Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Drug Selection
2.3. Participant Recruitment
2.4. Health Screening
2.5. Prior to Experimental Visits
2.6. Experimental Visit Protocol
2.6.1. Drug Administration
2.6.2. Eye Blink Rate
2.6.3. Pain Stimuli
2.6.4. Psychophysics
2.6.5. Main Experiment
2.6.6. EEG Recording
2.7. Analysis Methods
2.7.1. Statistical Analysis of Behavioural Data
2.7.2. EEG Analysis Method
2.7.3. Blink Rate
2.7.4. EEGLAB Pre-Processing
2.7.5. Source Localisation Analysis Parameters
2.7.6. EEG Analysis Statistical Analysis
3. Results
3.1. Behavioural Results
3.2. EEG Results
3.2.1. Blink Rate Analysis
3.2.2. Scalp-Level EEG Analysis
3.2.3. Source EEG Analysis
Anticipatory Time Window of Interest
Post-Laser Time Window of Interest
4. Discussion
4.1. Antagonist Effect on Pain Rating
4.2. D2R Modulation Reduced Neural Activity during Anticipation
4.3. Antagonist Reduced Neural Activity during Receipt of Pain
4.4. No Change in Pain Sensitivity or Unpleasantness
4.5. Clinical Impact
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Jääskeläinen, S.K.; Rinne, J.O.; Forssell, H.; Tenovuo, O.; Kaasinen, V.; Sonninen, P.; Bergman, J. Role of the dopaminergic system in chronic pain—A fluorodopa-PET study. Pain 2001, 90, 257–260. [Google Scholar] [CrossRef]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef]
- Wood, P.B.; Patterson, J.C., II; Sunderland, J.J.; Tainter, K.H.; Glabus, M.F.; Lilien, D.L. Reduced Presynaptic Dopamine Activity in Fibromyalgia Syndrome Demonstrated with Positron Emission Tomography: A Pilot Study. J. Pain 2007, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W. Fibromyalgia syndrome: Basic knowledge, diagnosis and treatment. Med. Mon. Pharm. 2016, 39, 504–511. [Google Scholar]
- Albrecht, D.S.; Mackie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, N.; Forssell, H.; Aalto, S.; Rinne, J.O.; Scheinin, H.; Taiminen, T.; Någren, K.; Eskola, O.; Jääskeläinen, S.K. Altered dopamine D2 receptor binding in atypical facial pain. Pain 2003, 106, 43–48. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Brefel-Courbon, C. Chronic pain and pain processing in Parkinson’s disease. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2018, 87, 200–206. [Google Scholar] [CrossRef]
- Silverdale, M.A.; Kobylecki, C.; Kass-Iliyya, L.; Martinez-Martin, P.; Lawton, M.; Cotterill, S.; Chaudhuri, K.R.; Morris, H.; Baig, F.; Williams, N.; et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Park. Relat. Disord. 2018, 56, 27–32. [Google Scholar] [CrossRef]
- Taylor, A.M.W.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194–1198. [Google Scholar] [CrossRef]
- Yang, S.; Boudier-Revéret, M.; Choo, Y.J.; Chang, M.C. Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System. Brain Sci. 2020, 10, 701. [Google Scholar] [CrossRef]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef] [PubMed]
- van Holstein, M.; Aarts, E.; van der Schaaf, M.E.; Geurts, D.E.M.; Verkes, R.J.; Franke, B.; van Schouwenburg, M.R.; Cools, R. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology 2011, 218, 567–578. [Google Scholar] [CrossRef]
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, G. Investigating the Role of Striatal Dopamine in Attentional Inhibition; The University of Manchester: Manchester, UK, 2017. [Google Scholar]
- Arias-Carrión, Ó.; Pöppel, E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol. Exp. 2007, 67, 481–488. [Google Scholar]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Kapur, S.; Mizrahi, R.; Li, M. From dopamine to salience to psychosis—Linking biology, pharmacology and phenomenology of psychosis. Schizophr. Res. 2005, 79, 59–68. [Google Scholar] [CrossRef]
- Howes, O.D.; Nour, M. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry 2016, 15, 3–4. [Google Scholar] [CrossRef]
- Shiner, T.; Symmonds, M.; Guitart-Masip, M.; Fleming, S.M.; Friston, K.J.; Dolan, R.J. Dopamine, Salience, and Response Set Shifting in Prefrontal Cortex. Cereb. Cortex 2014, 25, 3629–3639. [Google Scholar] [CrossRef]
