Effect of Audiovisual Cross-Modal Conflict during Working Memory Tasks: A Near-Infrared Spectroscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
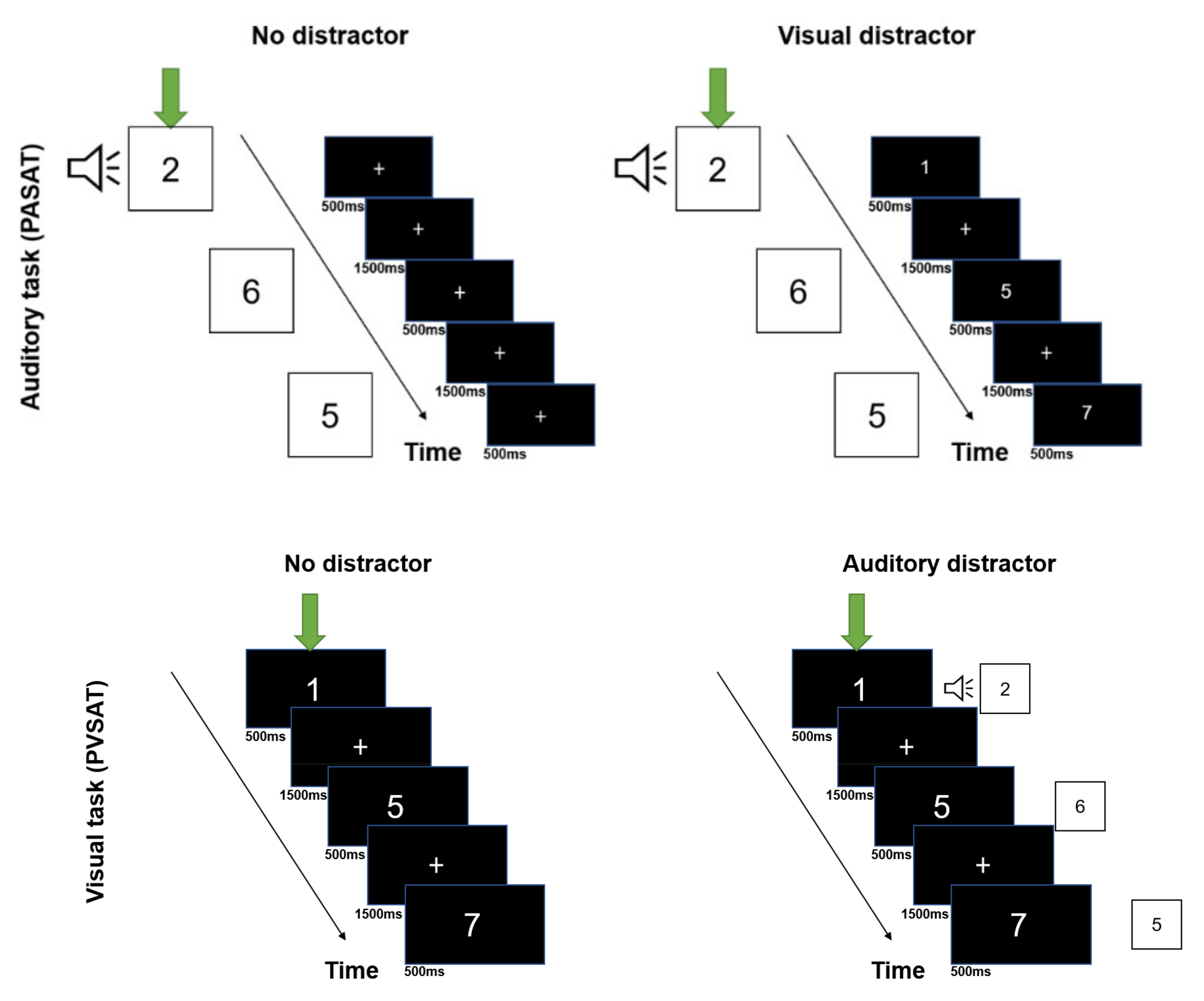
2.3. Experimental Task
2.4. Functional Near-Infrared Spectroscopy Instrument
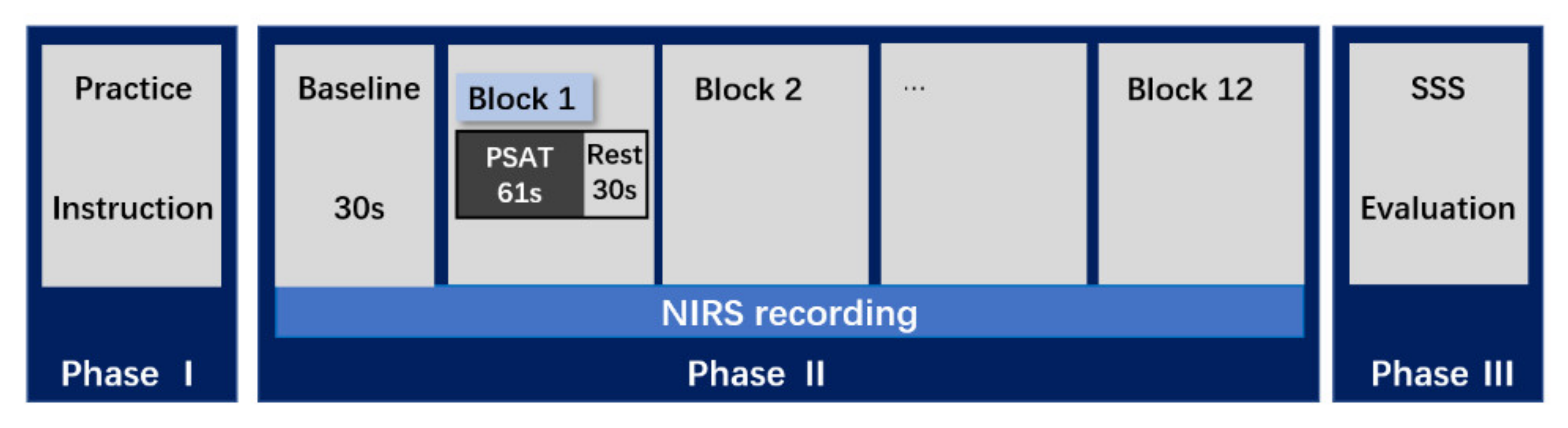
2.5. Experimental Procedure
2.6. Statistical Analysis
3. Results
3.1. Demographic Data
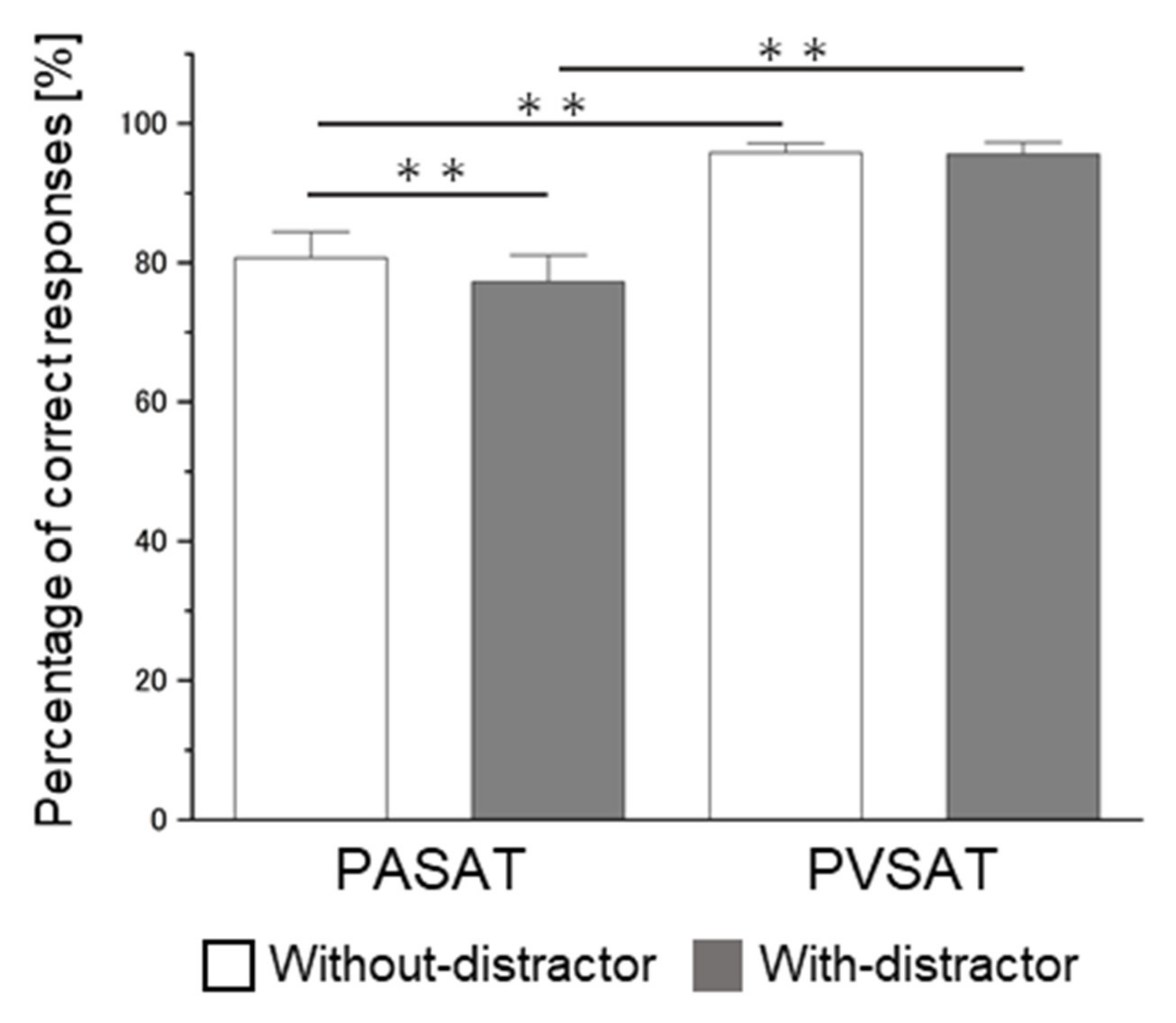
3.2. Behavioral Results
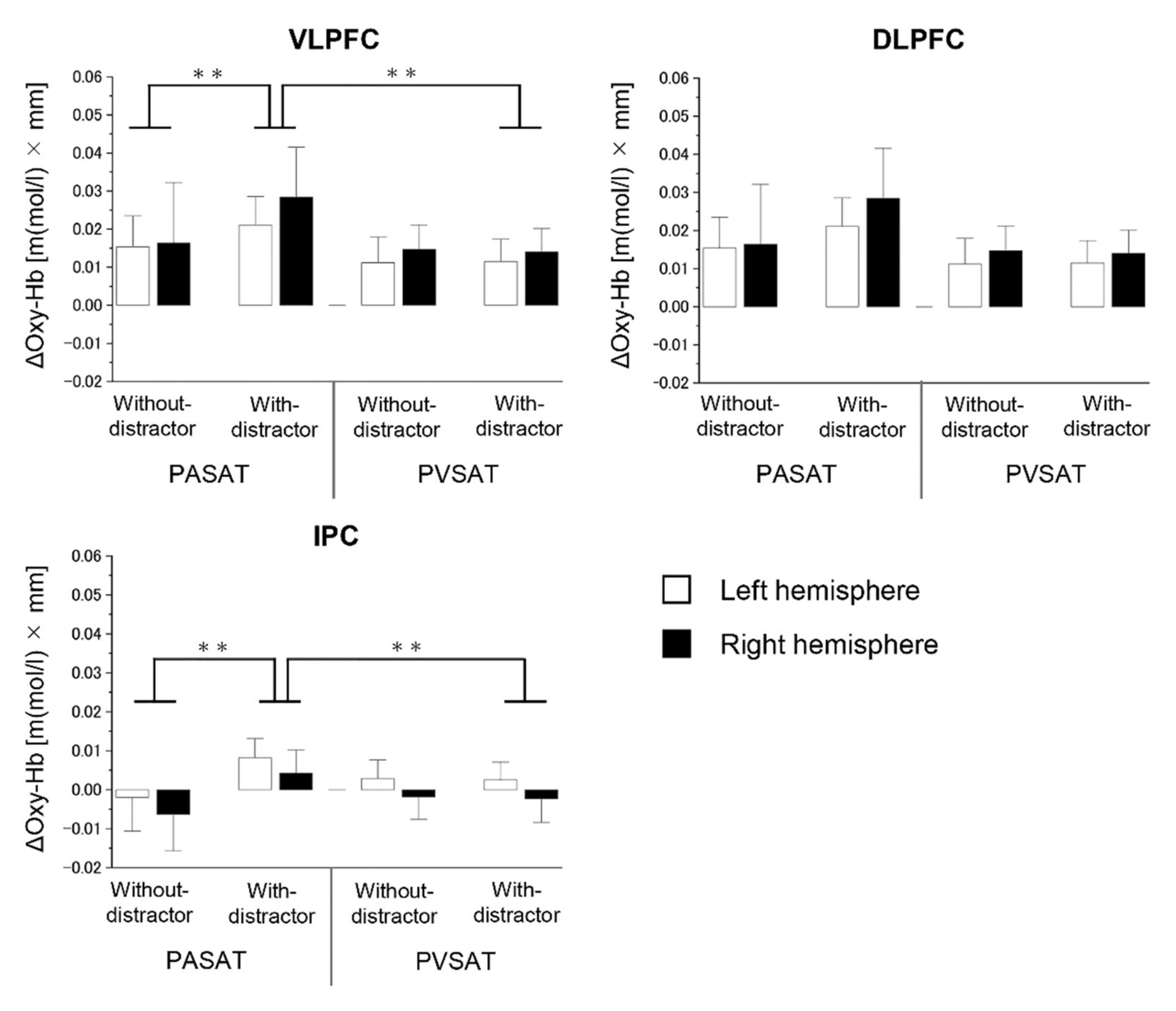
3.3. Brain Activity Results
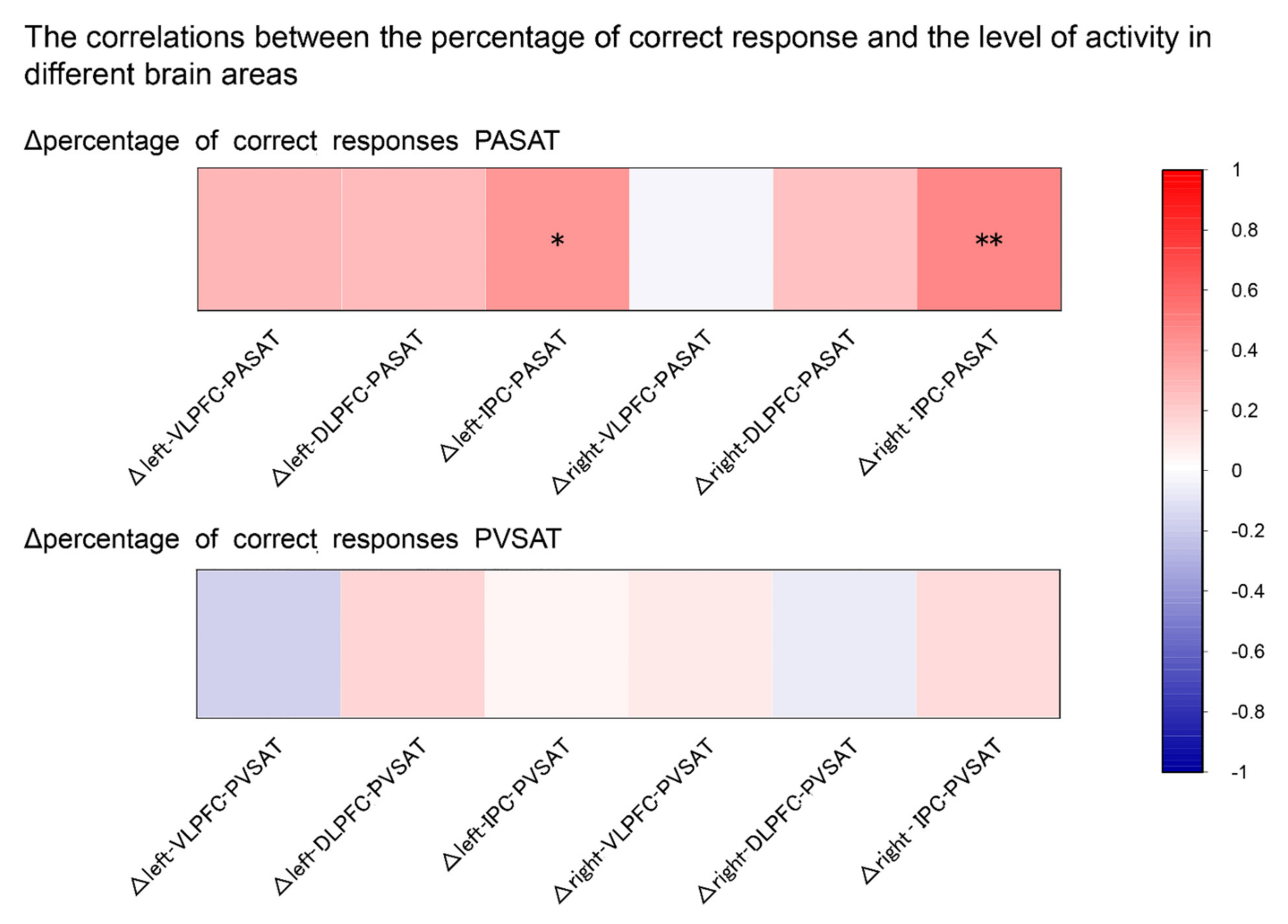
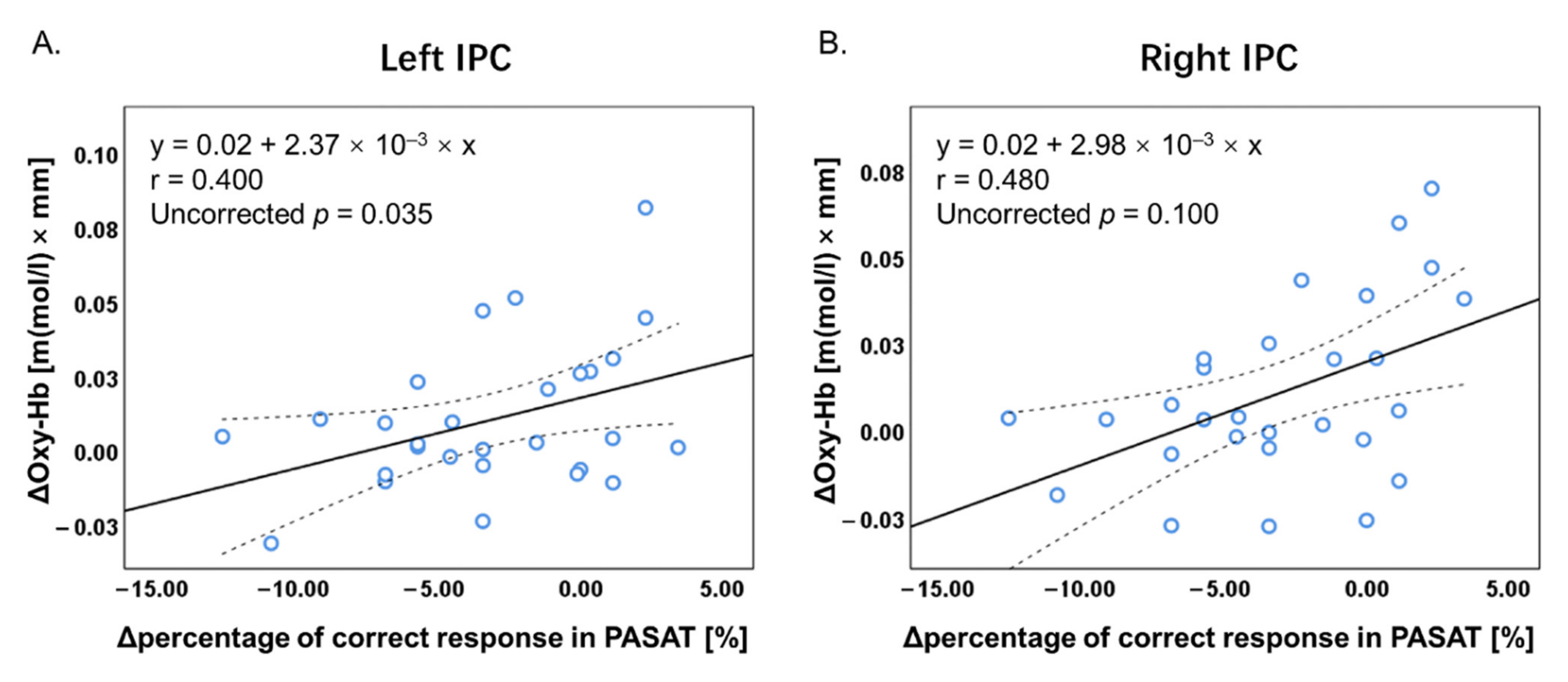
3.4. Correlation Analysis
4. Discussion
4.1. Task Performance
4.2. Brain Activity
4.3. Correlations between Task Performance and Brain Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gerven, P.W.M.; Guerreiro, M.J.S. Selective attention and sensory modality in aging: Curses and blessings. Front. Hum. Neurosci. 2016, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Lavie, N. Failures to ignore entirely irrelevant distractors: The role of load. J. Exp. Psychol. Appl. 2008, 14, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, V.; Carter, C.S. Conflict and cognitive control in the brain. Curr. Dir. Psychol. Sci. 2006, 15, 237–240. [Google Scholar] [CrossRef]
- Sugi, M.; Sakuraba, S.; Saito, H.; Miyazaki, M.; Yoshida, S.; Kamada, T.; Sakai, S.; Sawamura, D. Personality traits modulate the impact of emotional stimuli during a working memory task: A near-infrared spectroscopy study. Front. Behav. Neurosci. 2020, 14, 514414. [Google Scholar] [CrossRef] [PubMed]
- Bunge, S.A.; Ochsner, K.N.; Desmond, J.E.; Glover, G.H.; Gabrieli, J.D.E. Prefrontal regions involved in keeping information in and out of mind. Brain 2001, 124, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.T.; D’Esposito, M. Searching for ‘the top’ in top-down control. Neuron 2005, 48, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Quak, M.; London, R.E.; Talsma, D. A multisensory perspective of working memory. Front. Hum. Neurosci. 2015, 9, 197. [Google Scholar] [CrossRef]
