P3b Amplitude and Latency in Tic Disorders: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
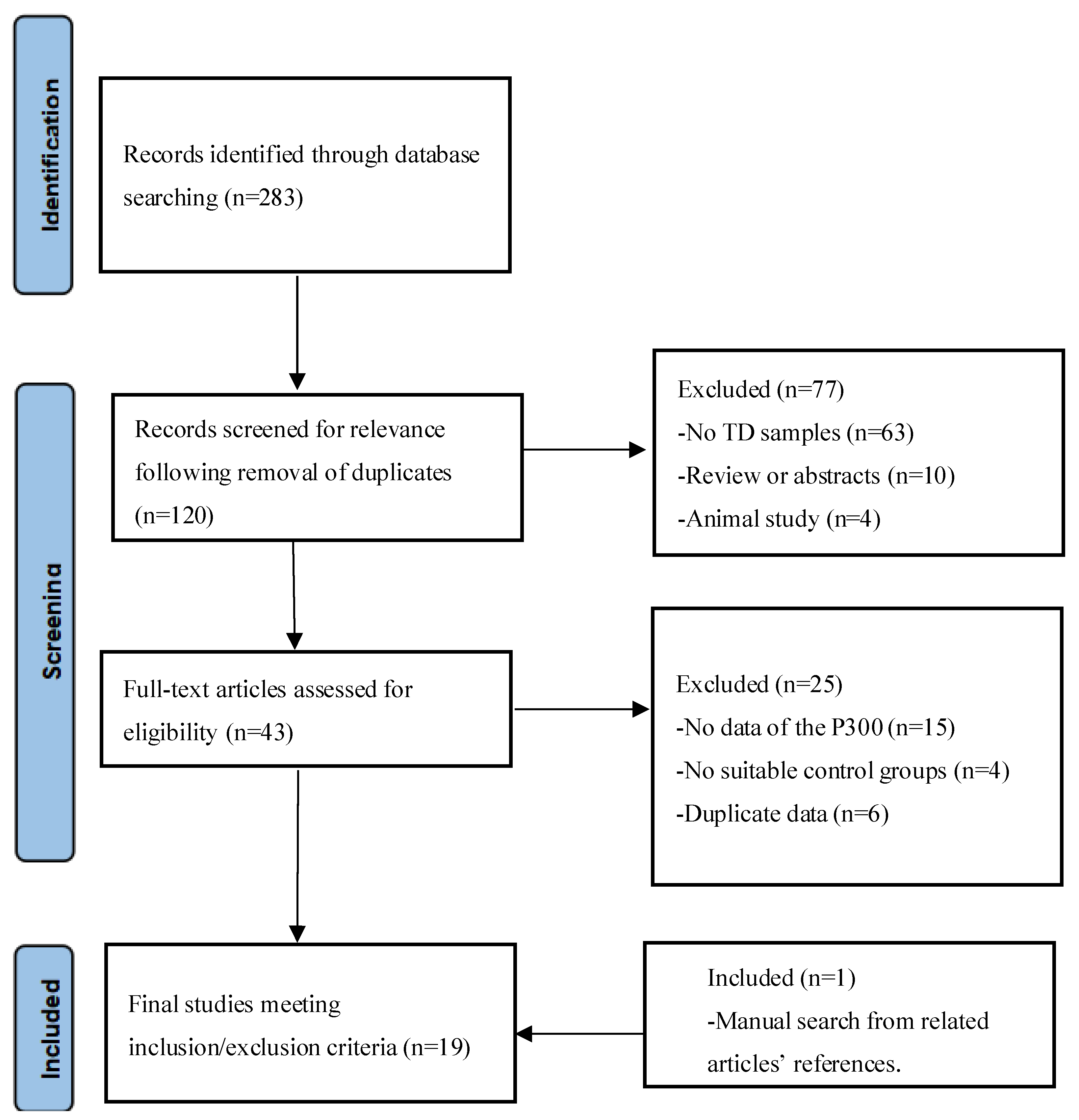
2.1. Literature Search
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
| Author | Year | Subjects (TD) | Subjects (HC) | Mean Age (TD) | Mean Age (HC) | Diagnosis | Diagnostic Criteria | Comorbidity | Modality | Reaction Method | Paradigm | Electrode (Average) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morand-Beaulieu S [41] | 2022 | 47 | 35 | 11.0 ± 1.7 | 11.4 ± 1.6 | TS | DSM-IV-TR | none | Visual | Keypress | Go/NoGo task | Pz |
| Kloft L [36] | 2019 | 12 | 21 | 33.6 ± 8.8 | 30.9 ± 7.8 | TD | DSM-IV | All patients with OCD | Visual | Keypress | Stop-signal task | Cz |
| Petruo V [38] | 2019 | 35 | 39 | 12.97 ± 2.52 | Matched with the TS | TS | ICD-10 | 3 patients with ADHD and 11 with OCD | Auditory | Keypress | Go/NoGo task | P7, P8 |
| Sauvé G [34] | 2017 | 12 | 15 | 33 ± 9 | 31 ± 9 | TD | DSM-IV-TR | none | Visual | Keypress | Oddball | AF4/FC4/CP6/AF2/FC2/CP2/F6/C6/P6/F4/C4/P4/F2/C2/P2 |
| Lange F [25] | 2017 | 23 | 26 | 32.7 ± 11.11 | 32.88 ± 11.23 | TS | DSM-5 | unknown | Visual | Keypress | Wisconsin Card Sorting Test | Pz |
| Morand-Beaulieu S [27] | 2016 | 26 | 27 | 38 ± 11.9 | 36 ± 13.0 | TS and chronic TD | DSM-IV-TR | none | Visual | Keypress | A reinforcement-based learning and reversal task | CP1/CP2/CP5/CP6/P1/P2/P3/P4/P5/P6 |
