Cranial Ultrasound Abnormalities in Small for Gestational Age or Growth-Restricted Infants Born over 32 Weeks Gestation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
1.1. Background
1.2. Aim
2. Methods
2.1. Eligibility Criteria
- (1)
- Inclusion criteria
- (2)
- Exclusion criteria
2.2. Population
- Moderate-late preterm and term infants, i.e., infants born from 32 weeks’ gestation to term age.
2.3. Exposure
- FGR/SGA.
2.4. Comparator/Control
- If available, to appropriately grown infants of the same gestation group.
2.5. Outcome Measures
2.6. Primary Outcomes
2.7. Secondary Outcomes
- Review of brain structure development and growth on cranial ultrasounds, of MLPT and term growth-restricted infants prior to discharge, by assessing:
- 2D- measurements of specific brain structures
- cerebellar vermis size
- transverse cerebellar diameter
- Review of cerebral artery Dopplers parameters including MCA peak systolic velocity, end diastolic velocity and resistive and pulsatility indices, prior to discharge on cranial ultrasounds of MLPT and term growth-restricted infants
2.8. Search Methodology
- A systematic search was performed after consultation with a clinical librarian, on three electronic databases PubMed (1996–December 2021), EMBASE (1974–December 2021) and MEDLINE via Ovid (1946–December 2021). Grey literature found through EMBASE was reviewed and included if subgroup analysis was available. Reference lists from relevant review articles and included studies were also manually reviewed to identify potentially relevant studies.
2.9. Study Selection
2.10. Data Extraction
2.11. Analysis
3. Results
3.1. Search Results
3.2. Methodological Quality
3.3. Study Characteristics
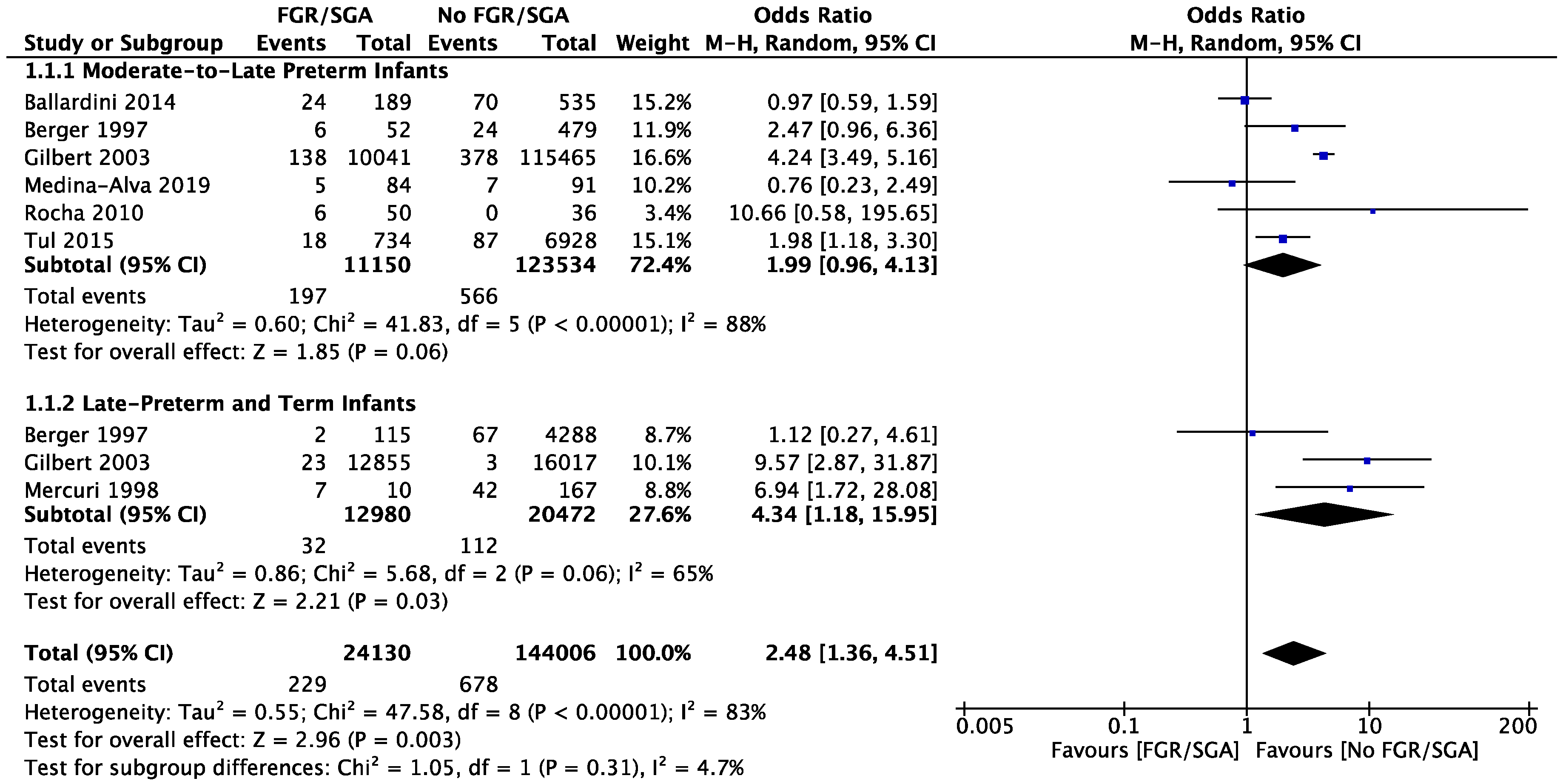
3.4. Cranial Ultrasound Abnormalities in Moderate to Late Preterm Infants
3.5. Cranial Ultrasound Abnormalities in Late Preterm and Term Growth-Restricted Infants
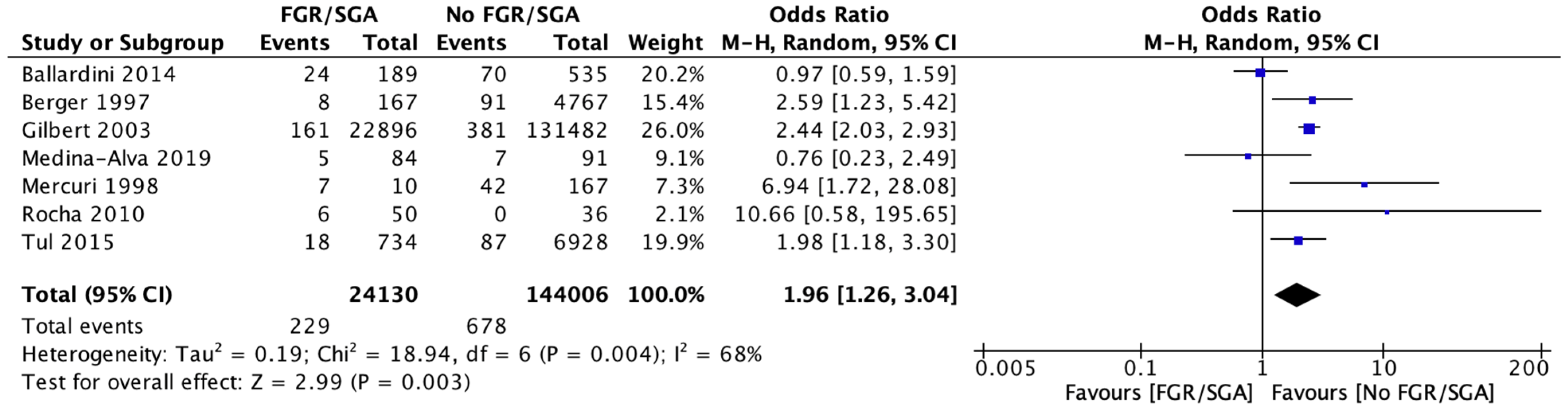
3.6. Meta-Analysis
3.7. Brain Structure Growth in SGA Infants
3.8. MCA Dopplers in Infants with Fetal Growth Restriction
4. Discussion
4.1. Incidence of Cranial Ultrasound Abnormalities
4.2. FGR versus AGA Cranial Ultrasound Result Comparison
5. Limitations
Implications for Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLean, G.; Malhotra, A.; Lombardo, P.; Schneider, M. Cranial Ultrasound Screening Protocols for Very Preterm Infants. Ultrasound Med. Biol. 2021, 47, 1645–1656. [Google Scholar] [CrossRef]
- de Vries, L.S.; Benders, M.J.; Groenendaal, F. Imaging the premature brain: Ultrasound or MRI? Neuroradiology 2013, 55 (Suppl. S2), 13–22. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; de Vries, L.S.; Ferriero, D.M.; Grant, P.E.; Ment, L.R.; Miller, S.P.; Volpe, J.J. Neuroimaging of the Preterm Brain: Review and Recommendations. J. Pediatr. 2021, 237, 276–287.e4. [Google Scholar] [CrossRef] [PubMed]