- Fu, D.; Weber, C.; Yang, G.; Kerzel, M.; Nan, W.; Barros, P.; Wu, H.; Liu, X.; Wermter, S. What can computational models learn from human selective attention? A review from an audiovisual unimodal and crossmodal perspective. Front. Integr. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Gazzaley, A.; Nobre, A.C. Top-down modulation: Bridging selective attention and working memory. Trends Cogn. Sci. 2012, 16, 129–135. [Google Scholar] [CrossRef]
- Dolcos, F.; Miller, B.; Kragel, P.; Jha, A.; McCarthy, G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007, 1152, 171–181. [Google Scholar] [CrossRef]
- Dolcos, F.; Iordan, A.D.; Kragel, J.; Stokes, J.; Campbell, R.; McCarthy, G.; Cabeza, R. Neural correlates of opposing effects of emotional distraction on working memory and episodic memory: An event-related fMRI investigation. Front. Psychol. 2013, 4, 293. [Google Scholar] [CrossRef]
- Hughes, M.E.; Budd, T.W.; Fulham, W.R.; Lancaster, S.; Woods, W.; Rossell, S.L.; Michie, P.T. Sustained brain activation supporting stop-signal task performance. Eur. J. Neurosci. 2014, 39, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, D.; Ikoma, K.; Yoshida, K.; Inagaki, Y.; Ogawa, K.; Sakai, S. Active inhibition of task-irrelevant sounds and its neural basis in patients with attention deficits after traumatic brain injury. Brain Inj. 2014, 28, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Yahav, P.H.; Golumbic, E.Z. Linguistic processing of task-irrelevant speech at a cocktail party. Elife 2001, 10, e65096. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Dyson, B.J. Cross-modal perceptual load: The impact of modality and individual differences. Exp. Brain Res. 2016, 234, 1279–1291. [Google Scholar] [CrossRef]
