Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Psychometric Questionnaires
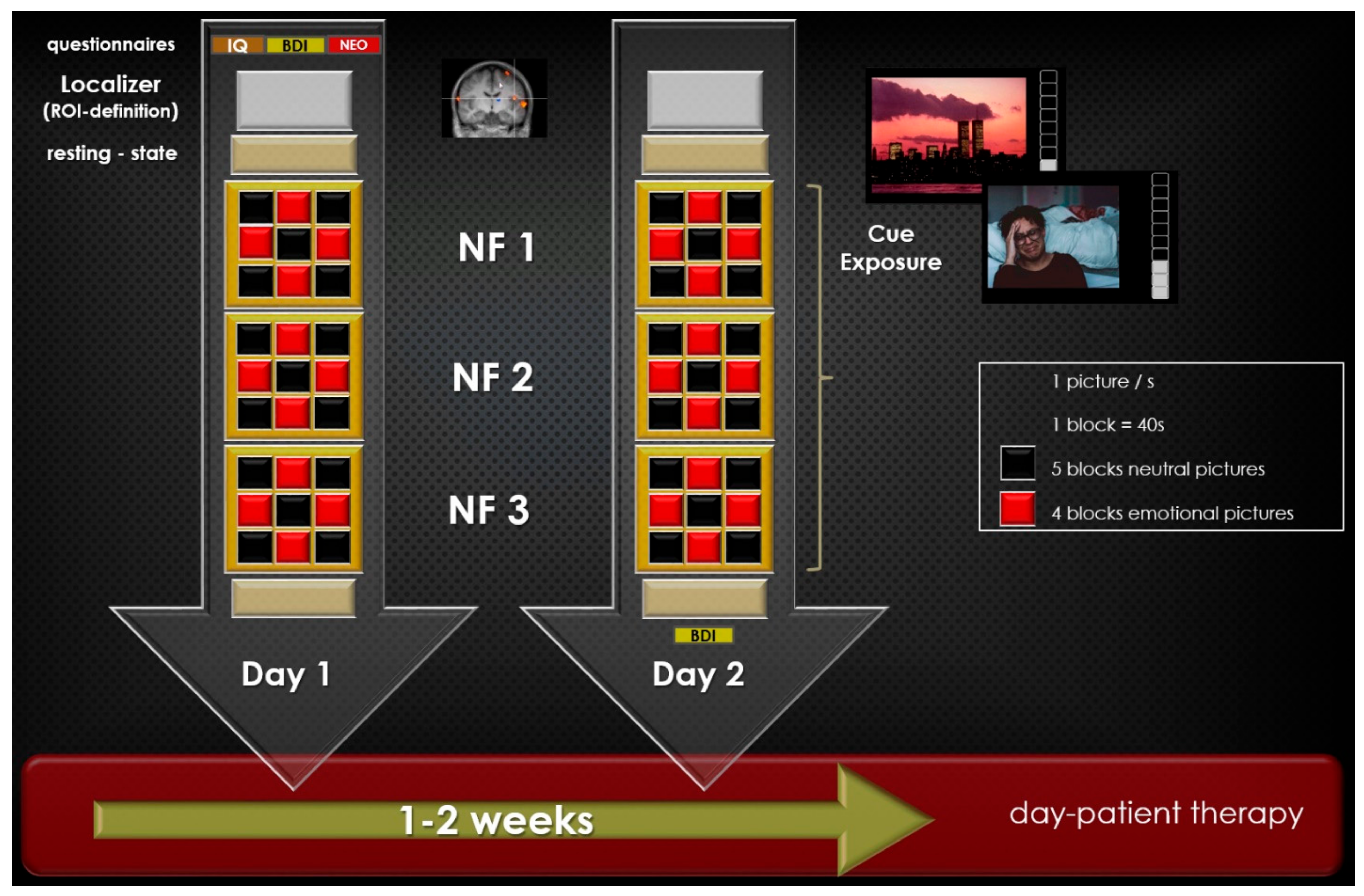
2.3. Paradigm
2.4. MRI and fMRI Data Acquisition
2.5. MRI and fMRI Data Pre- and Post-Processing
2.6. Statistical Analysis of Psychometric Data
3. Results
3.1. Comparison of Psychometric Data between HC REAL and MDD REAL
3.2. Correlations
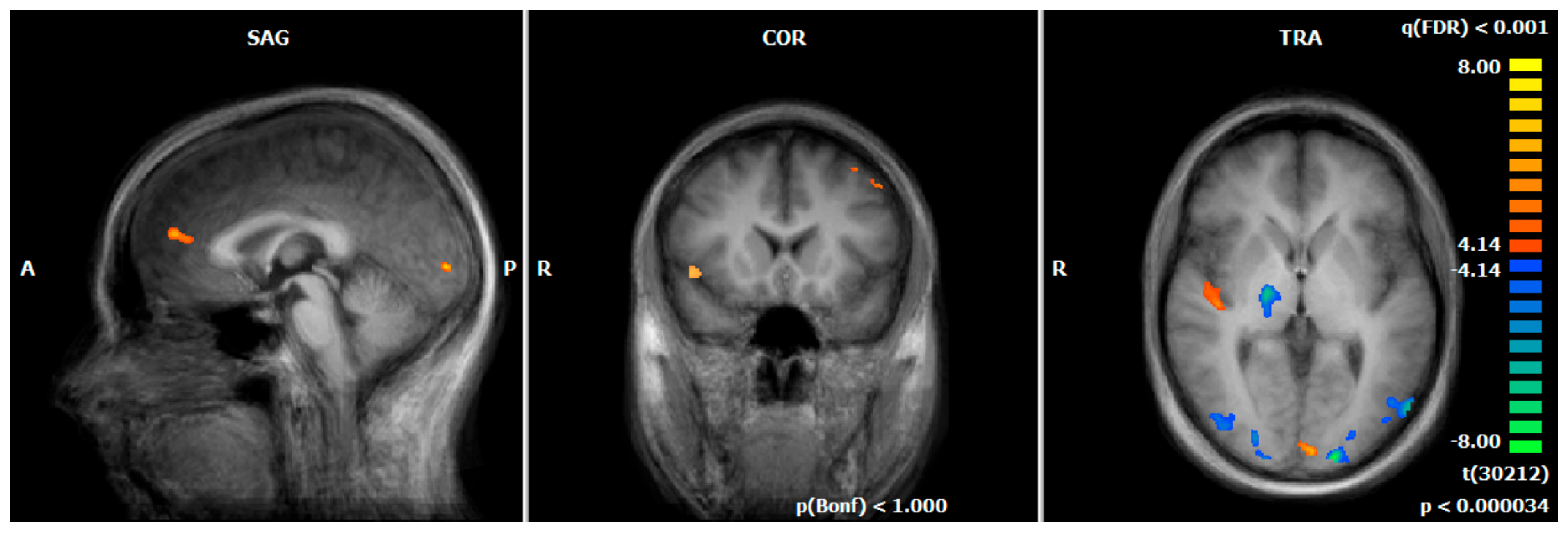
3.3. Hemodynamic Responses during the Emotion-Associated Task during the Functional Localizer on Day One: MDD REAL vs. HC REAL
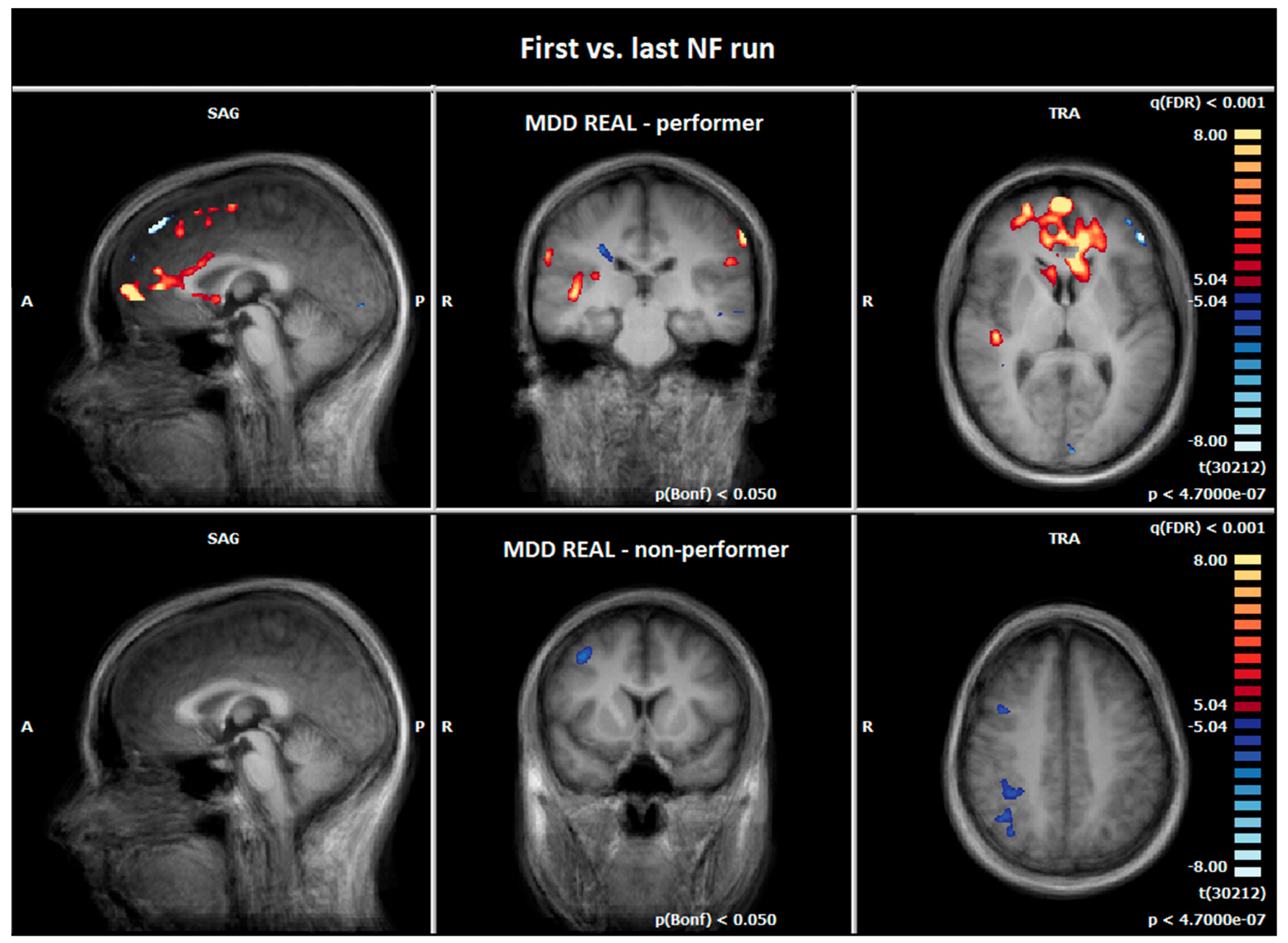
3.4. Comparison of Hemodynamic Responses between the First NF Run on Day One and the Last NF Run on Day Two: MDD REAL and HC REAL
3.5. Comparison of Hemodynamic Responses between the First and the Last NF Run: MDD REAL Responder vs. MDD REAL Non-Responder
3.6. Comparison of Hemodynamic Responses between the MDD REAL and HC REAL: First vs. Last NF Run
4. Discussion
4.1. Clinical Outcome of Psychometric Data
4.2. Functional Imaging Data
4.2.1. Comparison of Hemodynamic Responses of MDD REAL and HC REAL Group during the Emotion-Associated Task before the NF Training
4.2.2. Comparison of Hemodynamic Responses between the First and the Last NF Run
4.2.3. Comparison of Hemodynamic Responses between the MDD REAL Responder and MDD REAL Non-Responder: First vs. Last NF Run
4.2.4. Comparison of Hemodynamic Responses between the MDD REAL and HC REAL: First vs. Last NF Run
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassano, P.; Fava, M. Depression and public health: An overview. J. Psychosom. Res. 2002, 53, 849–857. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuijpers, P.; van Straten, A.; Schuurmans, J.; van Oppen, P.; Hollon, S.D.; Andersson, G. Psychotherapy for chronic major depression and dysthymia: A meta-analysis. Clin. Psychol. Rev. 2010, 30, 51–62. [Google Scholar] [CrossRef]
- Imel, Z.E.; Malterer, M.B.; McKay, K.M.; Wampold, B.E. A meta-analysis of psychotherapy and medication in unipolar depression and dysthymia. J. Affect Disord. 2008, 110, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Von Wolff, A.; Holzel, L.P.; Westphal, A.; Harter, M.; Kriston, L. Combination of pharmacotherapy and psychotherapy in the treatment of chronic depression: A systematic review and meta-analysis. BMC Psychiatry 2012, 12, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, I.; Szpindel, I.; Katzman, M.A. Pharmacological approaches to manage persistent symptoms of major depressive disorder: Rationale and therapeutic strategies. Psychiatry Res. 2014, 220 (Suppl 1), S15–S33. [Google Scholar] [CrossRef]
- Machmutow, K.; Meister, R.; Jansen, A.; Kriston, L.; Watzke, B.; Harter, M.C.; Liebherz, S. Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults. Cochrane Database Syst. Rev. 2019, 5, CD012855. [Google Scholar] [CrossRef]
- Vittengl, J.R.; Clark, L.A.; Jarrett, R.B. Continuation-phase cognitive therapy’s effects on remission and recovery from depression. J. Consult Clin. Psychol. 2009, 77, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Nutt, D.; Demyttenaere, K.; Janka, Z.; Aarre, T.; Bourin, M.; Canonico, P.L.; Carrasco, J.L.; Stahl, S. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. J. Psychopharmacol. 2007, 21, 461–471. [Google Scholar] [CrossRef]