- van Wezel-Meijler, G.; de Vries, L.S. Cranial Ultrasound—Optimizing Utility in the NICU. Curr. Pediatr. Rev. 2014, 10, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sauv, R. Routine screening cranial ultrasound examinations for the prediction of long term neurodevelopmental outcomes in preterm infant. Paediatr. Child Health 2001, 6, 39–43. [Google Scholar]
- Boswinkel, V.; Krüse-Ruijter, M.F.; Nijboer-Oosterveld, J.; Nijholt, I.M.; Edens, M.A.; Mulder-de Tollenaer, S.M.; Smit-Wu, M.N.; Boomsma, M.F.; de Vries, L.S.; van Wezel-Meijler, G. Incidence of brain lesions in moderate-late preterm infants assessed by cranial ultrasound and MRI: The BIMP-study. Eur. J. Radiol. 2021, 136, 109500. [Google Scholar] [CrossRef]
- Walsh, J.M.; Doyle, L.W.; Anderson, P.J.; Lee, K.J.; Cheong, J.L.Y. Moderate and late Preterm Birth: Effect on Brain Size and Maturation at Term-Equivalent Age. Radiology 2014, 273, 232–240. [Google Scholar] [CrossRef]
- Mohammad, K.; Scott, J.N.; Leijser, L.M.; Zein, H.; Afifi, J.; Piedboeuf, B.; de Vries, L.S.; van Wezel-Meijler, G.; Lee, S.K.; Shah, P.S. Consensus Approach for Standardizing the Screening and Classification of Preterm Brain Injury Diagnosed with Cranial Ultrasound: A Canadian Perspective. Front. Pediatr. 2021, 9, 618236. [Google Scholar] [CrossRef]
- Guillot, M.; Chau, V.; Lemyre, B. Routine imaging of the preterm neonatal brain. Paediatr. Child Health 2020, 25, 249–262. [Google Scholar] [CrossRef]
- Shapiro-Mendoza, C.K.; Lackritz, E.M. Epidemiology of late and moderate preterm birth. Semin. Fetal Neonatal Med. 2012, 17, 120–125. [Google Scholar] [CrossRef]
- Voigt, B.; Pietz, J.; Pauen, S.; Kliegel, M.; Reuner, G. Cognitive development in very vs. moderately to late preterm and full-term children: Can effortful control account for group differences in toddlerhood? Early Hum. Dev. 2012, 88, 307–313. [Google Scholar] [CrossRef]
- Woythaler, M.A.; McCormick, M.C.; Smith, V.C. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics 2011, 127, e622–e629. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Kaciroti, N.; Richards, B.; Oh, W.; Lumeng, J.C. Developmental Outcomes of Late Preterm Infants From Infancy to Kindergarten. Pediatrics 2016, 138, e20153496. [Google Scholar] [CrossRef]
- Cheong, J.L.; Doyle, L.; Burnett, A.C.; Lee, K.J.; Walsh, J.M.; Potter, C.R.; Treyvaud, K.; Thompson, D.; Olsen, J.E.; Anderson, P.; et al. Association Between Moderate and Late Preterm Birth and Neurodevelopment and Social-Emotional Development at Age 2 Years. JAMA Pediatr. 2017, 171, e164805. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef]