- Rienäcker, F.; Van Gerven, P.W.M.; Jacobs, H.I.L.; Eck, J.; Van Heugten, C.M.; Guerreiro, M.J.S. The neural correlates of visual and auditory cross-modal selective attention in aging. Front. Aging Neurosci. 2020, 12, 498978. [Google Scholar] [CrossRef]
- Calvert, G.A. Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb. Cortex 2001, 11, 1110–1123. [Google Scholar] [CrossRef]
- Vohn, R.; Fimm, B.; Weber, J.; Schnitker, R.; Thron, A.; Spijkers, W.; Willmes, K.; Sturm, W. Management of attentional resources in within-modal and cross-modal divided attention tasks: An fMRI study. Hum. Brain Mapp. 2007, 28, 1267–1275. [Google Scholar] [CrossRef]
- Michie, P.T.; LePage, E.L.; Solowij, N.; Haller, M.; Terry, L. Evoked otoacoustic emissions and auditory selective attention. Hear. Res. 1996, 98, 54–67. [Google Scholar] [CrossRef]
- Meric, C.; Collet, L. Attention and otoacoustic emissions: A review. Neurosci. Biobehav. Rev. 1994, 18, 215–222. [Google Scholar] [CrossRef]
- Guerreiro, M.J.S.; Anguera, J.A.; Mishra, J.; Van Gerven, P.W.M.; Gazzaley, A. Age-equivalent top–down modulation during cross-modal selective attention. J. Cogn. Neurosci. 2014, 26, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Gisselgård, J.; Petersson, K.M.; Ingvar, M. The irrelevant speech effect and working memory load. Neuroimage 2004, 22, 1107–1116. [Google Scholar] [CrossRef][Green Version]
- Rees, G.; Frith, C.; Lavie, N. Processing of irrelevant visual motion during performance of an auditory attention task. Neuropsychologia 2001, 39, 937–949. [Google Scholar] [CrossRef]
- Sörqvist, P.; Dahlström, Ö.; Karlsson, T.; Rönnberg, J. Concentration: The neural underpinnings of how cognitive load shields against distraction. Front. Hum. Neurosci. 2016, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Moisala, M.; Salmela, V.; Salo, E.; Carlson, S.; Vuontela, V.; Salonen, O.; Alho, K. Brain activity during divided and selective attention to auditory and visual sentence comprehension tasks. Front. Hum. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.J.S.; Van Gerven, P.W.M. Now you see It, now you don’t: Evidence for age-dependent and age-independent cross-modal distraction. Psychol. Aging 2011, 26, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.J.S.; Murphy, D.R.; Van Gerven, P.W.M. Making sense of age-related distractibility: The critical role of sensory modality. Acta Psychol. 2013, 142, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Rienäcker, F.; Jacobs, H.I.L.; Van Heugten, C.M.; Van Gerven, P.W.M. Practice makes perfect: High performance gains in older adults engaged in selective attention within and across sensory modalities. Acta Psychol. 2018, 191, 101–111. [Google Scholar] [CrossRef]
- Sherman, E.M.S.; Strauss, E.; Spellacy, F. Validity of the Paced Auditory Serial Addition Test (PASAT) in adults referred for neuropsychological assessment after head injury. Clin. Neuropsychol. 1997, 11, 34–45. [Google Scholar] [CrossRef]
- Nagels, G.; Geentjens, L.; Kos, D.; Vleugels, L.; D’hooghe, M.B.; Van Asch, P.; Vuylsteke, K.; De Deyn, P.P. Paced Visual Serial Addition Test in multiple sclerosis. Clin. Neurol. Neurosurg. 2005, 107, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; Rees, L.; Baird, B.; Kost, J. The effects of list difficulty and modality of presentation on a computerized version of the paced serial addition test (PSAT). J. Clin. Exp. Neuropsychol. 2004, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gielen, J.; Wiels, W.; Schependom, J.V.; Laton, J.; Hecke, W.V.; Parizel, P.M.; D’hooghe, M.B.; Nagels, G. The effect of task modality and stimulus frequency in paced serial addition tests on functional brain activity. PLoS ONE 2018, 13, e0194388. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Tachtsidis, I.; Hamilton, A.F.C. The role of parietal cortex in overimitation: A study with fNIRS. Soc. Neurosci. 2018, 13, 214–225. [Google Scholar] [CrossRef]