| Morand-Beaulieu S [27] | 2016 | 26 | 27 | 38 ± 11.9 | 36 ± 13.0 | TS and chronic TD | DSM-IV-TR | none | Visual | Counts | A reinforcement-based learning and reversal task | CP1/CP2/CP5/CP6/P1/P2/P3/P4/P5/P6 |
| Eichele H [33] | 2016 | 25 | 35 | 9.87 ± 0.24 | 10.04 ± 0.21 | TS | DSM-IV | 14 patients with ADHD | Visual | Keypress | A modified Eriksen-Flanker task | FC1/FC2/Cz/CP1/CP2 |
| Shephard E [42] | 2016 | 18 | 20 | 13.18 ± 2.78 | 13.03 ± 2.9 | TS | unknown | none | Visual | Keypress | A reinforcement-based learning and reversal task | Pz |
| Thibault G [28] | 2009 | 15 | 20 | 37 ± 8 | 40 ± 12 | TS | DSM-IV-TR | none | Visual | Keypress | A stimulus–response compatibility paradigm | P3, P4, CP3, CP4 |
| Thibault G [37] | 2008 | 14 | 14 | 32 ± 10 | 37 ± 13 | TS | DSM-IV-TR | none | Visual | Counts | Oddball | CP3/TP7/P3/T5/O1/CP4/TP8/P4/T6/O2 |
| Zhu Y [26] | 2006 | 19 | 20 | 11.169 ± 1.214 | 11.459 ± 1.146 | TS | DSM-IV | none | Auditory | Keypress | Oddball | Pz |
| Johannes S [43] | 2003 | 10 | 10 | 34.4 ± 15.3 | 33.7 ± 13.7 | TS | DSM-IV | 2 patients with ADHD, 2 with OCD and 1 with both disorders. | Visual | Keypress | Stroop-paradigm | Pz |
| Rothenberger A [39] | 2000 | 11 | 11 | 12.1 ± 2.5 | 11.5 ± 2.1 | TD | DSM-III-R | All patients with ADHD | Auditory | Keypress | An auditory selective-attention task | P3, P4 |