- Blair, E.M.; Nelson, K.B. Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks’ gestation. Am. J. Obstet. Gynecol. 2015, 212, 520.e1–520.e7. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Hüppi, P.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef]
- Malhotra, A.; Yahya, Z.; Sasi, A.; Jenkin, G.; Ditchfield, M.; Polglase, G.R.; Miller, S.L. Does fetal growth restriction lead to increased brain injury as detected by neonatal cranial ultrasound in premature infants? J. Paediatr. Child Health 2015, 51, 1103–1108. [Google Scholar] [CrossRef]
- Vollmer, B.; Edmonds, C.J. School Age Neurological and Cognitive Outcomes of Fetal Growth Retardation or Small for Gestational Age Birth Weight. Front. Endocrinol. 2019, 10, 186. [Google Scholar] [CrossRef]
- Tanis, J.C.; Van Braeckel, K.N.; Kerstjens, J.M.; Bocca-Tjeertes, I.F.; Reijneveld, S.A.; Bos, A.F. Functional outcomes at age 7 years of moderate preterm and full term children born small for gestational age. J. Pediatr. 2015, 166, 552–558.e1. [Google Scholar] [CrossRef]
- Korzeniewski, S.J.; Allred, E.N.; Joseph, R.M.; Heeren, T.; Kuban, K.C.; O’Shea, T.M.; Leviton, A.; ELGAN Study Investigators. Neurodevelopment at Age 10 Years of Children Born <28 Weeks with Fetal Growth Restriction. Pediatrics 2017, 140, e20170697. [Google Scholar]
- Polat, A.; Barlow, S.; Ber, R.; Achiron, R.; Katorza, E. Volumetric MRI study of the intrauterine growth restriction fetal brain. Eur. Radiol. 2017, 27, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Tolsa, C.B.; Zimine, S.; Warfield, S.K.; Freschi, M.; Rossignol, A.S.; Lazeyras, F.; Hanquinet, S.; Pfizenmaier, M.; Hüppi, P.S. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr. Res. 2004, 56, 132–138. [Google Scholar] [CrossRef]
- Dubois, J.; Benders, M.; Borradori-Tolsa, C.; Cachia, A.; Lazeyras, F.; Leuchter, R.H.-V.; Sizonenko, S.V.; Warfield, S.K.; Mangin, J.F.; Hüppi, P.S. Primary cortical folding in the human newborn: An early marker of later functional development. Brain 2008, 131 Pt 8, 2028–2041. [Google Scholar] [CrossRef]
- Ramenghi, L.A.; Martinelli, A.; De Carli, A.; Brusati, V.; Mandia, L.; Fumagalli, M.; Triulzi, F.; Mosca, F.; Cetin, I. Cerebral maturation in IUGR and appropriate for gestational age preterm babies. Reprod. Sci. 2011, 18, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Ditchfield, M.; Fahey, M.C.; Castillo-Melendez, M.; Allison, B.; Polglase, G.; Wallace, E.; Hodges, R.; Jenkin, G.; Miller, S. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr. Res. 2017, 82, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 15 June 2022).