- Weder, S.; Zhou, X.; Shoushtarian, M.; Innes-Brown, H.; McKay, C. Cortical processing related to intensity of a modulated noise stimulus—A functional near-infrared study. J. Assoc. Res. Otolaryngol. 2018, 19, 273–286. [Google Scholar] [CrossRef]
- Zhang, M.; Mary Ying, Y.L.; Ihlefeld, A. Spatial release from informational masking: Evidence from functional near infrared spectroscopy. Trends Hear. 2018, 22, 2331216518817464. [Google Scholar] [CrossRef]
- Hill, A.C.; Laird, A.R.; Robinson, J.L. Gender differences in working memory networks: A BrainMap meta-analysis. Biol. Psychol. 2014, 102, 18–29. [Google Scholar] [CrossRef]
- Okubo, M.; Suzuki, H.; Nicholls, M.E.R. A Japanese version of the Flanders handedness questionnaire. Jpn. J. Psychol. 2014, 85, 474–481. [Google Scholar] [CrossRef]
- Forster, K.I.; Forster, J.C. DMDX: A Windows display program with millisecond accuracy. Behav. Res. Methods Instrum. Comput. 2003, 35, 116–124. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxis Atlas of the Human Brain: 3-D Proportional System; Thieme Medical: New York, NY, USA, 1988. [Google Scholar]
- Cope, M.; Delpy, D.T.; Reynolds, E.O.; Wray, S.; Wyatt, J.; van der Zee, P. Methods of quantitating cerebral near infrared spectroscopy data. Adv. Exp. Med. Biol. 1988, 222, 183–189. [Google Scholar]
- Strangman, G.; Culver, J.P.; Thompson, J.H.; Boas, D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 2002, 17, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Christ, S.E.; Cowan, N. Domain-general and domain-specific functional networks in working memory. Neuroimage 2014, 102, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya, E.; Johansen-Berg, H.; Kischka, U.; Zarei, M.; Cockburn, J.; Dawes, H. Prefrontal cortex activation while walking under dual-task conditions in stroke: A multimodal imaging study. Neurorehabil. Neural Repair 2016, 30, 591–599. [Google Scholar] [CrossRef]
- Miles, C.; Jones, D.M.; Madden, C.A. Locus of the irrelevant speech effect in short-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 1991, 17, 578–584. [Google Scholar] [CrossRef]