- Parr, T.; Friston, K.J. Working memory, attention, and salience in active inference. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Pultorak, K.J.; Schelp, S.; Isaacs, D.P.; Krzystyniak, G.; Oleson, E.B. A Transient Dopamine Signal Represents Avoidance Value and Causally Influences the Demand to Avoid. Eneuro 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Elliot, A.J.; Covington, M.V. Approach and Avoidance Motivation. Educ. Psychol. Rev. 2001, 13, 73–92. [Google Scholar] [CrossRef]
- Salimpoor, V.N.; Benovoy, M.; Larcher, K.; Dagher, A.; Zatorre, R.J. Anatomically distinct dopamine release during an-ticipation and experience of peak emotion to music. Nat. Neurosci. 2011, 14, 257–262. [Google Scholar] [CrossRef]
- Baixauli, E. Happiness: Role of Dopamine and Serotonin on Mood and Negative Emotions. Emerg. Med. Open Access 2017, 7, 1–3. [Google Scholar] [CrossRef]
- Peciña, M.; Mickey, B.J.; Love, T.; Wang, H.; Langenecker, S.; Hodgkinson, C.; Shen, P.-H.; Villafuerte, S.; Hsu, D.; Weisenbach, S.L.; et al. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex 2013, 49, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Schwartenbeck, P.; Fitz, G.T.; Moutoussis, M.; Behrens, T.; Dolan, R. The anatomy of choice: Dopamine and decision-making. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130481. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.H.B.; Dolan, R.J.; Friston, K. Dopamine, reward learning, and active inference. Front. Comput. Neurosci. 2015, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Seymour, B.; El-Deredy, W.; Jones, A.K. Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain 2008, 139, 324–332. [Google Scholar] [CrossRef]
- Tiemann, L.; Heitmann, H.; Schulz, E.; Baumkötter, J.; Ploner, M. Dopamine Precursor Depletion Influences Pain Affect Rather than Pain Sensation. PLoS ONE 2014, 9, e96167. [Google Scholar] [CrossRef]
- Martikainen, I.K.; Hagelberg, N.; Jääskeläinen, S.K.; Hietala, J.; Pertovaara, A. Dopaminergic and serotonergic mechanisms in the modulation of pain: In vivo studies in human brain. Eur. J. Pharmacol. 2018, 834, 337–345. [Google Scholar] [CrossRef]
- Urien, L.; Wang, J. Top-Down Cortical Control of Acute and Chronic Pain. Psychosom. Med. 2019, 81, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Bhagyashree, A.; Manikkoth, S.; Sequeira, M.; Nayak, R.; Rao, S.N. Central dopaminergic system plays a role in the analgesic action of paracetamol: Preclinical evidence. Indian J. Pharmacol. 2017, 49, 21–25. [Google Scholar] [PubMed]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Romandini, S.; Merlo-Pich, E.; Mennini, T.; Samanin, R. Evidence of the involvement of dopamine in the anal-gesic effect of nefopam. Eur. J. Pharmacol. 1986, 128, 157–164. [Google Scholar] [CrossRef]
- Cooper, J.C.; Knutson, B. Valence and salience contribute to nucleus accumbens activation. NeuroImage 2008, 39, 538–547. [Google Scholar] [CrossRef]
- Wulff, S.; Nielsen, M.Ø.; Rostrup, E.; Svarer, C.; Jensen, L.T.; Pinborg, L.; Glenthøj, B.Y. The relation between dopamine D2 receptor blockade and the brain reward system: A longitudinal study of first-episode schizophrenia patients. Psychol. Med. 2020, 50, 220–228. [Google Scholar] [CrossRef]
- Gentry, R.N.; Schuweiler, D.R.; Roesch, M.R. Dopamine signals related to appetitive and aversive events in paradigms that manipulate reward and avoidability. Brain Res. 2018, 1713, 80–90. [Google Scholar] [CrossRef]
- Roughley, S.; Killcross, S. Differential involvement of dopamine receptor subtypes in the acquisition of Pavlovian sign-tracking and goal-tracking responses. Psychopharmacology 2019, 236, 1853–1862. [Google Scholar] [CrossRef]
- Jensen, J.; Mc, I.A.; Crawley, A.P.; Mikulis, D.; Remington, G.; Kapur, S. Direct Activation of the Ventral Striatum in Anticipation of Aversive Stimuli. Neuron 2003, 40, 1251–1257. [Google Scholar] [CrossRef]
- Horvitz, J.C. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav. Brain Res. 2002, 137, 65–74. [Google Scholar] [CrossRef][Green Version]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.C.; Filippini, B.; Pazo, J.H. The Striatum and Pain Modulation. Cell. Mol. Neurobiol. 2011, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, N.; Martikainen, I.K.; Mansikka, H.; Hinkka, S.; Någren, K.; Hietala, J.; Scheinin, H.; Pertovaara, A. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 2002, 99, 273–279. [Google Scholar] [CrossRef]