- Schmaal, L.; Hibar, D.P.; Samann, P.G.; Hall, G.B.; Baune, B.T.; Jahanshad, N.; Cheung, J.W.; van Erp, T.G.M.; Bos, D.; Ikram, M.A.; et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 2017, 22, 900–909. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Goodkind, M.; Eickhoff, S.B.; Oathes, D.J.; Jiang, Y.; Chang, A.; Jones-Hagata, L.B.; Ortega, B.N.; Zaiko, Y.V.; Roach, E.L.; Korgaonkar, M.S. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015, 72, 305–315. [Google Scholar] [CrossRef]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.P.; Müller, V.I.; Eickhoff, S.B.; Fox, P.T. Multimodal abnormalities of brain structure and function in major depressive disorder: A meta-analysis of neuroimaging studies. Am. J. Psychiatry 2020, 177, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Wager, T.D.; Mechelli, A.; He, Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry 2019, 85, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Janiri, D.; Moser, D.A.; Doucet, G.E.; Luber, M.J.; Rasgon, A.; Lee, W.H.; Murrough, J.W.; Sani, G.; Eickhoff, S.B.; Frangou, S. Shared neural phenotypes for mood and anxiety disorders: A meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020, 77, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Linn, K.A.; Shinohara, R.T.; Oathes, D.J.; Cook, P.A.; Duprat, R.; Moore, T.M.; Oquendo, M.A.; Phillips, M.L.; McInnis, M. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc. Natl. Acad. Sci. USA 2019, 116, 8582–8590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86. [Google Scholar] [CrossRef] [Green Version]
- Bray, S.; Shimojo, S.; O’Doherty, J.P. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J. Neurosci. 2007, 27, 7498–7507. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Young, K.D.; Phillips, R.; Zotev, V.; Misaki, M.; Bodurka, J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014, 4, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, S.J.; Boehm, S.G.; Healy, D.; Goebel, R.; Linden, D.E. Neurofeedback: A promising tool for the self-regulation of emotion networks. Neuroimage 2010, 49, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Posse, S.; Fitzgerald, D.; Gao, K.; Habel, U.; Rosenberg, D.; Moore, G.J.; Schneider, F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage 2003, 18, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Caria, A.; Veit, R.; Sitaram, R.; Lotze, M.; Weiskopf, N.; Grodd, W.; Birbaumer, N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage 2007, 35, 1238–1246. [Google Scholar] [CrossRef]
- Lawrence, E.J.; Su, L.; Barker, G.J.; Medford, N.; Dalton, J.; Williams, S.C.; Brammer, M. Self-regulation of the anterior insula: Reinforcement learning using real-time fMRI neurofeedback. Neuroimage 2014, 88, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Glover, G.H.; Hsu, J.J.; Johnson, R.F.; Gotlib, I.H. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum. Brain Mapp. 2011, 32, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Karch, S.; Paolini, M.; Gschwendtner, S.; Jeanty, H.; Reckenfelderbäumer, A.; Yaseen, O.; Maywald, M.K.U.; Fuchs, C.; Rauchmann, B.-S.; Chrobok, A. Real-time fMRI neurofeedback in patients with tobacco use disorder during smoking cessation: Functional differences and implications of the first training session in regard to future abstinence or relapse. Front. Hum. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Karch, S.; Keeser, D.; Hümmer, S.; Paolini, M.; Kirsch, V.; Karali, T.; Kupka, M.; Rauchmann, B.-S.; Chrobok, A.; Blautzik, J. Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS One 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Hampson, M.; Stoica, T.; Saksa, J.; Scheinost, D.; Qiu, M.; Bhawnani, J.; Pittenger, C.; Papademetris, X.; Constable, T. Real-time fMRI biofeedback targeting the orbitofrontal cortex for contamination anxiety. JoVE (J. Vis. Exp.) 2012, e3535. [Google Scholar] [CrossRef] [Green Version]
