Prolonged Longitudinal Transcutaneous Auricular Vagus Nerve Stimulation Effect on Striatal Functional Connectivity in Patients with Major Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. MRI Data Acquisition
2.4. Resting-State fMRI Data Preprocessing
2.5. Seed Based on FC Analyses
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Within-Group Patterns in Striatum rsFC
3.3. Between-Group Difference in Striatum rsFC
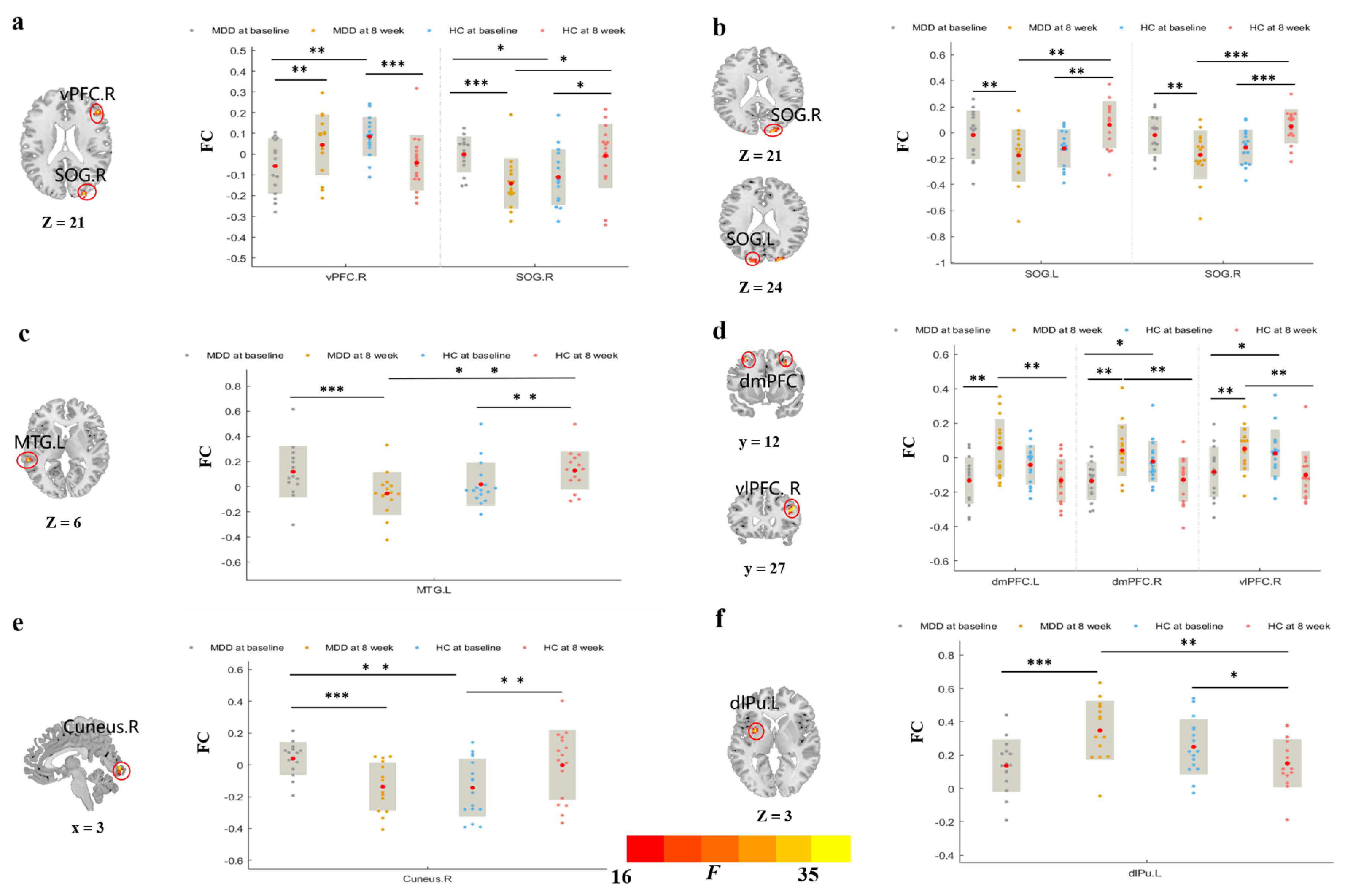
3.3.1. rsFC of Brain Regions Based on vCa
3.3.2. rsFC of Brain Regions Based on GP
3.3.3. rsFC of Brain Regions Based on NAc
3.3.4. rsFC of Brain Regions Based on dCa
4. Discussion
4.1. Prolonged taVNS Can Enhance the Intra-Striatal FC of Patients with MDD
4.2. Prolonged taVNS Can Upregulate the rsFC of Patients with MDD in the Frontal–Striatal Reward Network
4.3. Prolonged taVNS Downregulates the rsFC of Patients with MDD between the Striatum and Visual and Auditory Areas
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sealock, J.M.; Lee, Y.H.; Moscati, A.; Venkatesh, S.; Voloudakis, G.; Straub, P.; Singh, K.; Feng, Y.A.; Ge, T.; Roussos, P.; et al. Use of the PsycheMERGE Network to Investigate the Association Between Depression Polygenic Scores and White Blood Cell Count. JAMA Psychiatry 2021, 78, 1365–1374. [Google Scholar] [CrossRef]
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for united action on depression: A Lancet-World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef]
- Geugies, H.; Mocking, R.J.T.; Figueroa, C.A.; Groot, P.F.C.; Marsman, J.C.; Servaas, M.N.; Steele, J.D.; Schene, A.H.; Ruhé, H.G. Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain 2019, 142, 2510–2522. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Timimi, S.; Moncrieff, J.; Gøtzche, P.; Davies, J.; Kinderman, P.; Byng, R.; Montagu, L.; Read, J. Network meta-analysis of antidepressants. Lancet 2018, 392, 1011–1012. [Google Scholar] [CrossRef] [Green Version]
- Holtzheimer, P.E.; Mayberg, H.S. Stuck in a rut: Rethinking depression and its treatment. Trends Neurosci. 2011, 34, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.J. The medical management of depression. N. Engl. J. Med. 2005, 353, 1819–1834. [Google Scholar] [CrossRef] [Green Version]
- Cristancho, P.; Cristancho, M.A.; Baltuch, G.H.; Thase, M.E.; O’Reardon, J.P. Effectiveness and safety of vagus nerve stimulation for severe treatment-resistant major depression in clinical practice after FDA approval: Outcomes at 1 year. J. Clin. Psychiatry 2011, 72, 1376–1382. [Google Scholar] [CrossRef]
- Kraus, T.; Hösl, K.; Kiess, O.; Schanze, A.; Kornhuber, J.; Forster, C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural Transm. 2007, 114, 1485–1493. [Google Scholar] [CrossRef]
- Ben-Menachem, E. Vagus nerve stimulation, side effects, and long-term safety. J. Clin. Neurophysiol. 2001, 18, 415–418. [Google Scholar] [CrossRef]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Rong, P.; Wang, Y.; Jin, G.; Hou, X.; Li, S.; Xiao, X.; Zhou, W.; Wu, Y.; Liu, Y.; et al. Comparative Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation vs Citalopram for Major Depressive Disorder: A Randomized Trial. Neuromodulation 2022, 25, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm. 2013, 120, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Bora, E.; Harrison, B.J.; Davey, C.G.; Yücel, M.; Pantelis, C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012, 42, 671–681. [Google Scholar] [CrossRef]