| Johannes S [29] | 1997 | 12 | 12 | 32.9 | Matched with the TS | TS | DSM III -R | All patients with OCD and 3 with ADHD | Visual | Keypress | Oddball | Pz |
| Oades RD [44] | 1996 | 10 | 12 | 11.7 ± 2.2 | 10.4 ± 1.3 | TS | DSM III -R | unknown | Auditory | None | Oddball | Pz |
| Van Woerkom (1) [45] | 1994 | 24 | 24 | 27 ± 3.5 | 28 ± 3.3 | TS | DSM III -R | unknown | Auditory | Keypress | Oddball | Pz |
| Van Woerkom (1) [45] | 1994 | 24 | 24 | 27 ± 3.5 | 28 ± 3.3 | TS | DSM III -R | unknown | Auditory | None | Oddball | Pz |
| Van Woerkom (2) [45] | 1994 | 29 | 17 | 12.5 ± 1.52 | 13.4 ± 1.35 | TS | DSM III -R | unknown | Auditory | Keypress | Oddball | Pz |
| Van Woerkom (2) [45] | 1994 | 29 | 17 | 12.5 ± 1.52 | 13.4 ± 1.35 | TS | DSM III -R | unknown | Auditory | None | Oddball | Pz |
| Drake ME [35] | 1992 | 20 | 20 | 8–20 | 10–30 | TS | DSM-IIIR | 10 patients with ADHD and 6 with OCD | Auditory | Keypress | Oddball | Cz/A1/A2 |
| Van Woerkom (1) [46] | 1988 | 20 | 20 | 27 | 28 | TS | DSM-III | unknown | Auditory | Keypress | Oddball | Pz |
| Van Woerkom (2) [46] | 1988 | 20 | 20 | 27 | 28 | TS | DSM-III | unknown | Auditory | Counts | Oddball | Pz |
| van de Wetering [47] | 1985 | 6 | 16 | 28 | 23 | TS | DSM-III | unknown | Auditory | Counts | Oddball | Pz |
3. Results
3.1. Characteristics of Individual Studies
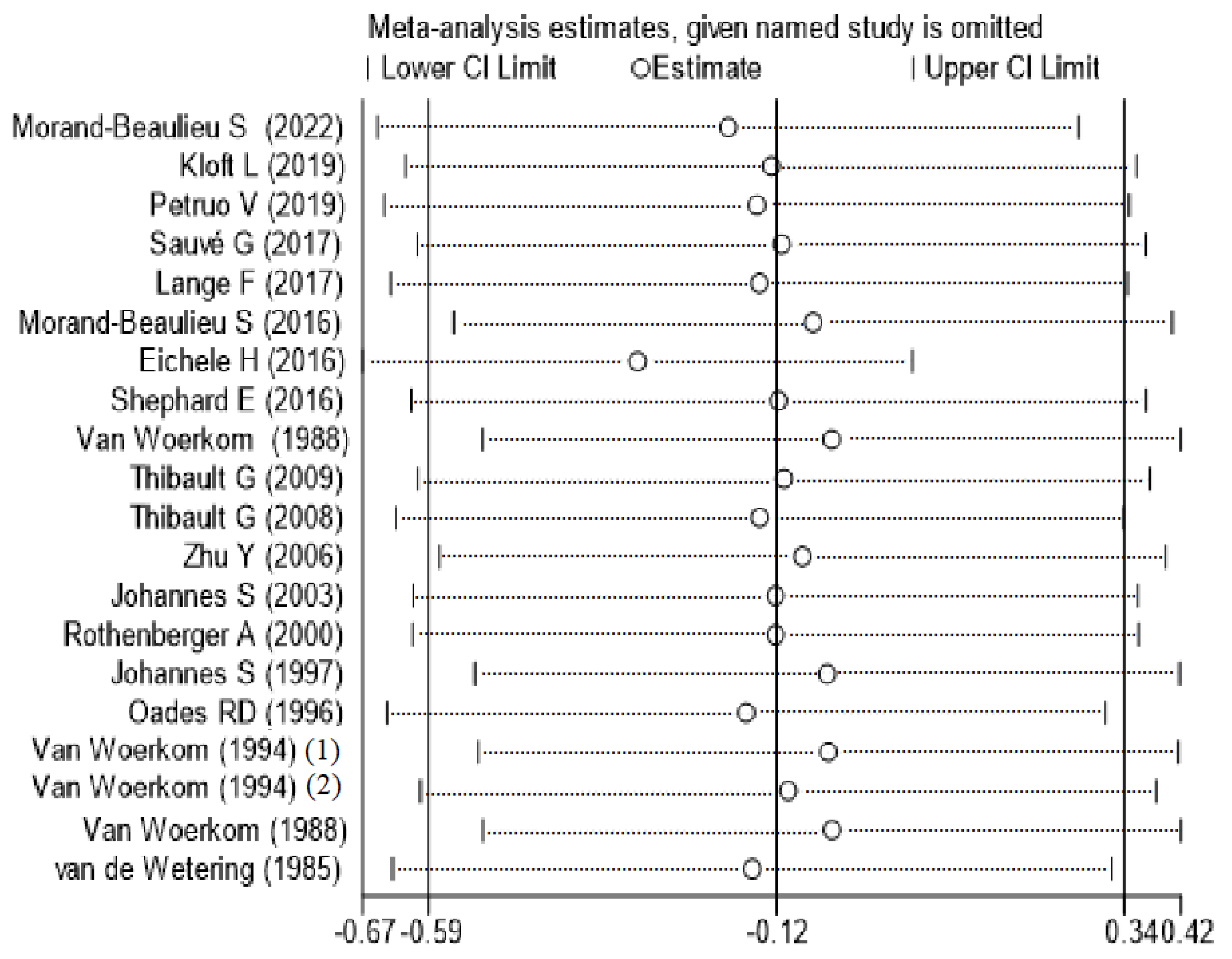
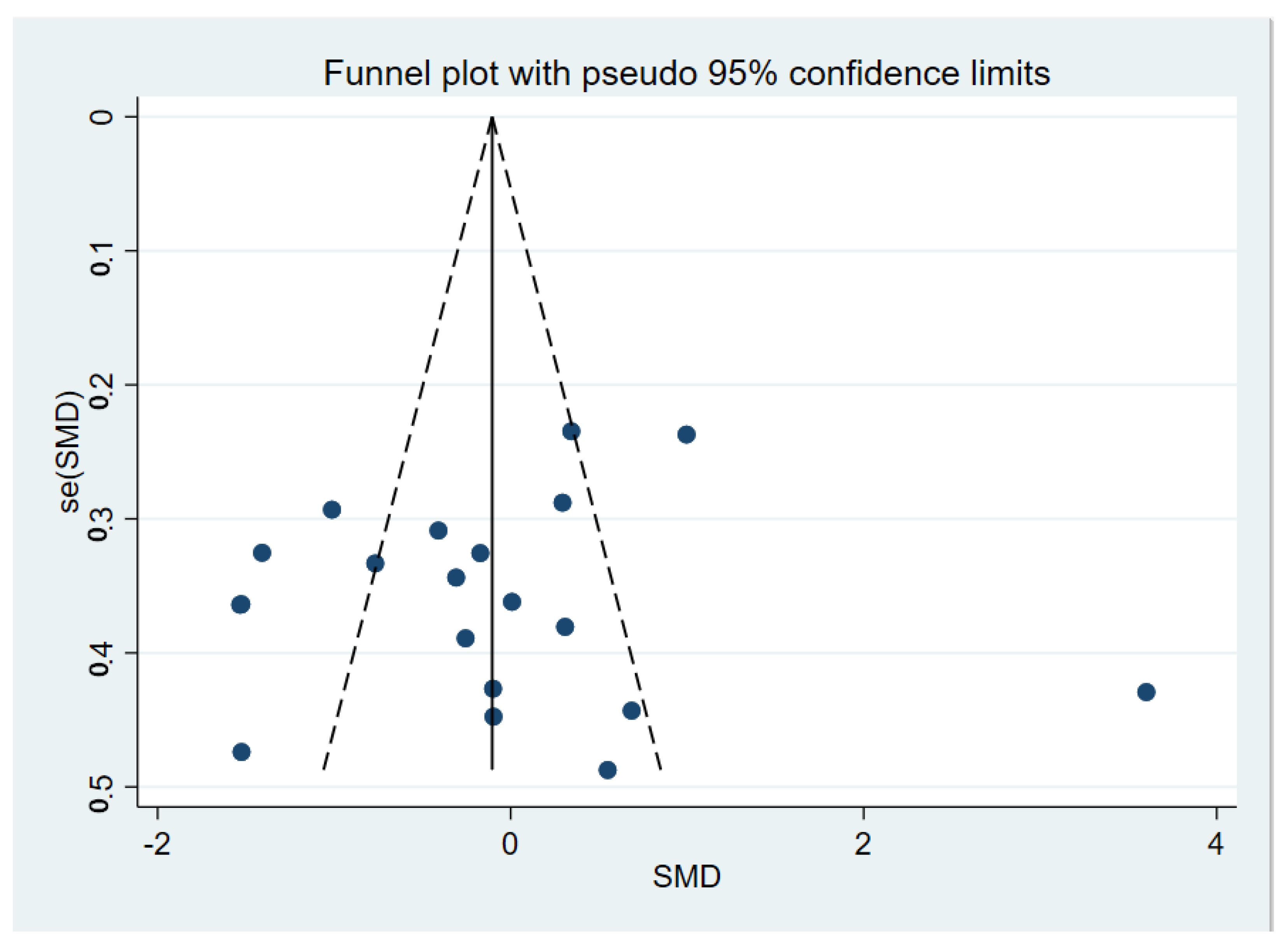
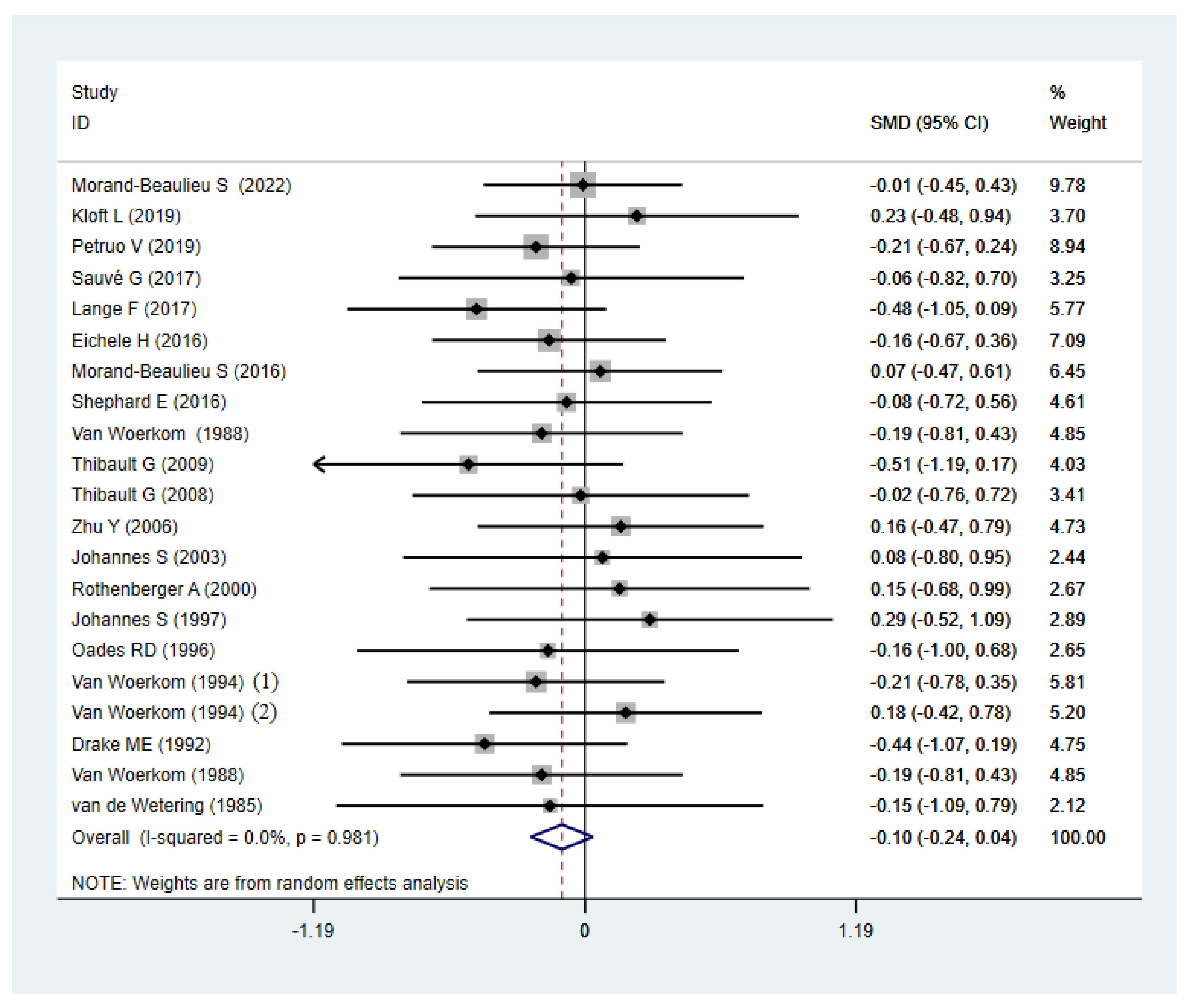