- Zilverstand, A.; Sorger, B.; Sarkheil, P.; Goebel, R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front. Behav. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef]
- Zhao, Z.; Yao, S.; Li, K.; Sindermann, C.; Zhou, F.; Zhao, W.; Li, J.; Lührs, M.; Goebel, R.; Kendrick, K.M. Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychother. Psychosom. 2019, 88, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PloS ONE 2014, 9, e88785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, D.E.; Habes, I.; Johnston, S.J.; Linden, S.; Tatineni, R.; Subramanian, L.; Sorger, B.; Healy, D.; Goebel, R. Real-time self-regulation of emotion networks in patients with depression. PloS ONE 2012, 7, e38115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamura, M.; Okamoto, Y.; Shibasaki, C.; Yoshino, A.; Okada, G.; Ichikawa, N.; Yamawaki, S. Antidepressive effect of left dorsolateral prefrontal cortex neurofeedback in patients with major depressive disorder: A preliminary report. J. Affect. Disord. 2020, 271, 224–227. [Google Scholar] [CrossRef]
- Young, K.D.; Siegle, G.J.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. Am. J. Psychiatry 2017, 174, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehler, D.M.; Sokunbi, M.O.; Habes, I.; Barawi, K.; Subramanian, L.; Range, M.; Evans, J.; Hood, K.; Lührs, M.; Keedwell, P. Targeting the affective brain—a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology 2018, 43, 2578–2585. [Google Scholar] [CrossRef]
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Drevets, W.C.; Bodurka, J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin. Neurosci. 2018, 72, 466–481. [Google Scholar] [CrossRef] [Green Version]
- Ahrweiler, N.; Santana-Gonzalez, C.; Zhang, N.; Quandt, G.; Ashtiani, N.; Liu, G.; Engstrom, M.; Schultz, E.; Liengswangwong, R.; Teoh, J.Y. Neural Activity Associated with Symptoms Change in Depressed Adolescents following Self-Processing Neurofeedback. Brain Sci. 2022, 12, 1128. [Google Scholar] [CrossRef]
- Fede, S.J.; Dean, S.F.; Manuweera, T.; Momenan, R. A Guide to Literature Informed Decisions in the Design of Real Time fMRI Neurofeedback Studies: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-Y.; Yoo, S.-S.; Tegethoff, M.; Meinlschmidt, G.; Lee, J.-H. The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. . J. Cogn. Neurosci. 2015, 27, 1552–1572. [Google Scholar] [CrossRef]
- Kandilarova, S.; Stoyanov, D.; Kostianev, S.; Specht, K. Altered resting state effective connectivity of anterior insula in depression. Front. Psychiatry 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Manoliu, A.; Meng, C.; Brandl, F.; Doll, A.; Tahmasian, M.; Scherr, M.; Schwerthöffer, D.; Zimmer, C.; Förstl, H.; Bäuml, J. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2014, 7, 930. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L. The human frontal lobe: An introduction. In The Human Frontal Lobes: Functions and Disorders; The Guilford Press: New York, NY, USA, 2007. [Google Scholar]