- Kang, G.; Chang, W.; Wang, L.; Wei, P.; Zhou, X. Reward enhances cross-modal conflict control in object categorization: Electrophysiological evidence. Psychophysiology 2018, 55, e13214. [Google Scholar] [CrossRef]
- Guerreiro, M.J.S.; Murphy, D.R.; Van Gerven, P.W.M. The role of sensory modality in age-related distraction: A critical review and a renewed view. Psychol. Bull. 2010, 136, 975–1022. [Google Scholar] [CrossRef]
- Tiego, J.; Testa, R.; Bellgrove, M.A.; Pantelis, C.; Whittle, S. A hierarchical model of inhibitory control. Front. Psychol. 2018, 9, 1339. [Google Scholar] [CrossRef]
- Ciaramitaro, V.M.; Buračas, G.T.; Boynton, G.M. Spatial and cross-modal attention alter responses to unattended sensory information in early visual and auditory human cortex. J. Neurophysiol. 2007, 98, 2399–2413. [Google Scholar] [CrossRef]
- Diaconescu, A.O.; Hasher, L.; McIntosh, A.R. Visual dominance and multisensory integration changes with age. Neuroimage 2013, 65, 152–166. [Google Scholar] [CrossRef]
- Diehl, M.M.; Romanski, L.M. Responses of prefrontal multisensory neurons to mismatching faces and vocalizations. J. Neurosci. 2014, 34, 11233–11243. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, C.; Humphreys, G.W.; Shalev, L. Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nat. Neurosci. 2006, 9, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, C.; Hodsoll, J.; Allen, H.; Shalev, L.; Humphreys, G. Ignoring the elephant in the room: A neural circuit to downregulate salience. J. Neurosci. 2010, 30, 6072–6079. [Google Scholar] [CrossRef] [PubMed]
- Friedman-Hill, S.R.; Robertson, L.C.; Desimone, R.; Ungerleider, L.G. Posterior parietal cortex and the filtering of distractors. Proc. Natl Acad. Sci. USA 2003, 100, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Knight, R.T. Contribution of human prefrontal cortex to delay performance. J. Cogn. Neurosci. 1998, 10, 167–177. [Google Scholar] [CrossRef]