3.2. Meta-Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Freeman, R.D. Tourette Syndrome International Database Consortium. Tic disorders and ADHD: Answers from a world-wide clinical dataset on Tourette syndrome. Eur. Child Adolesc. Psychiatry 2007, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Mathews, C.A.; Scharf, J.M.; Neale, B.M.; Davis, L.K.; Gamazon, E.R.; Derks, E.M.; Evans, P.; Edlund, C.K.; Crane, J.; et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette’s syndrome and OCD. Am. J. Psychiatry 2015, 172, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Seri, S.; Cavanna, A.E. The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: A systematic review. Eur. Child Adolesc. Psychiatry 2016, 25, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Eapen, V.; Cavanna, A.E.; Robertson, M.M. Comorbidities, Social Impact, and Quality of Life in Tourette Syndrome. Front. Psychiatry 2016, 7, 97. [Google Scholar] [CrossRef]
- Wang, Z.; Maia, T.V.; Marsh, R.; Colibazzi, T.; Gerber, A.; Peterson, B.S. The neural circuits that generate tics in Tourette’s syndrome. Am. J. Psychiatry 2011, 168, 1326–1337. [Google Scholar] [CrossRef]
- Hampson, M.; Tokoglu, F.; King, R.A.; Constable, R.T.; Leckman, J.F. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol. Psychiatry 2009, 65, 594–599. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. Int. J. Psychophysiol. 2015, 97, 189–209. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the event-related potential. Int. J. Med. Sci. 2005, 2, 147. [Google Scholar] [CrossRef]