- Smith, E.E.; Jonides, J. Storage and executive processes in the frontal lobes. Science 1999, 283, 1657–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompus, K.; Hugdahl, K.; Öhman, A.; Marklund, P.; Nyberg, L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci. Lett. 2009, 467, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Eippert, F.; Veit, R.; Weiskopf, N.; Erb, M.; Birbaumer, N.; Anders, S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007, 28, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Lévesque, J.; Eugene, F.; Joanette, Y.; Paquette, V.; Mensour, B.; Beaudoin, G.; Leroux, J.-M.; Bourgouin, P.; Beauregard, M. Neural circuitry underlying voluntary suppression of sadness. Biol. Psychiatry 2003, 53, 502–510. [Google Scholar] [CrossRef]
- Reinecke, A.; Thilo, K.; Filippini, N.; Croft, A.; Harmer, C.J. Predicting rapid response to cognitive-behavioural treatment for panic disorder: The role of hippocampus, insula, and dorsolateral prefrontal cortex. Behav. Res. Ther. 2014, 62, 120–128. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef]
- Siegle, G.J.; Thompson, W.; Carter, C.S.; Steinhauer, S.R.; Thase, M.E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol. Psychiatry 2007, 61, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Opel, N.; Redlich, R.; Grotegerd, D.; Dohm, K.; Zaremba, D.; Meinert, S.; Bürger, C.; Plümpe, L.; Alferink, J.; Heindel, W. Prefrontal brain responsiveness to negative stimuli distinguishes familial risk for major depression from acute disorder. J. Psychiatry Neurosci. JPN 2017, 42, 343. [Google Scholar] [CrossRef] [Green Version]
- Stratmann, M.; Konrad, C.; Kugel, H.; Krug, A.; Schöning, S.; Ohrmann, P.; Uhlmann, C.; Postert, C.; Suslow, T.; Heindel, W. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PloS One 2014, 9, e102692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprengelmeyer, R.; Steele, J.D.; Mwangi, B.; Kumar, P.; Christmas, D.; Milders, M.; Matthews, K. The insular cortex and the neuroanatomy of major depression. J. Affect. Disord. 2011, 133, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Lockwood, P.L. The anatomy of empathy: Vicarious experience and disorders of social cognition. Behav. Brain Res. 2016, 311, 255–266. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Etkin, A.; Furman, D.J.; Lemus, M.G.; Johnson, R.F.; Gotlib, I.H. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry 2012, 169, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Cooney, R.E.; Joormann, J.; Eugène, F.; Dennis, E.L.; Gotlib, I.H. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci. 2010, 10, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Sliz, D.; Hayley, S. Major depressive disorder and alterations in insular cortical activity: A review of current functional magnetic imaging research. Front. Hum. Neurosci. 2012, 6, 323. [Google Scholar] [CrossRef]
- Delaveau, P.; Jabourian, M.; Lemogne, C.; Guionnet, S.; Bergouignan, L.; Fossati, P. Brain effects of antidepressants in major depression: A meta-analysis of emotional processing studies. J. Affect. Disord. 2011, 130, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-T.; Cho, S.W.; Khang, H.S.; Lee, B.-C.; Choi, I.-G.; Lyoo, I.K.; Ham, B.-J. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1487–1492. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory; Harcourt Brace Jovanovich: New York, NY, USA, 1987. [Google Scholar]
- Schmidt, K.; Metzler, P. WST-Wortschatztest; Beltz Test: Göttingen, Germany, 1992. [Google Scholar]
- Costa, P.T.; McCrae, R.R. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol. Assess. 1992, 4, 5. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain Innovation. Turbo-BrainVoyager. Available online: https://www.brainvoyager.com/TurboBrainVoyager.html (accessed on 1 September 2022).