- McNab, F.; Klingberg, T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008, 11, 103–107. [Google Scholar] [CrossRef]
- Todd, J.J.; Marois, R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 2004, 428, 751–754. [Google Scholar] [CrossRef]
- Todd, J.J.; Marois, R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn. Affect. Behav. Neurosci. 2005, 5, 144–155. [Google Scholar] [CrossRef]
- Campbell, T. The cognitive neuroscience of auditory distraction. Trends Cogn. Sci. 2005, 9, 3–5. [Google Scholar] [CrossRef][Green Version]
- Gisselgård, J.; Petersson, K.M.; Baddeley, A.; Ingvar, M. The irrelevant speech effect: A PET study. Neuropsychologia 2003, 41, 1899–1911. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Zhang, W.; Zhang, B.; Liu, X.; Mo, L.; Chen, Q. Multisensory competition is modulated by sensory pathway interactions with fronto-sensorimotor and default-mode network regions. J. Neurosci. 2015, 35, 9064–9077. [Google Scholar] [CrossRef] [PubMed]
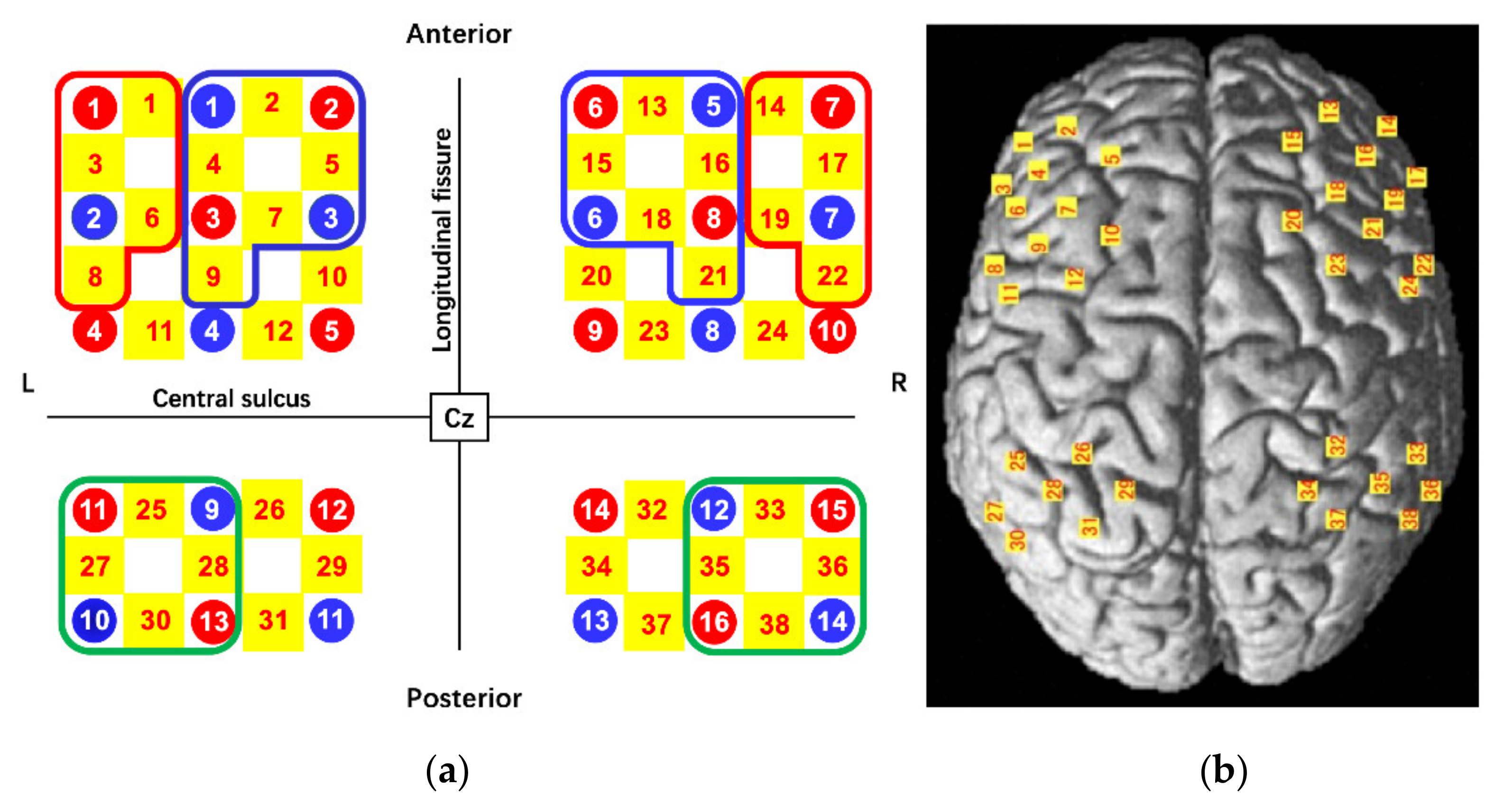
| Anatomical Labeling | Average Overlap Probability (%) | Channel Number | ||
|---|---|---|---|---|
| Talairach Daemon | Brodmann Area | Left | Right | |
| Ventrolateral prefrontal cortex | 44, 45, 47 | 71.57 (3.5) | 1, 3, 6, 8 | 14, 17, 19, 22 |
| Dorsolateral prefrontal cortex | 9,46 | 70.78 (3.5) | 2, 4, 5, 7, 9 | 13, 15, 16, 18, 21 |
| Includes Frontal eye field | 8 | 65.18 (3.8) | 10 | 20 |
| Pre-Motor and Supplementary Motor Cortex | 6 | 73.07 (3.2) | 11, 12 | 23,24 |
| Inferior parietal cortex | 39, 40 | 88.29 (3.3) | 25, 27, 28, 30 | 33, 35, 36, 38 |
| Primary Somatosensory Cortex | 1,2,3 | 58.15 (3.4) | 26 | 32 |
| Somatosensory Association Cortex | 5,7 | 76.41 (4.5) | 29, 31 | 34,37 |
| VLPFC | DLPFC | IPC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | η2p | F | p | η2p | F | p | η2p | |
| Task type | 6.387 | 0.018 * | 0.191 | 4.185 | 0.051 | 0.134 | 0.100 | 0.754 | 0.004 |