- Cobacho, N.; de la Calle, J.L.; Paíno, C.L. Dopaminergic modulation of neuropathic pain: Analgesia in rats by a D2-type receptor agonist. Brain Res. Bull. 2014, 106, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, I.K.; Hagelberg, N.; Mansikka, H.; Hietala, J.; Någren, K.; Scheinin, H.; Pertovaara, A. Association of striatal dopamine D2/D3 receptor binding potential with pain but not tactile sensitivity or placebo analgesia. Neurosci. Lett. 2005, 376, 149–153. [Google Scholar] [CrossRef]
- Clark, J.A.; Brown, C.A.; Jones, A.K.P.; El-Deredy, W. Dissociating nociceptive modulation by the duration of pain antic-ipation from unpredictability in the timing of pain. Clin. Neurophysiol. 2008, 119, 2870–2878. [Google Scholar] [CrossRef]
- Taylor, A.G.; Goehler, L.E.; Galper, D.I.; Innes, K.E.; Bourguignon, C. Top-Down and Bottom-Up Mechanisms in Mind-Body Medicine: Development of an Integrative Framework for Psychophysiological Research. Explore 2010, 6, 29–41. [Google Scholar] [CrossRef]
- Forkmann, K.; Grashorn, W.; Schmidt, K.; Fründt, O.; Buhmann, C.; Bingel, U. Altered neural responses to heat pain in drug-naive patients with Parkinson disease. Pain 2017, 158, 1408–1416. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhang, H.-T.; Chang, J.-Y.; Woodward, D.J.; Baccalá, L.A.; Luo, F. Anticipation of Pain Enhances the Nociceptive Transmission and Functional Connectivity within Pain Network in Rats. Mol. Pain 2008, 4, 34. [Google Scholar] [CrossRef]
- Brown, C.A.; Seymour, B.; Boyle, Y.; El-Deredy, W.; Jones, A.K.P.P. Modulation of pain ratings by expectation and uncertainty: Behavioral characteristics and anticipatory neural correlates. Pain 2008, 135, 240–250. [Google Scholar] [CrossRef]
- Atlas, L.Y.; Bolger, N.; Lindquist, M.A.; Wager, T.D. Brain Mediators of Predictive Cue Effects on Perceived Pain. J. Neurosci. 2010, 30, 12964–12977. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; El-Deredy, W.; Jones, A.K.P. When the brain expects pain: Common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur. J. Neurosci. 2013, 39, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, O.V.; Zeidan, F.; Mc, H.J.G.; Kraft, R.A.; Coghill, R.C. From cue to meaning: Brain mechanisms supporting the construction of expectations of pain. Pain 2014, 155, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Atlas, L.Y.; Wager, T.D. How expectations shape pain. Neurosci. Lett. 2012, 520, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, S.W. Exploring the ‘Pain Neuromatrix’. Curr. Rev. Pain 2000, 4, 467–477. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Suhara, T.; Okubo, Y.; Yasuno, F.; Sudo, Y.; Inoue, M.; Ichimiya, T.; Nakashima, Y.; Nakayama, K.; Tanada, S.; Suzuki, K.; et al. Decreased Dopamine D2 Receptor Binding in the Anterior Cingulate Cortex in Schizophrenia. Arch. Gen. Psychiatry 2002, 59, 25–30. [Google Scholar] [CrossRef]
- Wang, S.; Hu, S.-H.; Shi, Y.; Li, B.-M. The roles of the anterior cingulate cortex and its dopamine receptors in self-paced cost–benefit decision making in rats. Anim. Learn. Behav. 2016, 45, 89–99. [Google Scholar] [CrossRef]
- Suhara, T.; Yasuno, F.; Sudo, Y.; Yamamoto, M.; Inoue, M.; Okubo, Y.; Suzuki, K. Dopamine D2 Receptors in the Insular Cortex and the Personality Trait of Novelty Seeking. NeuroImage 2001, 13, 891–895. [Google Scholar] [CrossRef]
- Govindaiah, G.; Wang, Y.; Cox, C. Dopamine enhances the excitability of somatosensory thalamocortical neurons. Neuroscience 2010, 170, 981–991. [Google Scholar] [CrossRef]
- Fariello, R.G.; Fariello, R. Pharmacodynamic and Pharmacokinetic Features of Cabergoline. Drugs 1998, 55, 10–16. [Google Scholar] [CrossRef] [PubMed]
- CABERGOLINE. Available online: https://drugs.ncats.io/drug/LL60K9J05T (accessed on 21 February 2022).
- AMISULPRIDE. Available online: https://drugs.ncats.io/drug/8110R61I4U (accessed on 21 February 2022).