- Yu, D.; Sul, J.H.; Tsetsos, F.; Nawaz, M.S.; Huang, A.Y.; Zelaya, I.; Illmann, C.; Osiecki, L.; Darrow, S.M.; Hirschtritt, M.E.; et al. Interrogating the Genetic Determinants of Tourette’s Syndrome and Other Tic Disorders Through Genome-Wide Association Studies. Am. J. Psychiatry 2019, 176, 217–227. [Google Scholar] [CrossRef]
- Qi, Y.; Zheng, Y.; Li, Z.; Liu, Z.; Xiong, L. Genetic Studies of Tic Disorders and Tourette Syndrome. Methods Mol. Biol. 2019, 2011, 547–571. [Google Scholar] [CrossRef]
- Eddy, C.M.; Cavanna, A.E.; Rickards, H.E.; Hansen, P.C. Temporo-parietal dysfunction in Tourette syndrome: Insights from an fMRI study of Theory of Mind. J. Psychiatr. Res. 2016, 81, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhang, J.; Wen, H.; Zhang, Y.; Kang, H.; Wang, X.; Li, W.; He, H.; Peng, Y. Altered Spontaneous Brain Activity in Children with Early Tourette Syndrome: A Resting-state fMRI Study. Sci. Rep. 2017, 7, 4808. [Google Scholar] [CrossRef] [PubMed]
- Bodatsch, M.; Brockhaus-Dumke, A.; Klosterkotter, J.; Ruhrmann, S. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol. Psychiatry 2015, 77, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.; Braren, M.; Zubin, J.; John, E. Evoked-potential correlates of stimulus uncertainty. Science 1965, 150, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.-Q.; Hu, Y. Localizing P300 generators in high-density event-related potential with fMRI. Med. Sci. Monit. 2009, 15, MT47–MT53. [Google Scholar] [PubMed]