| Distractor | 10.525 | 0.003 ** | 0.280 | 1.809 | 0.190 | 0.063 | 4.278 | 0.048 * | 0.137 |
| Hemisphere | 0.600 | 0.445 | 0.022 | 0.315 | 0.579 | 0.012 | 1.570 | 0.221 | 0.055 |
| Task type × distractor | 4.643 | 0.040 * | 0.147 | 1.698 | 0.204 | 0.059 | 6.008 | 0.021 * | 0.182 |
| Task type × hemisphere | 0.973 | 0.333 | 0.035 | 0.070 | 0.794 | 0.003 | 0.144 | 0.708 | 0.005 |
| Distractor ×hemisphere | 1.237 | 0.276 | 0.044 | 0.423 | 0.521 | 0.015 | 0.010 | 0.919 | 0.000 |
| Task type × distractor × hemisphere | 1.126 | 0.298 | 0.040 | 0.720 | 0.404 | 0.026 | 0.017 | 0.898 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Sawamura, D.; Sakuraba, S.; Saito, R.; Tanabe, Y.; Miura, H.; Sugi, M.; Yoshida, K.; Watanabe, A.; Tokikuni, Y.; et al. Effect of Audiovisual Cross-Modal Conflict during Working Memory Tasks: A Near-Infrared Spectroscopy Study. Brain Sci. 2022, 12, 349. https://doi.org/10.3390/brainsci12030349
Cui J, Sawamura D, Sakuraba S, Saito R, Tanabe Y, Miura H, Sugi M, Yoshida K, Watanabe A, Tokikuni Y, et al. Effect of Audiovisual Cross-Modal Conflict during Working Memory Tasks: A Near-Infrared Spectroscopy Study. Brain Sciences. 2022; 12(3):349. https://doi.org/10.3390/brainsci12030349
Chicago/Turabian StyleCui, Jiahong, Daisuke Sawamura, Satoshi Sakuraba, Ryuji Saito, Yoshinobu Tanabe, Hiroshi Miura, Masaaki Sugi, Kazuki Yoshida, Akihiro Watanabe, Yukina Tokikuni, and et al. 2022. "Effect of Audiovisual Cross-Modal Conflict during Working Memory Tasks: A Near-Infrared Spectroscopy Study" Brain Sciences 12, no. 3: 349. https://doi.org/10.3390/brainsci12030349
APA StyleCui, J., Sawamura, D., Sakuraba, S., Saito, R., Tanabe, Y., Miura, H., Sugi, M., Yoshida, K., Watanabe, A., Tokikuni, Y., Yoshida, S., & Sakai, S. (2022). Effect of Audiovisual Cross-Modal Conflict during Working Memory Tasks: A Near-Infrared Spectroscopy Study. Brain Sciences, 12(3), 349. https://doi.org/10.3390/brainsci12030349