- Möller, H.-J. Amisulpride: Limbic specificity and the mechanism of antipsychotic atypicality. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, H.; Claustre, Y.; Fage, D.; Rouquier, L.; Chergui, K.; Curet, O.; Oblin, A.; Gonon, F.; Carter, C.; Benavides, J.; et al. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J. Pharmacol. Exp. Ther. 1997, 280, 83–97. [Google Scholar]
- Abbas, A.I.; Hedlund, P.B.; Huang, X.-P.; Tran, T.B.; Meltzer, H.Y.; Roth, B.L. Amisulpride is a potent 5-HT7 antagonist: Relevance for antidepressant actions in vivo. Psychopharmacology 2009, 205, 119–128. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zhuang, D.-B.; Lunderberg, T.; Yu, L.-C. Involvement of 5-hydroxytryptamine1A receptors in the descending anti-nociceptive pathway from periaqueductal gray to the spinal dorsal horn in intact rats, rats with nerve injury and rats with inflammation. Neuroscience 2002, 112, 399–407. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernández, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Viguier, F.; Michot, B.; Hamon, M.; Bourgoin, S. Multiple roles of serotonin in pain control mechanisms—Implications of 5-HT7 and other 5-HT receptor types. Eur. J. Pharmacol. 2013, 716, 8–16. [Google Scholar] [CrossRef]
- Amaya-Castellanos, E.; Pineda-Farias, J.B.; Castañeda-Corral, G.; Vidal-Cantú, G.C.; Murbartián, J.; Rocha-González, H.I.; Granados-Soto, V. Blockade of 5-HT7 receptors reduces tactile allodynia in the rat. Pharmacol. Biochem. Behav. 2011, 99, 591–597. [Google Scholar] [CrossRef]
- Viguier, F.; Michot, B.; Kayser, V.; Bernard, J.-F.; Vela, J.-M.; Hamon, M.; Bourgoin, S. GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology 2012, 63, 1093–1106. [Google Scholar] [CrossRef]
- Maruya, H.; Watanabe, Y.; Okita, M.; Lawlor, G.F.; Utsumi, H.; Niitsuma, T. Inhibitory effects of d2 agonists by striatal injection on excessive release of dopamine and hyperactivity induced by bay k 8644 in rats. Neuroscience 2003, 118, 1091–1098. [Google Scholar] [CrossRef]
- Ford, C. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; O’Reilly, R.C. A mechanistic account of striatal dopamine function in human cognition: Psychopharmaco-logical studies with cabergoline and haloperidol. Behav. Neurosci. 2006, 120, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Nandam, L.S.; Hester, R.; Wagner, J.; Dean, A.J.; Messer, C.; Honeysett, A.; Nathan, P.J.; Bellgrove, M.A. Dopamine D2 Receptor Modulation of Human Response Inhibition and Error Awareness. J. Cogn. Neurosci. 2013, 25, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Park, T.-W.; Yang, J.-C.; Huang, G.-B.; Zhao, T.; Oh, K.-Y.; Kim, M.-G. Cognitive Effects of a Single Dose of Atypical Antipsychotics in Healthy Volunteers Compared with Placebo or Haloperidol. J. Clin. Psychopharmacol. 2012, 32, 778–786. [Google Scholar] [CrossRef]
- Dostinex—FDA Prescribing Information, Side Effects and Uses. Available online: https://www.drugs.com/pro/dostinex.html (accessed on 6 September 2019).
- Benfield, A.C.S. Amisulpride: A Review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy in the Management of Schizophrenia. Adis Int. 1994, 6, 3. [Google Scholar]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Bucker, B.; Theeuwes, J. The effect of reward on orienting and reorienting in exogenous cuing. Cogn. Affect. Behav. Neurosci. 2014, 14, 635–646. [Google Scholar] [CrossRef]