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Berger, R.; Bender, S.; Sefkow, S.; Klingmüller, V.; Künzel, W.; Jensen, A. Peri/intraventricular haemorrhage: A cranial ultrasound study on 5286 neonates. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 75, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Dubowitz, L.; Brown, S.P.; Cowan, F. Incidence of cranial ultrasound abnormalities in apparently well neonates on a postnatal ward: Correlation with antenatal and perinatal factors and neurological status. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F185–F189. [Google Scholar] [CrossRef]
- Gilbert, W.M.; Danielsen, B. Pregnancy outcomes associated with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2003, 188, 1596–1601; discussion 9–601. [Google Scholar] [CrossRef]
- Marsoosi, V.; Bahadori, F.; Esfahani, F.; Ghasemi-Rad, M. The role of Doppler indices in predicting intra ventricular hemorrhage and perinatal mortality in fetal growth restriction. Med. Ultrason. 2012, 14, 125–132. [Google Scholar] [PubMed]
- Starčević, M.; Predojević, M.; Butorac, D.; Tumbri, J.; Konjevoda, P.; Kadić, A.S. Early functional and morphological brain disturbances in late-onset intrauterine growth restriction. Early Hum. Dev. 2016, 93, 33–38. [Google Scholar] [CrossRef]
- Medina-Alva, P.; Duque, K.R.; Zea-Vera, A.; Bellomo, S.; Cárcamo, C.; Guillen-Pinto, D.; Rivas, M.; Tori, A.; Zegarra, J.; Cam, L.; et al. Combined predictors of neurodevelopment in very low birth weight preterm infants. Early Hum. Dev. 2019, 130, 109–115. [Google Scholar] [CrossRef]
- Ortigosa Rocha, C.; Bittar, R.E.; Zugaib, M. Neonatal outcomes of late-preterm birth associated or not with intrauterine growth restriction. Obstet. Gynecol. Int. 2010, 2010, 231842. [Google Scholar] [CrossRef]
- Turcan, N.; Bohiltea, R.E.; Ionita-Radu, F.; Furtunescu, F.; Navolan, D.; Berceanu, C.; Nemescu, D.; Cirstoiu, M.M. Unfavorable influence of prematurity on the neonatal prognostic of small for gestational age fetuses. Exp. Ther. Med. 2020, 20, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Tul, N.; Lasic, M.; Bricelj, K.; Bregar, A.T.; Verdenik, I.; Lucovnik, M.; Blickstein, I. Outcome of small for gestational age preterm singletons: A population-based cohort study. J. Périnat. Med. 2016, 44, 941–944. [Google Scholar] [CrossRef]
- Baschat, A.A.; Gembruch, U.; Viscardi, R.M.; Gortner, L.; Harman, C.R. Antenatal prediction of intraventricular hemorrhage in fetal growth restriction: What is the role of Doppler? Ultrasound Obstet. Gynecol. 2002, 19, 334–339. [Google Scholar] [CrossRef]
- Valcamonico, A.; Accorsi, P.; Sanzeni, C.; Martelli, P.; La Boria, P.; Cavazza, A.; Frusca, T. Mid- and long-term outcome of extremely low birth weight (ELBW) infants: An analysis of prognostic factors. J. Matern. Neonatal Med. 2007, 20, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Štimac, T.; Šopić-Rahelić, A.-M.; Ivandić, J.; Ekinja, E.; Blickstein, I. Effect of gender on growth-restricted fetuses born preterm. J. Périnat. Med. 2019, 47, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.B.; Popiel, A.; Malhotra, A. Screening investigations in small-for-gestational-age near-term and term infants. Eur. J. Pediatr. 2017, 176, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, E.; Tarocco, A.; Baldan, A.; Antoniazzi, E.; Garani, G.; Borgna-Pignatti, C. Universal cranial ultrasound screening in preterm infants with gestational age 33–36 weeks. a retrospective analysis of 724 newborns. Pediatr. Neurol. 2014, 51, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Cosmi, E.; Bilardo, C.M.; Wolf, H.; Berg, C.; Rigano, S.; Germer, U.; Moyano, D.; Turan, S.; Hartung, J.; et al. Predictors of Neonatal Outcome in Early- Onset Placental Dysfunction. Obstet. Gynecol. 2007, 109 2 Pt 1, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Liu, C.-C. The differences in growth of cerebellar vermis between appropriate-for-gestational-age and small-for-gestational-age newborns. Early Hum. Dev. 1993, 33, 9–19. [Google Scholar] [CrossRef]
- Makhoul, I.R.; Goldstein, I.; Epelman, M.; Tamir, A.; Reece, E.A.; Sujov, P. Neonatal transverse cerebellar diameter in normal and growth-restricted infants. J. Matern. Fetal Med. 2000, 9, 155–160. [Google Scholar]