- Yeh, P.H.; Zhu, H.; Nicoletti, M.A.; Hatch, J.P.; Brambilla, P.; Soares, J.C. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: Differences in latent volumetric structure. Psychiatry Res. 2010, 184, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Sheline, Y.I. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biol. Psychiatry 2000, 48, 791–800. [Google Scholar] [CrossRef]
- Di Martino, A.; Scheres, A.; Margulies, D.S.; Kelly, A.M.; Uddin, L.Q.; Shehzad, Z.; Biswal, B.; Walters, J.R.; Castellanos, F.X.; Milham, M.P. Functional connectivity of human striatum: A resting state FMRI study. Cereb. Cortex 2008, 18, 2735–2747. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, H.; Han, D.; Mo, Y.; Li, Z.; Cheng, Y.; Xu, X.; Shen, Z.; Tan, C.; Zhao, W.; et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage Clin. 2016, 11, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Wacker, J.; Dillon, D.G.; Pizzagalli, D.A. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage 2009, 46, 327–337. [Google Scholar] [CrossRef]
- Drevets, W.C.; Videen, T.O.; Price, J.L.; Preskorn, S.H.; Carmichael, S.T.; Raichle, M.E. A functional anatomical study of unipolar depression. J. Neurosci. 1992, 12, 3628–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, E.E.; Dahl, R.E. Research Review: Altered reward function in adolescent depression: What, when and how? J. Child Psychol. Psychiatry Allied Discip. 2012, 53, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbay, V.; Ely, B.A.; Li, Q.; Bangaru, S.D.; Panzer, A.M.; Alonso, C.M.; Castellanos, F.X.; Milham, M.P. Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 628–641.e13. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, T.E.; Cohen, M.X.; Frick, C.; Kosel, M.; Brodesser, D.; Axmacher, N.; Joe, A.Y.; Kreft, M.; Lenartz, D.; Sturm, V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008, 33, 368–377. [Google Scholar] [CrossRef]
- Drobisz, D.; Damborská, A. Deep brain stimulation targets for treating depression. Behav. Brain Res. 2019, 359, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Záborszky, L.; Alheid, G.F.; Beinfeld, M.C.; Eiden, L.E.; Heimer, L.; Palkovits, M. Cholecystokinin innervation of the ventral striatum: A morphological and radioimmunological study. Neuroscience 1985, 14, 427–453. [Google Scholar] [CrossRef]
- Berendse, H.W.; Galis-de Graaf, Y.; Groenewegen, H.J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992, 316, 314–347. [Google Scholar] [CrossRef]
- Ferry, A.T.; Ongür, D.; An, X.; Price, J.L. Prefrontal cortical projections to the striatum in macaque monkeys: Evidence for an organization related to prefrontal networks. J. Comp. Neurol. 2000, 425, 447–470. [Google Scholar] [CrossRef]