- Costa, A.; la Fougère, C.; Pogarell, O.; Möller, H.-J.; Riedel, M.; Ettinger, U. Impulsivity is related to striatal dopamine transporter availability in healthy males. Psychiatry Res. Neuroimaging 2013, 211, 251–256. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; Smith, C.E.; et al. Dopaminergic Network Differences in Human Impulsivity. Science 2010, 329, 532. [Google Scholar] [CrossRef]
- Dalley, J.W.; Robbins, T. Fractionating impulsivity: Neuropsychiatric implications. Nat. Rev. Neurosci. 2017, 18, 158–171. [Google Scholar] [CrossRef]
- Stanford, M.S.; Mathias, C.W.; Dougherty, D.M.; Lake, S.L.; Anderson, N.E.; Patton, J.H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers. Individ. Dif. 2009, 47, 385–395. [Google Scholar] [CrossRef]
- Karson, C.N. Spontaneous Eye-Blink Rates and Dopaminergic Systems. Brain 1983, 106, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D.; Lawrence, M.S.; Roth, R.H.; Taylor, J.R.; Mailman, R.; Nichols, D.E.; Lewis, M.H.; Redmond, D.E. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J. Pharmacol. Exp. Ther. 1991, 259, 595–600. [Google Scholar] [PubMed]
- Lewis, T.; Pochin, E.E. The double pain response of the human skin to a single stimulus. Clin. Sci. 1937, 3, 50. [Google Scholar]
- Bromm, B.; Treede, R.-D. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum. Neurobiol. 1984, 3, 33–40. [Google Scholar] [PubMed]
- Brown, C.A.; Jones, A.K. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin. Neurophysiol. 2008, 119, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Jones, A.K. Meditation experience predicts less negative appraisal of pain: Electrophysiological evidence for the involvement of anticipatory neural responses. Pain 2010, 150, 428–438. [Google Scholar] [CrossRef]
- Martin, S.; Jones, A.K.P.; Brown, C.A.; Kobylecki, C.; Silverdale, M.A.; Sarah, M.L.; Anthony, J.K.P.; Christopher, B.A.; Christopher, K.; Monty, S.A. A neurophysiological investigation of anticipation to pain in Parkinson’s disease. Eur. J. Neurosci. 2020, 51, 611–627. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Nielsen, F.Å. The Brede database: A small database for functional neuroimaging. NeuroImage 2003, 19, 19–22. [Google Scholar]
- Karson, C.N.; Burns, R.S.; Le Witt, P.A.; Foster, N.L.; Newman, R.P. Blink rates and disorders of movement. Neurology 1984, 34, 677. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D. Blink rate as clinical indicator. Neurology 1985, 35, 286. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Elsworth, J.; Lawrence, M.; Sladek, J.; Roth, R.; Redmond, D. Spontaneous Blink Rates Correlate with Dopamine Levels in the Caudate Nucleus of MPTP-Treated Monkeys. Exp. Neurol. 1999, 158, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kleven, M.S.; Koek, W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 1996, 279, 1211–1219. [Google Scholar] [PubMed]
- Cousineau, D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutor. Quant. Methods Psychol. 2005, 1, 42–45. [Google Scholar] [CrossRef]
- Sescousse, G.; Ligneul, R.; van Holst, R.J.; Janssen, L.K.; de Boer, F.; Janssen, M.; Berry, A.S.; Jagust, W.J.; Cools, R. Spontaneous eye blink rate and dopamine synthesis capacity: Preliminary evidence for an absence of positive correlation. Eur. J. Neurosci. 2018, 47, 1081–1086. [Google Scholar] [CrossRef]
- Dang, L.C.; Samanez-Larkin, G.R.; Castrellon, J.J.; Perkins, S.F.; Cowan, R.L.; Newhouse, P.A.; Zald, D.H. Spontaneous Eye Blink Rate (EBR) Is Uncorrelated with Dopamine D2 Receptor Availability and Unmodulated by Dopamine Agonism in Healthy Adults. Eneuro 2017, 4, 5. [Google Scholar] [CrossRef]