- Krishnamurthy, M.B.; Pharande, P.; Whiteley, G.; Hodges, R.J.; Malhotra, A. Postnatal middle cerebral artery Dopplers in growth-restricted neonates. Eur. J. Pediatr. 2020, 179, 571–577. [Google Scholar] [CrossRef]
- Boswinkel, V.; Nijboer-Oosterveld, J.; Nijholt, I.M.; Edens, M.A.; Mulder-de Tollenaer, S.M.; Boomsma, M.F.; de Vries, L.S.; van Wezel-Meijler, G. A systematic review on brain injury and altered brain development in moderate-late preterm infants. Early Hum. Dev. 2020, 148, 105094. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Govaert, P.; Horsch, S.; Bravo, M.C.; Ramenghi, L.A.; eurUS Brain Group; Agut, T. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr. Res. 2020, 87 (Suppl. S1), 13–24. [Google Scholar] [CrossRef]
- Gleiβner, M.; Jorch, G.; Avenarius, S. Risk factors for intraventricular hemorrhage in a birth cohort of 3721 premature infants. J. Périnat. Med. 2000, 28, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Twomey, S.; Flatley, C.; Kumar, S. The association between a low cerebro-umbilical ratio at 30–34 weeks gestation, increased intrapartum operative intervention and adverse perinatal outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Khazardoost, S.; Ghotbizadeh, F.; Sahebdel, B.; Amiri, F.N.; Shafaat, M.; Akbarian-Rad, Z.; Pahlavan, Z. Predictors of Cranial Ultrasound Abnormalities in Intrauterine Growth-Restricted Fetuses Born between 28 and 34 Weeks of Gestation: A Prospective Cohort Study. Fetal Diagn. Ther. 2019, 45, 238–247. [Google Scholar] [CrossRef] [PubMed]


| Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow Up of Cohorts | ||
| Berger, 1997 [32] | ★ | ★ | ★ | - | ★ | ★ | - | ★ | ★★★★★★ Moderate Risk |
| Mercuri, 1998 [33] | - | ★ | ★ | - | ★ | ★ | - | - | ★★★★ Moderate Risk |
| Gilbert, 2003 [34] | ★ | ★ | ★ | - | ★ | ★ | - | ★ | ★★★★★★ Moderate Risk |
| Baschat, 2007 [46] | - | - | ★ | ★ | - | ★ | - | - | ★★★ High Risk |
| Valcamonico, 2007 [42] | - | - | ★ | ★ | - | ★ | - | - | ★★★ High Risk |
| Marsoosi, 2012 [35] | - | - | ★ | ★ | ★ | ★ | ★ | ★ | ★★★★★★ Moderate Risk |
| Ballardini, 2014 [45] | ★ | ★ | ★ | ★ | ★ | ★ | - | ★ | ★★★★★★★ Low Risk |
| Tul, 2015 [40] | ★ | ★ | ★ | - | ★ | ★ | - | ★ | ★★★★★★ Moderate Risk |
| Starcevic, 2016 [36] | - | - | ★ | ★ | - | ★ | ★ | ★ | ★★★★★ Moderate Risk |
| Krishnamurthy, 2017 [44] | - | - | ★ | - | - | ★ | - | ★ | ★★★ High Risk |
| Stimac, 2019 [43] | - | - | ★ | - | - | ★ | - | - | ★★ High Risk |
| Medina-Alva, 2019 [37] | - | - | ★ | ★ | - | ★ | ★ | - | ★★★★ Moderate Risk |
| Turcan, 2020 [39] | ★ | ★ | ★ | - | ★ | ★ | ★ | - | ★★★★★★ Moderate Risk |
| Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | ||
| Rocha, 2010 [38] | ★ | - | - | ★ | ★ | ★ | ★ | - | ★★★★★ Moderate Risk |
| Author, Year | Gestation | Study Total | Study Design | Aims | Key Findings of Study | Relevant Findings Related to FGR or SGA |
|---|---|---|---|---|---|---|
| Berger, 1997 [32] | 24+0–43+0 weeks | 5286 | Prospective cohort study | To examine the incidence of brain injury on cranial ultrasound compared with obstetric risk factors | The most frequent abnormality was IVH, with the incidence increasing with decreasing gestational age | Growth restriction and acidosis (pH ≤ 7.29) on arterial cord gases was associated with an increased risk of IVH That rate of IVH in FGR infants born 35–37 weeks was 11.5% |
| Gilbert, 2003 [34] | 26+0–41+0 weeks | 1,347,788 | Retrospective cohort study | To examine incidence of FGR and associated neonatal outcomes | Until 28 weeks, prematurity associated with adverse neonatal outcomes (RDS, IVH, NEC, CHA) was largely unaffected by FGR | Of the FGR Infants born between 34–39 weeks there was a statistically significant increase in the rate of IVH compared to AGA counterparts |
| Valcamonico, 2007 [42] | 24+0–34+6 weeks | 183 | Prospective cohort study | To evaluate morbidity and long-term neurological outcomes in extremely low birthweight infants (i.e., <1000 g) | Extremely low birth weight increases the risk of perinatal morbidity and neonatal morbidity The most significant factor for long-term neurological outcomes was gestational age | Subgroup analysis of 10 infants born between 32–34 weeks gestation had 10% (n = 1) infant with IVH and 0 with PVL |