- Leaver, A.M.; Espinoza, R.; Joshi, S.H.; Vasavada, M.; Njau, S.; Woods, R.P.; Narr, K.L. Desynchronization and Plasticity of Striato-frontal Connectivity in Major Depressive Disorder. Cereb. Cortex 2016, 26, 4337–4346. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.W.; Xin, S.C.; Ou, Y.M.; Zhang, W.Y.; Liang, Y.L.; Chen, J.; Yang, X.Q.; Chen, X.Y.; Guo, T.W.; Yang, X.J.; et al. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J. Psychiatr. Res. 2016, 76, 111–120. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Mitchell, P.B.; Wang, C.Y.; Si, T.M. Striatal Resting-State Connectivity Abnormalities Associated with Different Clinical Stages of Major Depressive Disorder. J. Clin. Psychiatry 2020, 81, 19m12790. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.M.; Sato, J.R.; Salum, G.A.; Rohde, L.A.; Gadelha, A.; Zugman, A.; Mari, J.; Jackowski, A.; Picon, F.; Miguel, E.C.; et al. Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample. Am. J. Psychiatry 2017, 174, 1112–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; An, J.; Gao, H.M.; Zhang, P.; Chen, C.; Li, K.; Mitchell, P.B.; Si, T.M. Duloxetine effects on striatal resting-state functional connectivity in patients with major depressive disorder. Hum. Brain Mapp. 2019, 40, 3338–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, X.; Liu, J.; Chen, J.; Liu, X.; Nie, G.; Jorgenson, K.; Sohn, K.C.; Huang, R.; Liu, M.; et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 2017, 84, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Fang, J.; Liu, J.; Rong, P.; Jorgenson, K.; Park, J.; Lang, C.; Hong, Y.; Zhu, B.; Kong, J. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J. Psychiatr. Res. 2018, 102, 123–131. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar]
- Connolly, M.E.; Gollan, J.K.; Cobia, D.; Wang, X. Reduced striatal activation in females with major depression during the processing of affective stimuli. J. Psychiatr. Res. 2015, 68, 384–391. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Holmes, A.J.; Dillon, D.G.; Goetz, E.L.; Birk, J.L.; Bogdan, R.; Dougherty, D.D.; Iosifescu, D.V.; Rauch, S.L.; Fava, M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Groves, S.J.; Pitcher, T.L.; Melzer, T.R.; Jordan, J.; Carter, J.D.; Malhi, G.S.; Johnston, L.C.; Porter, R.J. Brain activation during processing of genuine facial emotion in depression: Preliminary findings. J. Affect. Disord. 2018, 225, 91–96. [Google Scholar] [CrossRef]
- Li, A.; Zalesky, A.; Yue, W.; Howes, O.; Yan, H.; Liu, Y.; Fan, L.; Whitaker, K.J.; Xu, K.; Rao, G.; et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 2020, 26, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, I.N. Anatomizing the “king of neurosciences”. World J. Neurol. 2013, 3, 3. [Google Scholar] [CrossRef]
- Cox, J.; Witten, I.B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 2019, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.S.; Claussen, C.M.; Kharas, N.; Dafny, N. The prefrontal cortex and the caudate nucleus respond conjointly to methylphenidate (Ritalin). Concomitant behavioral and neuronal recording study. Brain Res. Bull. 2020, 157, 77–89. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Zhang, W.N.; Chang, S.H.; Guo, L.Y.; Zhang, K.L.; Wang, J. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord. 2013, 151, 531–539. [Google Scholar] [CrossRef]
- Li, B.J.; Friston, K.; Mody, M.; Wang, H.N.; Lu, H.B.; Hu, D.W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef] [Green Version]
- Etkin, A.; Büchel, C.; Gross, J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015, 16, 693–700. [Google Scholar] [CrossRef]