- Ulmer, S. Neuroanatomy and cortical landmarks. In fMRI; Springer: Berlin/Heidelberg, Germany, 2010; pp. 5–13. [Google Scholar]
- Young, K.D.; Misaki, M.; Harmer, C.J.; Victor, T.; Zotev, V.; Phillips, R.; Siegle, G.J.; Drevets, W.C.; Bodurka, J. Real-time functional magnetic resonance imaging amygdala neurofeedback changes positive information processing in major depressive disorder. Biol. Psychiatry 2017, 82, 578–586. [Google Scholar] [CrossRef]
- Rosellini, A.J.; Brown, T.A. The NEO Five-Factor Inventory: Latent structure and relationships with dimensions of anxiety and depressive disorders in a large clinical sample. Assessment 2011, 18, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koorevaar, A.; Comijs, H.; Dhondt, A.; Van Marwijk, H.; Van Der Mast, R.; Naarding, P.; Voshaar, R.O.; Stek, M. Big Five personality and depression diagnosis, severity and age of onset in older adults. J. Affect. Disord. 2013, 151, 178–185. [Google Scholar] [CrossRef]
- Matthews, S.C.; Strigo, I.A.; Simmons, A.N.; Yang, T.T.; Paulus, M.P. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord. 2008, 111, 13–20. [Google Scholar] [CrossRef]
- Öngür, D.; Price, J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 2000, 10, 206–219. [Google Scholar] [CrossRef]
- Öngür, D.; Ferry, A.T.; Price, J.L. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003, 460, 425–449. [Google Scholar] [CrossRef]
- Milad, M.R.; Wright, C.I.; Orr, S.P.; Pitman, R.K.; Quirk, G.J.; Rauch, S.L. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 2007, 62, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Quirk, G.J.; Garcia, R.; González-Lima, F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry 2006, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sotres-Bayon, F.; Quirk, G.J. Prefrontal control of fear: More than just extinction. Curr. Opin. Neurobiol. 2010, 20, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayberg, H.S.; Brannan, S.K.; Tekell, J.L.; Silva, J.A.; Mahurin, R.K.; McGinnis, S.; Jerabek, P.A. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol. Psychiatry 2000, 48, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Keedwell, P.; Drapier, D.; Surguladze, S.; Giampietro, V.; Brammer, M.; Phillips, M. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: Subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J. Psychopharmacol. 2009, 23, 775–788. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Adolphs, R.; Oya, H.; Kovach, C.; Damasio, H.; Kaufman, O.; Howard Iii, M. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. J. Cogn. Neurosci. 2005, 17, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Zald, D.H.; Mattson, D.L.; Pardo, J.V. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc. Natl. Acad. Sci. 2002, 99, 2450–2454. [Google Scholar] [CrossRef] [Green Version]
- Maunsell, J.H.; Treue, S. Feature-based attention in visual cortex. Trends Neurosci. 2006, 29, 317–322. [Google Scholar] [CrossRef]
- Engel, S.A.; Rumelhart, D.E.; Wandell, B.A.; Lee, A.T.; Glover, G.H.; Chichilnisky, E.-J.; Shadlen, M.N. fMRI of human visual cortex. Nature 1994, 326, 525. [Google Scholar] [CrossRef] [PubMed]
- Shobe, E.R. Independent and collaborative contributions of the cerebral hemispheres to emotional processing. Front. Hum. Neurosci. 2014, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Lemke, H.; Klute, H.; Skupski, J.; Thiel, K.; Waltemate, L.; Winter, A.; Breuer, F.; Meinert, S.; Klug, M.; Enneking, V.; et al. Brain structural correlates of recurrence following the first episode in patients with major depressive disorder. Transl Psychiatry 2022, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Zhu, H.; Zhao, Y.; Wu, M.; Kuang, W.; Bi, F.; Kemp, G.J.; Gong, Q. Characterization of brain blood flow and the amplitude of low-frequency fluctuations in major depressive disorder- a multimodal meta-analysis. J. Affect. Disord. 2017, 210, 303–311. [Google Scholar] [CrossRef]
- Thibault, R.T.; MacPherson, A.; Lifshitz, M.; Roth, R.R.; Raz, A.J.N. Neurofeedback with fMRI: A critical systematic review. Neuroimage 2018, 172, 786–807. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, S.; Okamoto, Y.; Onoda, K.; Matsunaga, M.; Ueda, K.; Suzuki, S.-i. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J. Affect. Disord. 2010, 122, 76–85. [Google Scholar] [CrossRef]
- Phelps, E.A.; Delgado, M.R.; Nearing, K.I.; LeDoux, J.E. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 2004, 43, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, S.; Price, J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995, 363, 615–641. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef]
- Cavada, C.; Compañy, T.; Tejedor, J.; Cruz-Rizzolo, R.J.; Reinoso-Suárez, F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 2000, 10, 220–242. [Google Scholar] [CrossRef]
- Beauregard, M. Functional neuroimaging studies of the effects of psychotherapy. Dialogues Clin. Neurosci. 2014, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, A.L.; Hagland, P.; Radua, J.; Mataix-Cols, D.; Kvale, G.; Hansen, B.; van den Heuvel, O.A. Emotional processing in obsessive-compulsive disorder: A systematic review and meta-analysis of 25 functional neuroimaging studies. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 563–571. [Google Scholar] [CrossRef]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Horwitz, B.; Herscovitch, P.; Post, R.M. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 1995, 152, 341–351. [Google Scholar] [PubMed]