- Yoshida, W.; Seymour, B.; Koltzenburg, M.; Dolan, R.J. Uncertainty increases pain: Evidence for a novel mechanism of pain modulation involving the periaqueductal gray. J. Neurosci. 2013, 33, 5638–5646. [Google Scholar] [CrossRef]
- Andreou, C.; Bozikas, V.P.; Luedtke, T.; Moritz, S. Associations between visual perception accuracy and confidence in a dopaminergic manipulation study. Front. Psychol. 2015, 6, 414. [Google Scholar] [CrossRef]
- Schwartenbeck, P.; Fitz, G.T.; Mathys, C.; Dolan, R.; Friston, K. The Dopaminergic Midbrain Encodes the Expected Certainty about Desired Outcomes. Cereb. Cortex 2015, 25, 3434–3445. [Google Scholar] [CrossRef]
- Tomassini, A.; Ruge, D.; Galea, J.M.; Penny, W.; Bestmann, S. The Role of Dopamine in Temporal Uncertainty. J. Cogn. Neurosci. 2016, 28, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Altin, M.; Duenas, H.; Alev, L. The Role of Descending Inhibitory Pathways on Chronic Pain Modulation and Clinical Implications. Pain Pract. 2014, 14, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Power, A.; Boyle, Y.; Anderson, I.M.; Silverdale, M.A.; Jones, A.K.P. 5-HT modulation of pain perception in humans. Psychopharmacology 2017, 234, 2929–2939. [Google Scholar] [CrossRef]
- Berger, B.; Trottier, S.; Verney, C.; Gaspar, P.; Alvarez, C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: A radioautographic study. J. Comp. Neurol. 1988, 273, 99–119. [Google Scholar] [CrossRef]
- Bergson, C.; Mrzljak, L.; Smiley, J.F.; Pappy, M.; Levenson, R.; Goldman-Rakic, P.S. Regional, cellular, and subcellular vari-ations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. 1995, 15, 7821–7836. [Google Scholar] [CrossRef]
- Goldsmith, S.; Joyce, J. Dopamine D2 receptors are organized in bands in normal human temporal cortex. Neuroscience 1996, 74, 435–451. [Google Scholar] [CrossRef]
- Herath, P.; Kinomura, S.; Roland, P.E. Visual recognition: Evidence for two distinctive mechanisms from a PET study. Hum. Brain Mapp. 2001, 12, 110–119. [Google Scholar] [CrossRef]
- Ishai, A.; Ungerleider, L.G.; Martin, A.; Schouten, J.L.; Haxby, J.V. Distributed representation of objects in the human ventral visual pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 9379–9384. [Google Scholar] [CrossRef]
- Chao, L.L.; Haxby, J.V.; Martin, A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat. Neurosci. 1999, 2, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Tranel, D.; Damasio, H.; Damasio, A.R. A neural basis for the retrieval of conceptual knowledge. Neuropsychology 1997, 35, 1319–1327. [Google Scholar] [CrossRef]
- Kluger, D.S.; Schubotz, R.I. Strategic adaptation to non-reward prediction error qualities and irreducible uncertainty in f, MRI. Cortex 2017, 97, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L.; Fagan, E.; Price, C. Functional Subdivisions in the Left Angular Gyrus Where the Semantic System Meets and Diverges from the Default Network. J. Neurosci. 2010, 30, 16809–16817. [Google Scholar] [CrossRef]
- Kincade, J.M.; Abrams, R.A.; Astafiev, S.V.; Shulman, G.L.; Corbetta, M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J. Neurosci. 2005, 25, 4593–4604. [Google Scholar] [CrossRef]
- Rushworth, M.F.S.; Ellison, A.; Walsh, V. Complementary localization and lateralization of orienting and motor attention. Nat. Neurosci. 2001, 4, 656–661. [Google Scholar] [CrossRef]
- Gottlieb, J. From Thought to Action: The Parietal Cortex as a Bridge between Perception, Action, and Cognition. Neuron 2007, 53, 9–16. [Google Scholar] [CrossRef]