- Rive, M.M.; van Rooijen, G.; Veltman, D.J.; Phillips, M.L.; Schene, A.H.; Ruhé, H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 2529–2553. [Google Scholar] [CrossRef]
- Furman, D.J.; Hamilton, J.P.; Gotlib, I.H. Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 2011, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Pan, H.; Kocsis, J.H.; Yang, Y.; Butler, T.; Chusid, J.; Hochberg, H.; Murrough, J.; Strohmayer, E.; Stern, E.; et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry 2006, 163, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.S.; Johnstone, T.; Shackman, A.J.; Light, S.N.; Peterson, M.J.; Kolden, G.G.; Kalin, N.H.; Davidson, R.J. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci. USA 2009, 106, 22445–22450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. NeuroImage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawetz, C.; Bode, S.; Derntl, B.; Heekeren, H.R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2017, 72, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Zilverstand, A.; Parvaz, M.A.; Goldstein, R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage 2017, 151, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Gotlib, I.H.; Hamilton, J.P. Neuroimaging and Depression: Current Status and Unresolved Issues. Curr. Dir. Psychol. Sci. 2008, 17, 159–163. [Google Scholar] [CrossRef]
- Ray, R.D.; Ochsner, K.N.; Cooper, J.C.; Robertson, E.R.; Gabrieli, J.D.; Gross, J.J. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 2005, 5, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.; Gross, J.J. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 2004, 23, 483–499. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Liu, J.H.; Wang, Y.P. Altered Default Mode and Sensorimotor Network Connectivity with Striatal Subregions in Primary Insomnia: A Resting-State Multi-Band fMRI Study. Front. Neurosci. 2018, 12, 917. [Google Scholar] [CrossRef] [Green Version]
- Schmaal, L.; Hibar, D.P.; Sämann, P.G.; Hall, G.B.; Baune, B.T.; Jahanshad, N.; Cheung, J.W.; van Erp, T.G.M.; Bos, D.; Ikram, M.A.; et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 2017, 22, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Arnone, D.; Job, D.; Selvaraj, S.; Abe, O.; Amico, F.; Cheng, Y.; Colloby, S.J.; O’Brien, J.T.; Frodl, T.; Gotlib, I.H.; et al. Computational meta-analysis of statistical parametric maps in major depression. Hum. Brain Mapp. 2016, 37, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Won, E.; Kang, J.; Chang, H.S.; Yoon, H.K.; Tae, W.S.; Kim, Y.K.; Lee, M.S.; Joe, S.H.; Kim, H.; et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci. Rep. 2016, 6, 21089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desseilles, M.; Balteau, E.; Sterpenich, V.; Dang-Vu, T.T.; Darsaud, A.; Vandewalle, G.; Albouy, G.; Salmon, E.; Peters, F.; Schmidt, C.; et al. Abnormal neural filtering of irrelevant visual information in depression. J. Neurosci. 2009, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.T.; Cho, S.W.; Khang, H.S.; Lee, B.C.; Choi, I.G.; Lyoo, I.K.; Ham, B.J. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1487–1492. [Google Scholar] [CrossRef]
- Shuler, M.G.; Bear, M.F. Reward timing in the primary visual cortex. Science 2006, 311, 1606–1609. [Google Scholar] [CrossRef]