- Schienle, A.; Schäfer, A.; Stark, R.; Vaitl, D. Long-term effects of cognitive behavior therapy on brain activation in spider phobia. Psychiatry Res. Neuroimaging 2009, 172, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. A non-reward attractor theory of depression. Neurosci. Biobehav. Rev. 2016, 68, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshel, N.; Roiser, J.P. Reward and punishment processing in depression. Biol. Psychiatry 2010, 68, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Whitton, A.E.; Treadway, M.T.; Pizzagalli, D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 2015, 28, 7. [Google Scholar] [CrossRef] [Green Version]
- Schutter, D.J.; van Honk, J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. J. Psychiatry Neurosci. 2006, 31, 101. [Google Scholar]
- Harmer, C.J.; Hill, S.A.; Taylor, M.J.; Cowen, P.J.; Goodwin, G.M. Toward a neuropsychological theory of antidepressant drug action: Increase in positive emotional bias after potentiation of norepinephrine activity. Am. J. Psychiatry 2003, 160, 990–992. [Google Scholar] [CrossRef] [Green Version]
- Lewinsohn, P.M. A behavioral approach to depression. In Essential Papers on Depression; NYU Press: New York, NY, USA, 1974; pp. 150–172. [Google Scholar]
- Brody, A.L.; Saxena, S.; Mandelkern, M.A.; Fairbanks, L.A.; Ho, M.L.; Baxter, L.R., Jr. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 2001, 50, 171–178. [Google Scholar] [CrossRef]
- Sherwood, M.S.; Kane, J.H.; Weisend, M.P.; Parker, J.G. Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage 2016, 124, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Müller, J.L.; Sommer, M.; Klein, H.E.; Hajak, G. Changes in the emotional processing in depressive patients: A study with functional magnetoresonance tomography under the employment of pictures with affective contents. Psychiatr. Prax. 2004, 31, S70–S72. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; McRae, K.; Ramel, W.; Gross, J.J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol. Psychiatry 2008, 63, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayberg, H.S. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 2003, 65, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M. The prefrontal–limbic network in depression: Modulation by hypothalamus, basal ganglia and midbrain. Prog. Neurobiol. 2011, 93, 468–487. [Google Scholar] [CrossRef]
- Zeng, L.-L.; Shen, H.; Liu, L.; Wang, L.; Li, B.; Fang, P.; Zhou, Z.; Li, Y.; Hu, D. Identifying major depression using whole-brain functional connectivity: A multivariate pattern analysis. Brain 2012, 135, 1498–1507. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.M.; Schultz, A.P.; Huijbers, W.; Van Dijk, K.R.; Hedden, T.; Sperling, R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2014, 35, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Wessa, M.; Lois, G. Brain functional effects of psychopharmacological treatment in major depression: A focus on neural circuitry of affective processing. Curr. Neuropharmacol. 2015, 13, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Delplanque, S.; N’diaye, K.; Scherer, K.; Grandjean, D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J. Neurosci. Methods 2007, 165, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.; Cahill, L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage 2003, 20, 2091–2099. [Google Scholar] [CrossRef]
- Fu, C.H.; Williams, S.C.; Cleare, A.J.; Scott, J.; Mitterschiffthaler, M.T.; Walsh, N.D.; Donaldson, C.; Suckling, J.; Andrew, C.; Steiner, H. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry 2008, 64, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hägele, C.; Friedel, E.; Schlagenhauf, F.; Sterzer, P.; Beck, A.; Bermpohl, F.; Stoy, M.; Held-Poschardt, D.; Wittmann, A.; Ströhle, A. Affective responses across psychiatric disorders—A dimensional approach. Neurosci. Lett. 2016, 623, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Huang, F.; Zhou, Y.; Zhuang, L.; Xu, J.; Gao, C.; Qin, S.; Luo, J. The function of the hippocampus and middle temporal gyrus in forming new associations and concepts during the processing of novelty and usefulness features in creative designs. Neuroimage 2020, 214, 116751. [Google Scholar] [CrossRef] [PubMed]


| MDD REAL (N = 16) | HC REAL (N = 19) | |
|---|---|---|
| Age at entry, M (SD) | 33.13 (12.36) | 24.35 (3.06) |
| male, n (%) | 5 (31.3) | 9 (47.4) |
| female, n (%) | 11 (68.8) | 10 (52.6) |
| Medication 1, n (%) | ||
| SSRI | 5 (31.3) | |
| SSNRI | 3 (18.8) | |
| TCA | 1 (6.3) | |
| Medication 2, n (%) | ||
| TeCA | 1 (6.3) | |
| atypical AP | 1 (6.3) | |
| Questionnaire | HC REAL | MDD REAL | p-Value | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| IQ-Test (WST) | 113.47 | 6.92 | 107.13 | 11.87 | 0.080 |
| NEO-FFI-N | 19.00 | 6.57 | 33.80 | 6.44 | ≤0.001 |
| NEO-FFI-E | 29.74 | 6.70 | 21.13 | 8.50 | 0.002 * |
| NEO-FFI-N | 31.16 | 7.05 | 30.93 | 5.30 | 0.919 |
| NEO-FFI-A | 34.04 | 6.14 | 32.07 | 6.39 | 0.364 |