- Watson, A.; El-Deredy, W.; Iannetti, G.D.; Lloyd, D.; Tracey, I.; Vogt, B.A.; Nadeau, V.; Jones, A.K. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 2009, 145, 24–30. [Google Scholar] [CrossRef]
- Peyron, R.; García-Larrea, L.; Grégoire, M.-C.; Costes, N.; Convers, P.; Lavenne, F.; Mauguière, F.; Michel, D.; Laurent, B. Haemodynamic brain responses to acute pain in humans. Brain 1999, 122, 1765–1780. [Google Scholar] [CrossRef]
- Kulkarni, B.; Bentley, D.E.; Elliott, R.; Youell, P.; Watson, A.; Derbyshire, S.W.G.; Frackowiak, R.; Friston, K.; Jones, A. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur. J. Neurosci. 2005, 21, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Yin, Y.; Sun, S.; Deng, X. Involvement of 5-HT7 receptors in the pathogenesis of temporal lobe epilepsy. Eur. J. Pharmacol. 2012, 685, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; D’Esposito, M. Inverted-U–Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Vijay, R.S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F.T. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar]
- Levy, F. Dopamine vs Noradrenaline: Inverted-U Effects and ADHD Theories. Aust. N. Z. J. Psychiatry 2009, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B. Prefrontal dopamine and behavioral flexibility: Shifting from an “inverted-U” toward a family of functions. Front. Neurosci. 2013, 7, 62. [Google Scholar] [CrossRef]
- Beggs, J.M.; Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003, 23, 11167–11177. [Google Scholar] [CrossRef]
- Finke, K.; Dodds, C.M.; Bublak, P.; Regenthal, R.; Baumann, F.; Manly, T.; Müller, U. Effects of modafinil and methylphenidate on visual attention capacity: A TVA-based study. Psychopharmacology 2010, 210, 317–329. [Google Scholar] [CrossRef]
- Li, S.-C.; Lindenberger, U.; Bäckman, L. Dopaminergic modulation of cognition across the life span. Neurosci. Biobehav. Rev. 2010, 34, 625–630. [Google Scholar] [CrossRef]
- Goto, Y.; Otani, S.; Grace, A. The Yin and Yang of dopamine release: A new perspective. Neuropharmacology 2007, 53, 583–587. [Google Scholar] [CrossRef]
- Friston, K.J.; Shiner, T.; Fitz, G.T.; Galea, J.M.; Adams, R.; Brown, H.; Dolan, R.; Moran, R.; Stephan, K.E.; Bestmann, S. Dopamine, Affordance and Active Inference. PLoS Comput. Biol. 2012, 8, e1002327. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, S.W.; Jones, A.; Gyulai, F.; Clark, S.; Townsend, D.; Firestone, L.L. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 1997, 73, 431–445. [Google Scholar] [CrossRef]
- Mazzola, V.; Latorre, V.; Petito, A.; Gentili, N.; Fazio, L.; Popolizio, T.; Blasi, G.; Arciero, G.; Bondolfi, G. Affective response to a loved one’s pain: Insula activity as a function of individual differences. PLoS ONE 2010, 5, e15268. [Google Scholar] [CrossRef]
- Lu, C.; Yang, T.; Zhao, H.; Zhang, M.; Meng, F.; Fu, H.; Xie, Y.; Xu, H. Insular Cortex is Critical for the Perception, Modulation, and Chronification of Pain. Neurosci. Bull. 2016, 32, 191–201. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef]
- Valdés-Baizabal, C.; Carbajal, G.V.; Pérez-González, D.; Malmierca, M.S. Dopamine modulates subcortical responses to surprising sounds. PLoS Biol. 2020, 18, e3000744. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Čeko, M.; Louis-Foster, M.; Elfassy, N.M.; Leyton, M.; Shir, Y.; Schweinhardt, P. Dopamine and Pain Sensitivity: Neither Sulpiride nor Acute Phenylalanine and Tyrosine Depletion Have Effects on Thermal Pain Sensations in Healthy Volunteers. PLoS ONE 2013, 8, e80766. [Google Scholar] [CrossRef]



| Pain Rating (NRS Score/10) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Uncertain Low | Uncertain High | |||||