| Baschat, 2007 [46] | 24+0–32+6 weeks | 604 | Prospective cohort study | To determine morbidity and mortality in growth-restricted infants with early onset placental dysfunction | Gestational age was the most significant predictor (p < 0.005) of survival until 26+6 weeks and intact survival until 29+2 weeks. Beyond 29+2 weeks and in infants > 600 g, ductus venosus Doppler and cord artery pH was predictive of neonatal mortality (p < 0.001) and Doppler alone was predictive of intact survival. | Of 76 infants born growth-restricted at 32 weeks 1.3% (n = 1) had a grade III-IV IVH |
| Rocha, 2010 [38] | 34+0–36+6 weeks | 86 | Case-control study | To compare neonatal outcomes between FGR and AGA infants | Late-preterm FGR infants require longer hospitalisation and are at higher risk of IVH and hypoglycaemia compared to AGA infants | Greater rates of IVH * seen in FGR population vs. AGA infants (12% vs. 0%, p = 0.037) |
| Marsoosi, 2012 [35] | 23+0–40+6 weeks | 41 | Prospective cohort study | To determine if there is a correlation between Doppler indices and IVH and perinatal mortality in pregnancies affected by FGR | Infants with AREDF had a 5 times greater chance of developing IVH The risk of IVH was associated with gestational age at delivery, birth weight, and acidosis | The rate of IVH was 33% in infants born between 32–33+6 weeks and 12.5% in infants born between 34–35+6 weeks |
| Ballardini, 2014 [45] | 33+0–36+6 weeks | 724 | Retrospective cohort study | To determine the number of neonates with abnormal cranial ultrasounds and evaluate universal ultrasound screening | Infants born 33+0–34+6 weeks were four times more likely to have CUAs compared with those born at 35+0–36+6 weeks A postnatal head circumference < 3rd centile, need for ventilation or surfactant, low APGAR at the 5th minute of life and neurological abnormalities were associated with an increase rate of CUAs | No significant increase in rate CUAs in SGA infants with birth weights < 10th or <3rd centiles. |
| Tul, 2015 [40] | 24+0–36+6 | 159,774 | Retrospective cohort study | To compare short term outcomes and morbidity of SGA and AGA infants by gestational age and maternal, antenatal and birth details | Infants born > 30 weeks and SGA had worse 5 min APGAR scores, and higher rates of IVH, RDS, neonatal death and ventilation compared with infants that were AGA. | Infants born 33–36+6 weeks and SGA had a 2-times increase rate of IVH compared to AGA infants. (2% (n = 18) vs. 1.3% (n = 87) OR 2 (1.2–3.3 95%CI) |
| Stimac, 2019 [43] | 23+0–>37 weeks | 2676 | Retrospective cohort study | To examine the effect on neonatal outcomes of gender in SGA infants | Both female and male infants had similar rates of RDS, IVH and admission to intensive care | The rate of IVH documented in SGA infants born 33–36+6 weeks was 5.1% (8 of 158 infants) |
| Medina-Alva, 2019 [37] | 24–36 weeks | 414 | Prospective cohort study | To assess the combined prognostic value of neurological examination, head circumference and cranial ultrasounds for neurodevelopmental delay in very low birth weight (VLBW, <1500 g) preterm infants | A combination of microcephaly and major ultrasound abnormalities (brain infarction, parenchymal haemorrhage, grade 3 or 4 IVH, post-haemorrhagic hydrocephalus with ventricular index > 14mm and PVL/or periventricular cysts) showed the highest positive predictive value (100%; 95% CI, 51%–100%) for poorer neurological outcomes. | 5.9% of SGA infants born between 32–36 weeks had major ultrasound abnormalities, vs. 7.7% of infants that were well grown |
| First Author | Subgroup Gestation | Subgroup Total | Definition of FGR/SGA Infants | Number of FGR/SGA Infants | Study Population Incidence of CUAs | CUAs Incidence in Non FGR/SGA Infants | CUAs Incidence in FGR/SGA |
|---|---|---|---|---|---|---|---|
| Berger [32] | 35+0–37+6 | 531 | <10th Centile | 52 | 3.6% | 5% | 11.5% |
| Gilbert [34] | 32+0–32+6 33+0–33+6 34+0–34+6 35+0–35+6 36+0–36+6 | 5891 9994 17,843 30,247 51,490 | Based on ICD coding at discharge | 1740 1874 2015 2157 2255 | Not reported | 1.8% 0.9% 0.4% 0.2% 0.1% | 2.7% 1.5% 1.3% 1.3% 0.4% |