| NEO-FFI-C | 31.21 | 6.61 | 26.73 | 8.80 | 0.100 |
| BDI | 1.84 | 1.68 | 23.33 | 1.68 | ≤0.001 * |
| ROI/ Questionnaire | dlPFC | Insula | Thalamus | Hippocampus | Amygdala | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| NEO-FFI-N | 0.32 | 0.24 | 0.55 | 0.04 * | 0.10 | 0.71 | 0.00 | 1.00 | 0.22 | 0.39 |
| NEO-FFI-E | −0.24 | 0.39 | −0.15 | 0.60 | −0.37 | 0.21 | −0.29 | 0.29 | −0.40 | 0.14 |
| NEO-FFI-O | −0.15 | 0.60 | 0.29 | 0.29 | 0.11 | 0.71 | −0.14 | 0.60 | −0.14 | 0.60 |
| NEO-FFI-A | −0.03 | 0.91 | −0.41 | 0.13 | 0.04 | 0.90 | 0.26 | 0.34 | 0.03 | 0.92 |
| NEO-FFI-C | −0.55 | 0.04 * | −0.40 | 0.14 | −0.40 | 0.17 | −0.31 | 0.25 | −0.31 | 0.25 |
| BDI-pre | 0.57 | 0.04 * | 0.37 | 0.17 | −0.14 | 0.62 | −0.11 | 0.68 | 0.11 | 0.68 |
| BDI-post | 0.05 | 0.89 | −0.03 | 0.93 | 0.11 | 0.74 | 0.14 | 0.68 | −0.30 | 0.36 |
| Questionnaire | Sex | Age | ||
|---|---|---|---|---|
| r | p | r | p | |
| BDI-pre | 0.14 | 0.70 | −0.20 | 0.54 |
| BDI-post | 0.37 | 0.36 | −0.20 | 0.57 |
| Centre of Gravity | Size | T-Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| HC REAL > MDD REAL | ||||||||
| Middle Frontal Gyrus | L | 6 | −28 | −11 | 47 | 577 | 4.80 | 5.92 |
| Inferior Frontal Gyrus | R | 46 | 37 | 33 | 13 | 611 | 4.82 | 6.97 |
| Precentral Gyrus | L | 6 | −44 | 2 | 33 | 1118 | 4.77 | 5.99 |
| Thalamus | R | − | 16 | −14 | 3 | 655 | 5.13 | 7.12 |
| Cuneus | R | 17 | 21 | −88 | 6 | 419 | 5.11 | 6.86 |
| Middle Occipital Gyrus | R | 19 | 36 | −74 | 9 | 992 | 5.03 | 7.72 |
| Middle Occipital Gyrus | L | 19 | −36 | −76 | 5 | 3843 | 5.21 | 9.21 |
| MDD REAL > HC REAL | ||||||||
| Middle Frontal Gyrus | R | 8 | 30 | 21 | 46 | 665 | 4.80 | 6.56 |
| L | 8 | −33 | 18 | 48 | 476 | 4.82 | 5.95 | |
| Medial Frontal Gyrus | L | 9 | 0 | 45 | 17 | 260 | 4.99 | 6.86 |
| Thalamus | L | − | −9 | −31 | 15 | 482 | 4.75 | 6.22 |
| Postcentral Gyrus | R | 2 | 50 | −18 | 27 | 584 | 4.82 | 6.08 |
| Precentral Gyrus | R | 6 | 54 | −9 | 38 | 288 | 4.67 | 5.88 |
| Posterior Insula | R | 13 | 46 | −11 | 1 | 517 | 4.58 | 5.90 |
| Anterior Insula | R | 13 | 46 | 11 | −5 | 339 | 4.92 | 6.30 |
| Superior Temporal Gyrus | R | 22 | 36 | −57 | 21 | 251 | 4.76 | 6.09 |
| L | 41 | −52 | −27 | 16 | 244 | 4.84 | 6.38 | |
| Middle Temporal Gyrus | L | 39 | −40 | −51 | 9 | 644 | 4.56 | 5.63 |
| Supramarginal Gyrus | R | 40 | 56 | −345 | 22 | 275 | 5.10 | 6.97 |
| Lingual Gyrus | L | 17 | −4 | −90 | 2 | 267 | 5.46 | 8.02 |
| Cuneus | L | 18 | −8 | −71 | 18 | 265 | 4.59 | 5.40 |
| HC REAL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Centre of Gravity | Size | T-Score | ||||||
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| (A) Last NF run vs. first NF run | ||||||||
| Medial Frontal Gyrus | L | 10 | −8 | 48 | 7 | 1562 | 4.84 | 7.35 |
| Middle Temporal Gyrus | R | 39 | 42 | −56 | 10 | 305 | 4.60 | 5.62 |
| (B) First NF run vs. last NF run | ||||||||
| Middle Frontal Gyrus | R | 9 | 33 | 36 | 28 | 2296 | −4.83 | −6.71 |
| R | 9 | 46 | 7 | 37 | 243 | −4.51 | −5.33 | |
| Precentral Gyrus | L | 6 | −43 | −3 | 50 | 316 | −5.15 | −6.81 |
| Cuneus | L | 18 | −17 | −67 | 18 | 1140 | −4.58 | −5.78 |
| Precuneus | R | 31 | 16 | −64 | 20 | 1427 | −4.85 | −6.75 |
| L | 19 | −28 | −72 | 30 | 286 | −4.67 | −6.24 | |
| Lingual Gyrus | L | 17 | −19 | −87 | −2 | 607 | −4.78 | −6.07 |
| L | 18 | −2 | −76 | 0 | 492 | −4.55 | −5.90 | |
| Middle Occipital Gyrus | L | 19 | −36 | −80 | 10 | 794 | −5.41 | −7.85 |
| Supramarginal Gyrus | L | 40 | −56 | −43 | 33 | 669 | −4.71 | −5,99 |
| R | 40 | 52 | −42 | 30 | 2122 | −4.72 | −6.62 | |
| Superior Occipital Gyrus | R | 19 | 34 | −72 | 21 | 1489 | −4.59 | −5.851 |
| Cingulate Gyrus | L/R | 23/24 | −1 | −14 | 31 | 3184 | −4.74 | −6.13 |
| Posterior Cingulate Gyrus | L/R | 23/31 | −1 | −63 | 16 | 261 | −4.66 | −5.72 |
| Insula | R | 13 | 29 | 16 | 17 | 1232 | −4.68 | −5.97 |
| L | 13 | −40 | 9 | 9 | 521 | −5.06 | −6.76 | |
| Claustrum | R | − | 30 | 15 | 5 | 246 | −4.47 | −5.01 |
| Parahippocampal Gyrus | R | 19 | 23 | −50 | −2 | 603 | −4.48 | −5.66 |
| R | 19 | −31 | −46 | −5 | 4549 | −4.86 | −7.57 | |
| Globus Pallidus | L | − | −16 | −8 | −2 | 1305 | −4.66 | −6.34 |
| Medial Globus Pallidus | L | − | −15 | −10 | −2 | 1467 | −4.62 | 6.34 |
| MDD REAL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Centre of Gravity | Size | T-Score | ||||||
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| (A) Last NF run vs. first NF run | ||||||||
| Medial Frontal Gyrus | L/R | 10 | 0 | 53 | 3 | 541 | 4.95 | 7.20 |
| Inferior Frontal Gyrus | L | 47 | −26 | 25 | −4 | 446 | 5.35 | 7.41 |
| Middle Temporal Gyrus | L | 37 | −45 | −51 | −6 | 471 | 5.05 | 6.13 |
| Anterior Cingulate Gyrus | L | 32/10 | −19 | 44 | 8 | 610 | 4.85 | 6.15 |
| (B) First NF run vs. last NF run | ||||||||
| Precentral Gyrus | R | 6 | 30 | −13 | 51 | 390 | −4,70 | −5.36 |
| Middle Frontal Gyrus | R | 6 | 35 | 14 | 47 | 610 | −4.84 | −6.05 |
| R | 8 | 25 | 26 | 37 | 336 | −4.29 | −5.89 | |
| Medial Frontal Gyrus | R | 9 | 18 | 38 | 31 | 462 | −5.52 | −8.04 |
| R | 9 | 7 | 49 | 32 | 286 | −5.31 | −7.85 | |
| Inferior Frontal Gyrus | L | 46 | −44 | 40 | 7 | 471 | −5.17 | −6.65 |
| Precuneus | R | 31 | 15 | −54 | 36 | 430 | −4.66 | −5.60 |
