, F.H.; Herrmann, C.S. Transcranial Alternating Current Stimulation (TACS) Enhances Mental Rotation Performance during and after Stimulation. Front. Hum. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef]
- Harada, T.; Hara, M.; Matsushita, K.; Kawakami, K.; Kawakami, K.; Anan, M.; Sugata, H. Off-Line Effects of Alpha-Frequency Transcranial Alternating Current Stimulation on a Visuomotor Learning Task. Brain Behav. 2020, 10, e01754. [Google Scholar] [CrossRef]
- Nakazono, H.; Ogata, K.; Takeda, A.; Yamada, E.; Kimura, T.; Tobimatsu, S. Transcranial Alternating Current Stimulation of α but Not β Frequency Sharpens Multiple Visual Functions. Brain Stimul. 2020, 13, 343–352. [Google Scholar] [CrossRef]
- Moliadze, V.; Sierau, L.; Lyzhko, E.; Stenner, T.; Werchowski, M.; Siniatchkin, M.; Hartwigsen, G. After-Effects of 10 Hz TACS over the Prefrontal Cortex on Phonological Word Decisions. Brain Stimul. 2019, 12, 1464–1474. [Google Scholar] [CrossRef]
- Berger, A.; Pixa, N.H.; Steinberg, F.; Doppelmayr, M. Brain Oscillatory and Hemodynamic Activity in a Bimanual Coordination Task Following Transcranial Alternating Current Stimulation (TACS): A Combined EEG-FNIRS Study. Front. Behav. Neurosci. 2018, 12, 67. [Google Scholar] [CrossRef]
- Vossen, A.; Gross, J.; Thut, G. Alpha Power Increase after Transcranial Alternating Current Stimulation at Alpha Frequency (α-TACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimul. 2015, 8, 499–508. [Google Scholar] [CrossRef]
- Strüber, D.; Rach, S.; Neuling, T.; Herrmann, C.S. On the Possible Role of Stimulation Duration for After-Effects of Transcranial Alternating Current Stimulation. Front. Cell. Neurosci. 2015, 9, 311. [Google Scholar] [CrossRef]
- Zanto, T.P.; Jones, K.T.; Ostrand, A.E.; Hsu, W.-Y.; Campusano, R.; Gazzaley, A. Individual Differences in Neuroanatomy and Neurophysiology Predict Effects of Transcranial Alternating Current Stimulation. Brain Stimul. 2021, 14, 1317–1329. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knepper, H.; Nolte, G.; Strüber, D.; Rach, S.; Herrmann, C.S.; Schneider, T.R.; Engel, A.K. Selective Modulation of Interhemispheric Functional Connectivity by HD-TACS Shapes Perception. PLoS Biol. 2014, 12, e1002031. [Google Scholar] [CrossRef]
- Strüber, D.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Antiphasic 40 Hz Oscillatory Current Stimulation Affects Bistable Motion Perception. Brain Topogr. 2014, 27, 158–171. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Schneider, T.R.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of Brain Oscillations by Transcranial Alternating Current Stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to Gamma TACS in Alzheimer’s Disease: A Randomized, Double-Blind, Sham-Controlled, Crossover, Pilot Study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef]
- Mellin, J.M.; Alagapan, S.; Lustenberger, C.; Lugo, C.E.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Randomized Trial of Transcranial Alternating Current Stimulation for Treatment of Auditory Hallucinations in Schizophrenia. Eur. Psychiatry 2018, 51, 25–33. [Google Scholar] [CrossRef]
- Ahn, S.; Mellin, J.M.; Alagapan, S.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Targeting Reduced Neural Oscillations in Patients with Schizophrenia by Transcranial Alternating Current Stimulation. Neuroimage 2019, 186, 126–136. [Google Scholar] [CrossRef]
- Strüber, D.; Herrmann, C.S. Modulation of Gamma Oscillations as a Possible Therapeutic Tool for Neuropsychiatric Diseases: A Review and Perspective. Int. J. Psychophysiol. 2020, 152, 15–25. [Google Scholar] [CrossRef]
- Wischnewski, M.; Engelhardt, M.; Salehinejad, M.A.; Schutter, D.J.L.G.; Kuo, M.-F.; Nitsche, M.A. NMDA Receptor-Mediated Motor Cortex Plasticity after 20 Hz Transcranial Alternating Current Stimulation. Cereb. Cortex 2019, 29, 2924–2931. [Google Scholar] [CrossRef]
- Antal, A.; Herrmann, C.S. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast. 2016, 2016, 3616807. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Chaieb, L.; Paulus, W.; Antal, A. Evaluating Aftereffects of Short-Duration Transcranial Random Noise Stimulation on Cortical Excitability. Neural Plast. 2011, 2011, 105927. [Google Scholar] [CrossRef]