| Valcamonico [42] | 32+0–34+6 | 10 | As infants < 1000 g—based on preterm growth charts would be <3rd centile | 10 | 28.9% | - | 10% |
| Baschat [46] | 32+0–32+6 | 76 | AC < 5th centile + elevated UAPI | 76 | 15.2% | - | 1.3% |
| Rocha [38] | 34+0–36+6 | 86 | <10th centile | 50 | 7% | 0% | 12% |
| Marsoosi [35] | 32+0–33+6 34+0–35+6 | 6 8 | EFW + AC < 10th percentile + UAPI + UARI > 2 SD | 6 8 | 17.1% | - | 33% 12.5% |
| Ballardini [45] | 33+0–36+6 | 724 | <10th Centile <3rd Centile | 189 31 | 13% | - | 12.6% 19.4% |
| Tul [40] | 24+0–36+6 | 7662 | <10th centile based on local birth weight data | 734 | 3.9% | 1.37% | 2.5% |
| Stimac [43] | 33+036+6 | 158 | <5th centile based on local birth weight data | 158 | 3.8% | - | 5.1% |
| Medina-Alva [37] | 32+0–36+6 | 175 | <10th centile | 84 | 7.7% | 5.9% |
| Berger, 1997 [32] | IVH |
| Gilbert, 2003 [34] | IVH |
| Valcamonico, 2007 [42] | IVH (classification by Volpe) PVL (classification by Pierrat) |
| Baschat, 2007 [46] | Grade III/IV IVH |
| Rocha, 2010 [38] | IVH |
| Marsoosi, 2012 [35] | IVH |
| Ballardini, 2014 [45] | IVH (classification by Volpe) PVL (classification by De Vries) Agenesis of corpus callosum Other haemorrhages Enlarged cisterna magna Hyperechogenicity of thalami Other major anomalies of the brain |
| Tul, 2015 [40] | IVH |
| Medina-Alva, 2019 [37] | Brain infarction Parenchymal haemorrhage Grade III/IV IVH Post-haemorrhagic hydrocephalus with ventricular index > 14mm PVL/or periventricular cysts |
| Stimac, 2019 [43] | IVH |
| Author, Year | Gestation | Study Total | Study Design | Aims | Key Findings of Study | Relevant Findings Related to FGR or SGA |
|---|---|---|---|---|---|---|
| Berger, 1997 [32] | 24+0–43+0 weeks | 5286 | Prospective cohort study | To examine the incidence of brain injury on cranial ultrasound compared with obstetric risk factors | The most frequent abnormality was IVH, with the incidence increasing with decreasing gestational age | 1.7% (n = 2) of 115 infants born < 10th Centile at 38–43 weeks were documented to have IVH |
| Mercuri, 1998 [33] | 36+0–42+0 weeks | 177 | Prospective cohort study | To evaluate cranial ultrasounds and neurological examinations in ‘normal’ neonates and correlate with perinatal factors | Ultrasound abnormalities were present in 20% (n = 35) of the infants studied The most common finding were ischaemic lesions (periventricular and thalamic densities) seen in 8% (n = 15); IVH in 5% (n = 9) of infants | 7 of the 10 infants that were FGR had abnormal ultrasound findings (specific findings not listed) |
| Gilbert, 2003 [34] | 26+0–41+0 weeks | 1,347,788 | Retrospective cohort study | To examine incidence of FGR and associated neonatal outcomes | Until 28 weeks, prematurity associated with adverse neonatal outcomes (RDS, IVH, NEC, CHA) was largely unaffected by FGR | Of the FGR Infants born between 34–39 weeks there was an increased rate of IVH compared to their well grown counterparts |
| Marsoosi, 2012 [35] | 23+0–40+6 weeks | 41 | Prospective cohort study | To determine if there is a correlation between Doppler indices, IVH and perinatal mortality in pregnancies affected by FGR | Infants with AREDF had a 5 times greater chance of developing IVH The risk of IVH was associated with gestational age at delivery, birth weight, and acidosis | Of the 13 infants born ≥ 36 weeks, no infants developed IVH |
| Starcevic, 2016 [36] | ≥37 weeks | 60 | Retrospective cohort study | To ascertain if infants born at term, diagnosed with late-onset FGR, have CUAs and abnormal neurological examinations and to assess predictive values of umbilical cord gases and umbilical Doppler indices | Abnormal umbilical Doppler indices were more predictive than cord blood gases of neurological outcomes, with C/U being most predictive | 53.37% (n = 32) of infants had had CUAs detected 38.33% (n = 23) had IVH, and 15.0% (n = 9) had PVL |
| Krishnamurthy, 2017 [44] | 35+0–43+0 weeks | 415 | Retrospective cohort study | To determine frequency of cranial US screening and incidence of abnormal ultrasonography in SGA infants | No significant increased yield when screening <3rd vs. < 10th centile infants. The majority of infants had positive minor findings (e.g., grade 1–2 IVH) The rest of the infants with abnormal cranial ultrasounds were known antenatal findings or those with significant risk factors (e.g., HIE) | 12.8% of infants with a birth weight < 10th centile had CUAs and 11% of infants with a birth weight < 3rd centile had CUAs (no control group) |