| Parahippocampal Gyrus | R | 19 | 38 | −49 | −2 | 309 | −4.62 | −5.28 |
| MDD REAL Responder | ||||||||
|---|---|---|---|---|---|---|---|---|
| Centre of Gravity | Size | T-Score | ||||||
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| (A) Last NF run vs. first NF run | ||||||||
| Superior Frontal Gyrus | R | 10 | 23.38 | 52.69 | 8.52 | 509 | 6.70 | 9.84 |
| Superior/Medial Frontal Gyrus | L | 6 | −2.53 | 14.98 | 46.33 | 15899 | 6.58 | 15.38 |
| Medial Frontal Gyrus | R | 10 | 6.38 | 52.85 | 5.58 | 1368 | 7.71 | 15.35 |
| L | 10 | −3.87 | 53.7 | 5.03 | 863 | 13.33 | 7.59 | |
| R | 6 | 13.19 | −17.48 | 57.34 | 313 | 5.65 | 6.52 | |
| Middle Frontal Gyrus | R | 10 | 29.15 | 40.03 | 14.92 | 1127 | 6.38 | 9.81 |
| Inferior Frontal Gyrus | R | 47 | 49.78 | 26.55 | −1.74 | 475 | 7.35 | 11.23 |
| L | 47 | −50.58 | 26.33 | −0.41 | 248 | 6.53 | 9.11 | |
| Inferior Parietal Lobule/ Supramarginal Gyrus | R | 40 | 54.52 | −32.23 | 32.25 | 2184 | 6.77 | 12.60 |
| R | 40 | 39.99 | −43.94 | 33.17 | 439 | 5.65 | 6.73 | |
| L | 40 | −55.31 | −38.27 | 37.65 | 3607 | 6.86 | 13.51 | |
| L | 40 | −52.59 | −25.05 | 23.63 | 248 | 6.20 | 8.79 | |
| Anterior Cingulate | L | 32 | −14.42 | 37.26 | 8.65 | 3050 | 6.73 | 9.6 |
| R | 24 | 6.51 | 35.18 | 10.95 | 2744 | 6.55 | 9.65 | |
| Cingulate Gyrus | L | 24 | −6.75 | 14.92 | 27.51 | 695 | 6.13 | 8.96 |
| R | 24 | 6.97 | 11.52 | 28.45 | 931 | 6.19 | 8.86 | |
| Lingual Gyrus | R | 19 | 31.46 | −72.89 | 0.81 | 914 | 6.13 | 9.43 |
| Inferior Occipital Gyrus | L | 19 | −34.66 | −71.24 | 1.26 | 848 | 5.79 | 7.51 |
| Cuneus | R | 18 | 17.9 | −84.39 | 17.06 | 399 | 5.89 | 8.46 |
| Middle Temporal Gyrus | L | 37 | −46.06 | −49.51 | −4.56 | 1411 | 7.10 | 10.63 |
| Insula | R | 13 | 54.52 | −32.23 | 32.25 | 2144 | 6.15 | 8.78 |
| Lentiform Nucleus | R | - | 16.32 | −0.72 | 12.24 | 294 | 5.52 | 6.74 |
| Caudate Head | L | - | −10.64 | 15.35 | 3.43 | 2538 | 7.18 | 13.05 |
| Caudate Head | R | - | 7.87 | 15.71 | 5.42 | 687 | 5.74 | 7.44 |
| Putamen | L | - | −22.86 | 15.33 | −2.11 | 1168 | 11.93 | 6.64 |
| Medial Globus Pallidus | R | - | 10.2 | −6.59 | −3.91 | 302 | 5.78 | 7.28 |
| (B) First NF run vs. last NF run | ||||||||
| Middle Frontal Gyrus | R | 8 | 33.05 | 18.09 | 48.66 | 317 | −6.41 | −9.96 |
| L | 9 | −44.32 | 21.07 | 31.84 | 6060 | −7.37 | −15.76 | |
| Superior Frontal Gyrus | R | 9 | 8.33 | 55.01 | 23.95 | 279 | −6.42 | −9.96 |
| L | 8 | −0.87 | 38.72 | 45.9 | 1124 | −8.76 | −16.22 | |
| Lingual Gyrus | L | 18 | −7.32 | −85.98 | −0.82 | 765 | −7.19 | −12.11 |
| Inferior Occipital Gyrus | L | 19 | −45.98 | −75.25 | −2.19 | 759 | −7.27 | −12.42 |
| Middle Temporal Gyrus | L | 21 | −53.07 | −17.9 | −11.03 | 416 | −5.91 | −7.51 |
| Parahippocampal Gyrus | R | 19 | 37.44 | −43.72 | −0.4 | 836 | −6.13 | −8.24 |
| Caudate Body | R | - | 19.77 | −18.58 | 28.87 | 1065 | −6.00 | −7.91 |
| MDD REAL Non-Responder | ||||||||
|---|---|---|---|---|---|---|---|---|
| Centre of Gravity | Size | T-Score | ||||||
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| (A) Last NF run vs. first NF run | ||||||||
| - | - | - | - | - | - | - | - | - |
| (B) First NF run vs. last NF run | ||||||||
| Middle Frontal Gyrus | R | 6 | 36.84 | 10.36 | 42.84 | 363 | −5.53 | −6.18 |
| Medial Frontal Gyrus | R | 9 | 17.6 | 32.71 | 30.42 | 505 | −5.51 | −6.76 |
| Inferior Parietal Lobule | L | 40 | 34.11 | −49.84 | 37.99 | 1523 | −5.54 | −6.90 |
| Centre of Gravity | Size | T-Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Side | BA | x | y | z | Ø | Max | |
| HC REAL > MDD patients | ||||||||
| Middle Temporal Gyrus | R | 39 | 42 | −53 | 10 | 739 | −4.94 | 6.14 |
| MDD patients > HC REAL | ||||||||
| Inferior Parietal Lobule | R | 40 | 58 | −29 | 30 | 424 | 5.57 | 7.54 |
| Supramarginal Gyrus | L | 40 | −57 | −42 | 34 | 298 | 4.71 | 5.31 |
| Parahippocampal Gyrus | L | 19/36 | −23 | −45 | −5 | 270 | 5.03 | 6.36 |
| Lateral Globus Pallidus | L | - | −20 | −4 | −2 | 702 | 4.96 | 6.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maywald, M.; Paolini, M.; Rauchmann, B.S.; Gerz, C.; Heppe, J.L.; Wolf, A.; Lerchenberger, L.; Tominschek, I.; Stöcklein, S.; Reidler, P.; et al. Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study. Brain Sci. 2022, 12, 1714. https://doi.org/10.3390/brainsci12121714
Maywald M, Paolini M, Rauchmann BS, Gerz C, Heppe JL, Wolf A, Lerchenberger L, Tominschek I, Stöcklein S, Reidler P, et al. Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study. Brain Sciences. 2022; 12(12):1714. https://doi.org/10.3390/brainsci12121714
Chicago/Turabian StyleMaywald, Maximilian, Marco Paolini, Boris Stephan Rauchmann, Christian Gerz, Jan Lars Heppe, Annika Wolf, Linda Lerchenberger, Igor Tominschek, Sophia Stöcklein, Paul Reidler, and et al. 2022. "Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study" Brain Sciences 12, no. 12: 1714. https://doi.org/10.3390/brainsci12121714
APA StyleMaywald, M., Paolini, M., Rauchmann, B. S., Gerz, C., Heppe, J. L., Wolf, A., Lerchenberger, L., Tominschek, I., Stöcklein, S., Reidler, P., Tschentscher, N., Ertl-Wagner, B., Pogarell, O., Keeser, D., & Karch, S. (2022). Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study. Brain Sciences, 12(12), 1714. https://doi.org/10.3390/brainsci12121714