- Chaieb, L.; Antal, A.; Paulus, W. Transcranial Random Noise Stimulation-Induced Plasticity Is NMDA-Receptor Independent but Sodium-Channel Blocker and Benzodiazepines Sensitive. Front. Neurosci. 2015, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Ghin, F.; O’Hare, L.; Pavan, A. Electrophysiological Aftereffects of High-Frequency Transcranial Random Noise Stimulation (Hf-TRNS): An EEG Investigation. Exp. Brain Res. 2021, 239, 2399–2418. [Google Scholar] [CrossRef] [PubMed]
- Pilar-Cuellar, F.; Vidal, R.; Díaz, A.; Castro, E.; Anjos, S.; Vargas, V.; Romero, B.; Valdizan, E. Signaling Pathways Involved in Antidepressant-Induced Cell Proliferation and Synaptic Plasticity. Curr. Pharm. Des. 2014, 20, 3776–3794. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.-J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041. [Google Scholar] [CrossRef]
- Von Conta, J.; Kasten, F.H.; Ćurčić-Blake, B.; Aleman, A.; Thielscher, A.; Herrmann, C.S. Interindividual Variability of Electric Fields during Transcranial Temporal Interference Stimulation (TTIS). Sci. Rep. 2021, 11, 20357. [Google Scholar] [CrossRef]
- Negahbani, E.; Kasten, F.H.; Herrmann, C.S.; Fröhlich, F. Targeting Alpha-Band Oscillations in a Cortical Model with Amplitude-Modulated High-Frequency Transcranial Electric Stimulation. Neuroimage 2018, 173, 3–12. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Kronberg, G.; Reato, D.; Parra, L.C.; Bikson, M. Temporal Interference Stimulation Targets Deep Brain Regions by Modulating Neural Oscillations. Brain Stimul. 2021, 14, 55–65. [Google Scholar] [CrossRef]
- Ma, R.; Xia, X.; Zhang, W.; Lu, Z.; Wu, Q.; Cui, J.; Song, H.; Fan, C.; Chen, X.; Zha, R.; et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor Cortex Improves Motor Functions. Front. Neurosci. 2021, 15, 800436. [Google Scholar] [CrossRef]
- Reuter, S.; Deuschl, G.; Falk, D.; Mehdorn, M.; Witt, K. Uncoupling of Dopaminergic and Subthalamic Stimulation: Life-Threatening DBS Withdrawal Syndrome. Mov. Disord. 2015, 30, 1407–1413. [Google Scholar] [CrossRef]
- Reuter, S.; Deuschl, G.; Berg, D.; Helmers, A.; Falk, D.; Witt, K. Life-Threatening DBS Withdrawal Syndrome in Parkinson’s Disease Can Be Treated with Early Reimplantation. Parkinsonism Relat. Disord. 2018, 56, 88–92. [Google Scholar] [CrossRef]
- Orendáčová, M.; Kvašňák, E. Effects of Transcranial Alternating Current Stimulation and Neurofeedback on Alpha (EEG) Dynamics: A Review. Front. Hum. Neurosci. 2021, 15, 628229. [Google Scholar] [CrossRef]
- Nagaoka, T.; Katayama, Y.; Kano, T.; Kobayashi, K.; Oshima, H.; Fukaya, C.; Yamamoto, T. Changes in Glucose Metabolism in Cerebral Cortex and Cerebellum Correlate with Tremor and Rigidity Control by Subthalamic Nucleus Stimulation in Parkinson’s Disease: A Positron Emission Tomography Study. Neuromodulation 2007, 10, 206–215. [Google Scholar] [CrossRef]
- Chen, H.-M.; Sha, Z.-Q.; Ma, H.-Z.; He, Y.; Feng, T. Effective Network of Deep Brain Stimulation of Subthalamic Nucleus with Bimodal Positron Emission Tomography/Functional Magnetic Resonance Imaging in Parkinson’s Disease. CNS Neurosci. Ther. 2018, 24, 135–143. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Ponto, L.L.B.; Kamholz, J.; Rudroff, T. Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities. Brain Sci. 2020, 10, 855. [Google Scholar] [CrossRef]
- Dhaynaut, M.; Sprugnoli, G.; Cappon, D.; Macone, J.; Sanchez, J.S.; Normandin, M.D.; Guehl, N.J.; Koch, G.; Paciorek, R.; Connor, A.; et al. Impact of 40Hz Transcranial Alternating Current Stimulation on Cerebral Tau Burden in Patients with Alzheimer’s Disease: A Case Series. J. Alzheimers Dis. 2022, 85, 1667–1676. [Google Scholar] [CrossRef]
- Sidtis, J.J.; Sidtis, D.V.L.; Dhawan, V.; Tagliati, M.; Eidelberg, D. Stimulation of the Subthalamic Nucleus Changes Cortical-Subcortical Blood Flow Patterns during Speech: A Positron Emission Tomography Study. Front. Neurol. 2021, 12, 684596. [Google Scholar] [CrossRef]
- Tremblay, S.; Tuominen, L.; Zayed, V.; Pascual-Leone, A.; Joutsa, J. The Study of Noninvasive Brain Stimulation Using Molecular Brain Imaging: A Systematic Review. Neuroimage 2020, 219, 117023. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakajima, W.; Hatano, M.; Shibata, Y.; Kuroki, Y.; Arisawa, T.; Serizawa, A.; Sano, A.; Kogami, S.; Yamanoue, T.; et al. Visualization of AMPA Receptors in Living Human Brain with Positron Emission Tomography. Nat. Med. 2020, 26, 281–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).