| Stimac, 2019 [43] | 23+0–>37 weeks | 2676 | Retrospective cohort study | To examine the effect on neonatal outcomes of gender in SGA infants | Both female and male infants had similar rates of RDS, IVH and admission to intensive care | The rate of IVH documented in SGA infants born > 37 weeks was 0.5% |
| Turcan, 2020 [39] | 230–410 weeks | 1405 | Retrospective cohort study | To compare the rate of short-term complications of preterm infants born with and without low birth weight and term low birth weight infants | Infants in the preterm SGA cohort had the highest frequency of neonatal complications | Subgroup analysis of 206 infants born with ‘low birth weight’ (< 2SD from curve for gestation) at 37–41 weeks showed 0.4% (n = 1) had cerebral haemorrhage and 1.4% (n = 3) had IVH |
| First Author | Subgroup Gestation | Subgroup Total | Definition of FGR/SGA Infants | Number of FGR/SGA Infants | Study Population Incidence of CUAs | CUAs Incidence in Non FGR/SGA Infants | FGR/SGA Gestation Incidence of CUAs |
|---|---|---|---|---|---|---|---|
| Berger [32] | 38+0–43+0 | 4403 | < 10th Centile | 115 | 3.6% | 1.6% | 1.7% |
| Mercuri [33] | 36+0–42+0 | 177 | As documented in patient clinical notes | 10 | 19.7% | - | 70% |
| Gilbert [34] | 37+0–37+6 38+0–38+6 39+0–39+6 40+0–40+6 41+0–41+6 | 106,939 220,170 351,279 340,887 202,058 | Based on ICD coding at discharge | 2386 2498 2598 2679 2694 | Not reported | 0.1% 0% 0% 0% 0% | 0.5% 0.2% 0.1% 0.1% 0.1% |
| Marsoosi [35] | 36+0–40+6 | 12 | EFW + AC < 10th percentile + UAPI + UARI > 2 SD | 12 | 17.1% | - | 0% |
| Starcevic [36] | ≥37+0 | - | EFW < 10th centile + elevated UARI | 60 | 53.4% | - | 53.4% |
| Krishnamurthy [44] | 35+0–43+0 | 415 | <10th centile <3rd centile | 201 214 | 12.8% 11% | - | 12.8% 11% |
| Stimac [43] | >37 | 2363 | <5th centile based on local birth weight data | 2363 | 3.8% | - | 0.5% |
| Turcan [39] | 370–410 | 206 | <2 SD for weight | 206 | 10% | - | 1% |
| Berger, 1997 [32] | IVH |
| Mercuri, 1998 [33] | Periventricular densities Unilateral thalamic densities Focal asymmetrical ventricular dilatation, paramedian or choroid cysts. IVH (classification by de Vries) White matter haemorrhagic changes Periventricular densities Full choroid |
| Gilbert, 2003 [34] | IVH |
| Marsoosi, 2012 [35] | IVH |
| Starcevic, 2016 [36] | PVL (classification by Pidcock) IVH (classification by Volpe) |
| Krishnamurthy, 2017 [44] | Cysts: in the caudothalamic groove, sub-ependymal, interhemispheric, or posterior fossa IVH (Grade I/II) Echogenicity in periventricular area (PVE) or basal ganglia Agenesis or dysgenesis of corpus callosum Mild ventricular dilatation Hydrocephalus Arnold Chiari malformation Cerebellar hypoplasia Colpocephaly |
| Turcan, 2020 [39] | IVH |
| Stimac, 2019 [43] | IVH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roufaeil, C.; Razak, A.; Malhotra, A. Cranial Ultrasound Abnormalities in Small for Gestational Age or Growth-Restricted Infants Born over 32 Weeks Gestation: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 1713. https://doi.org/10.3390/brainsci12121713
Roufaeil C, Razak A, Malhotra A. Cranial Ultrasound Abnormalities in Small for Gestational Age or Growth-Restricted Infants Born over 32 Weeks Gestation: A Systematic Review and Meta-Analysis. Brain Sciences. 2022; 12(12):1713. https://doi.org/10.3390/brainsci12121713
Chicago/Turabian StyleRoufaeil, Charlene, Abdul Razak, and Atul Malhotra. 2022. "Cranial Ultrasound Abnormalities in Small for Gestational Age or Growth-Restricted Infants Born over 32 Weeks Gestation: A Systematic Review and Meta-Analysis" Brain Sciences 12, no. 12: 1713. https://doi.org/10.3390/brainsci12121713
APA StyleRoufaeil, C., Razak, A., & Malhotra, A. (2022). Cranial Ultrasound Abnormalities in Small for Gestational Age or Growth-Restricted Infants Born over 32 Weeks Gestation: A Systematic Review and Meta-Analysis. Brain Sciences, 12(12), 1713. https://doi.org/10.3390/brainsci12121713