| Drug Condition | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Control | 2.97 | 0.88 | 6.30 | 0.71 | 3.28 | 1.07 | 5.86 | 0.62 |
| Agonist | 3.05 | 0.93 | 6.28 | 0.79 | 3.50 | 1.14 | 5.75 | 0.80 |
| Antagonist | 2.83 | 1.00 | 6.36 | 0.60 | 3.31 | 1.10 | 5.58 | 0.68 |
| Unpleasantness Rating (NRS Score/10) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Uncertain Low | Uncertain High | |||||
| Drug Condition | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Control | 2.39 | 1.30 | 6.49 | 0.91 | 2.56 | 1.44 | 7.08 | 1.30 |
| Agonist | 2.25 | 1.07 | 6.49 | 0.67 | 2.35 | 1.02 | 6.98 | 0.90 |
| Antagonist | 1.94 | 1.22 | 5.42 | 2.37 | 2.22 | 1.55 | 5.91 | 2.67 |
| Cluster-Level | Peak-Level | MNI Coordinates | Brain Region | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p(FWE) | K | F/T | Z | X | Y | Z | ||||
| Mid Anticipation | F-Contrast | |||||||||
| Drug | 0.004 | 909 | 11.62 | 4.20 | 32 | −44 | 34 | R | Inferior Parietal (48.0%) | |
| 0.013 | 700 | 9.79 | 3.79 | 34 | −54 | 14 | R | Mid Temporal (56.3%) | ||
| Expectation | 0.000 | 4098 | 25.90 | 4.85 | −44 | −48 | 6 | L L | Mid Temporal (35.3%) Mid Occipital (23.1%) | |
| 0.013 | 783 | 18.64 | 4.09 | −56 | 2 | −16 | L | Mid Temporal (69.1%) | ||
| 0.007 | 931 | 15.28 | 3.69 | −14 | −86 | 2 | L L | Calcarine (52.8%) Lingual (37.2%) | ||
| 0.005 | 390 | 15.65 | 3.73 | −30 | −18 | 8 | L | Insula (60.7%) | ||
| T-Contrast | ||||||||||
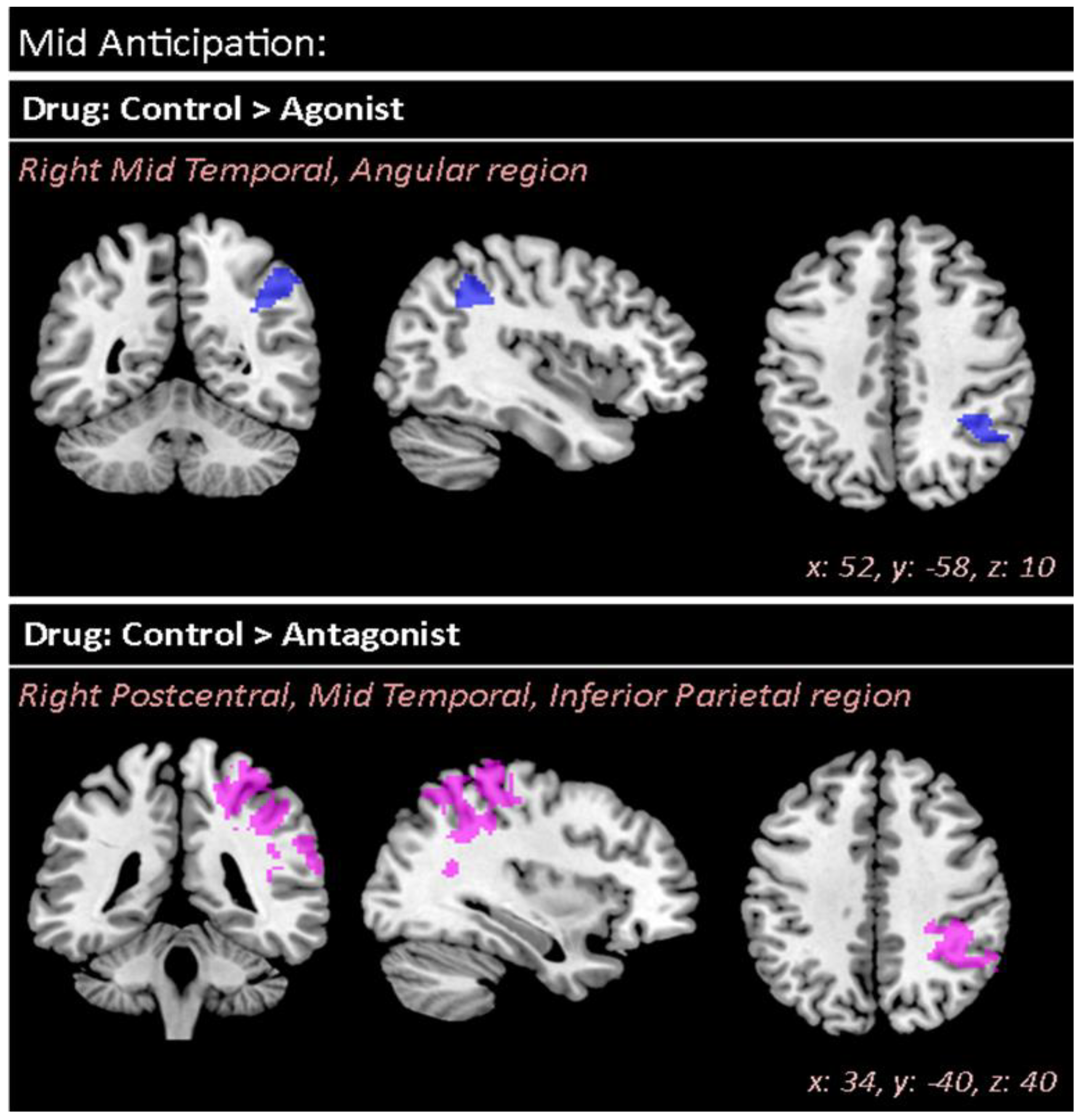
| Drug Control > Agonist | 0.031 | 693 | 3.92 | 3.87 | 52 | −58 | 10 | R R | Mid Temporal (49.9%) Angular gyrus (29.4%) | |
| Drug Control > Antag | 0.000 | 4184 | 4.60 | 4.52 | 34 | −40 | 40 | R R R | Postcentral (17.2%) Mid Temporal (16.9%) Inferior Parietal (15.0%) | |
| Expectation High > Low | 0.000 | 8555 | 5.09 | 4.98 | −44 | −48 | 6 | L L | Mid Temporal (30.4%) Mid Occipital (17.9%) | |
| 0.003◊ | 641 | 3.96 | 3.90 | −30 | −18 | 8 | L | Insula (58.8%) | ||
| Post-Stimulus | F-Contrast | |||||||||
| Drug | 0.022 | 164 | 10.82 | 4.02 | 28 | −26 | 14 | R | Insula (40.9%) | |
| T-Contrast | ||||||||||
| Drug Control > Antag | 0.033◊ | 131 | 3.73 | 3.68 | 28 | −26 | 14 | R | Insula (45.0%) | |
| Drug Agonist > Antag | 0.022 | 748 | 3.93 | 3.88 | 30 | −20 | −16 | R | Hippocampus (54.3%) | |
| 0.010 | 940 | 3.80 | 3.75 | 64 | −30 | −6 | R R | Mid Temporal (52.0%) Inferior Temporal (38.7%) | ||
| 0.008 ◊ | 347 | 4.14 | 4.07 | 28 | −26 | 14 | R R | Insula (32.6%) Heschl (18.9%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, S.L.; Jones, A.K.P.; Brown, C.A.; Kobylecki, C.; Whitaker, G.A.; El-Deredy, W.; Silverdale, M.A. Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers. Brain Sci. 2022, 12, 351. https://doi.org/10.3390/brainsci12030351
Martin SL, Jones AKP, Brown CA, Kobylecki C, Whitaker GA, El-Deredy W, Silverdale MA. Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers. Brain Sciences. 2022; 12(3):351. https://doi.org/10.3390/brainsci12030351
Chicago/Turabian StyleMartin, Sarah L., Anthony K. P. Jones, Christopher A. Brown, Christopher Kobylecki, Grace A. Whitaker, Wael El-Deredy, and Monty A. Silverdale. 2022. "Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers" Brain Sciences 12, no. 3: 351. https://doi.org/10.3390/brainsci12030351
APA StyleMartin, S. L., Jones, A. K. P., Brown, C. A., Kobylecki, C., Whitaker, G. A., El-Deredy, W., & Silverdale, M. A. (2022). Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers. Brain Sciences, 12(3), 351. https://doi.org/10.3390/brainsci12030351