- Zaremba, D.; Dohm, K.; Redlich, R.; Grotegerd, D.; Strojny, R.; Meinert, S.; Bürger, C.; Enneking, V.; Förster, K.; Repple, J.; et al. Association of Brain Cortical Changes with Relapse in Patients with Major Depressive Disorder. JAMA Psychiatry 2018, 75, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Rees, G. Comparing the temporal relationship of structural and functional connectivity changes in different adult human brain networks: A single-case study. Wellcome Open Res. 2018, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Poldrack, R.A.; Laumann, T.O.; Koyejo, O.; Gregory, B.; Hover, A.; Chen, M.Y.; Gorgolewski, K.J.; Luci, J.; Joo, S.J.; Boyd, R.L.; et al. Long-term neural and physiological phenotyping of a single human. Nat. Commun. 2015, 6, 8885. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Woelfer, M.; Kühn, S.; Zhang, Z.; Biswal, B.B. Estimations of the weather effects on brain functions using functional MRI: A cautionary note. Hum. Brain Mapp. 2022, 43, 3346–3356. [Google Scholar] [CrossRef]


| Characteristic | MDD (n = 15) | HCs (n = 16) | t/Z/x2 | p Value |
|---|---|---|---|---|
| Gender (M/F) | 3/12 | 7/9 | 1.998 | 0.157 # |
| Age (year) | 40.9 ± 14.4 | 35.5 ± 14.8 | −0.694 | 0.488 * |
| Education (year) | 14.3 ± 2.9 | 14.7 ± 2.2 | −0.531 | 0.595 * |
| 17-HAMD_pre | 16.6 ± 4.37 | 1.19 ± 1.42 | 4.778 | <0.001 a |
| 17-HAMD_aft | 6.53 ± 2.45 | 1.75 ± 1.48 | 6.634 | <0.001 * |
| HAMD scoreΔ | 10.07 ± 3.04 | - | 12.847 | <0.001 b |
| Seed Points | Brain Regions | BA | Peak Point MNI Coordinates | Cluster Size (Voxels) | F Value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left vCa | Right vPFC | 45, 48 | 48 | 33 | 21 | 15 | 27.992 |
| Right SOG | 18, 19 | 27 | −93 | 21 | 18 | 27.330 | |
| Right GP | Left SOG | 18 | −16 | −93 | 24 | 32 | 26.1023 |
| Right SOG | 18, 19 | 21 | −93 | 21 | 46 | 30.9472 | |
| Left NAc | Left MTG | 22, 21 | −60 | −36 | 6 | 14 | 27.518 |
| Right NAc | Left dmPFC | 8, 9 | −33 | 12 | 57 | 18 | 23.509 |
| Right dmPFC | 8, 9 | 30 | 18 | 42 | 26 | 24.526 | |
| Right vlPFC | 44 | 42 | 27 | 24 | 43 | 23.927 | |
| Left dCa | Right Cuneus | 17, 18 | 3 | −90 | −3 | 28 | 27.938 |
| Right dCa | Left dlPu | −30 | 3 | 3 | 30 | 35.8427 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; He, J.-K.; Zhong, G.-L.; Wang, Y.; Zhao, Y.-N.; Wang, L.; Li, S.-Y.; Xiao, X.; Yang, Z.-Y.; Zhao, B.; et al. Prolonged Longitudinal Transcutaneous Auricular Vagus Nerve Stimulation Effect on Striatal Functional Connectivity in Patients with Major Depressive Disorder. Brain Sci. 2022, 12, 1730. https://doi.org/10.3390/brainsci12121730
Zhang S, He J-K, Zhong G-L, Wang Y, Zhao Y-N, Wang L, Li S-Y, Xiao X, Yang Z-Y, Zhao B, et al. Prolonged Longitudinal Transcutaneous Auricular Vagus Nerve Stimulation Effect on Striatal Functional Connectivity in Patients with Major Depressive Disorder. Brain Sciences. 2022; 12(12):1730. https://doi.org/10.3390/brainsci12121730
Chicago/Turabian StyleZhang, Shuai, Jia-Kai He, Gang-Liang Zhong, Yu Wang, Ya-Nan Zhao, Lei Wang, Shao-Yuan Li, Xue Xiao, Zheng-Yi Yang, Bin Zhao, and et al. 2022. "Prolonged Longitudinal Transcutaneous Auricular Vagus Nerve Stimulation Effect on Striatal Functional Connectivity in Patients with Major Depressive Disorder" Brain Sciences 12, no. 12: 1730. https://doi.org/10.3390/brainsci12121730