- Kato, Y.; Endo, H.; Kizuka, T. Mental fatigue and impaired response processes: Event-related brain potentials in a Go/NoGo task. Int. J. Psychophysiol. 2009, 72, 204–211. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Kutas, M.; McCarthy, G.; Donchin, E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science 1977, 197, 792–795. [Google Scholar] [CrossRef]
- Verleger, R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology 1997, 34, 131–156. [Google Scholar] [CrossRef]
- Van Dinteren, R.; Arns, M.; Jongsma, M.L.; Kessels, R.P. P300 development across the lifespan: A systematic review and meta-analysis. PLoS ONE 2014, 9, e87347. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Verleger, R. Event-related potentials and cognition: A critique of the context updating hypothesis and an alternative interpretation of P3. Behav. Brain Sci. 1988, 11, 343–356. [Google Scholar] [CrossRef]
- Verleger, R.; Jaśkowski, P.; Wascher, E. Evidence for an integrative role of P3b in linking reaction to perception. J. Psychophysiol. 2005, 19, 165–181. [Google Scholar] [CrossRef]
- Lange, F.; Seer, C.; Muller-Vahl, K.; Kopp, B. Cognitive flexibility and its electrophysiological correlates in Gilles de la Tourette syndrome. Dev. Cogn. Neurosci. 2017, 27, 78–90. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, P.Z.; Leung, K.M.; Su, L.Y.; Wu, D.X.; Zhou, M. P300 differences exist between Tourette’s syndrome with and without attention deficiency and hyperactivity disorder in children. World J. Biol. Psychiatry 2006, 7, 91–98. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; O’Connor, K.P.; Richard, M.; Sauve, G.; Leclerc, J.B.; Blanchet, P.J.; Lavoie, M.E. The Impact of a Cognitive-Behavioral Therapy on Event-Related Potentials in Patients with Tic Disorders or Body-Focused Repetitive Behaviors. Front. Psychiatry 2016, 7, 81. [Google Scholar] [CrossRef]
- Thibault, G.; O’Connor, K.P.; Stip, E.; Lavoie, M.E. Electrophysiological manifestations of stimulus evaluation, response inhibition and motor processing in Tourette syndrome patients. Psychiatry Res. 2009, 167, 202–220. [Google Scholar] [CrossRef]
- Johannes, S.; Weber, A.; Müller-Vahl, K.; Kolbe, H.; Dengler, R.; Münte, T. Event-related brain potentials show changed attentional mechanisms in Gilles de la Tourette syndrome. Eur. J. Neurol. 1997, 4, 152–161. [Google Scholar] [CrossRef]
- Bohlhalter, S.; Goldfine, A.; Matteson, S.; Garraux, G.; Hanakawa, T.; Kansaku, K.; Wurzman, R.; Hallett, M. Neural correlates of tic generation in Tourette syndrome: An event-related functional MRI study. Brain 2006, 129, 2029–2037. [Google Scholar] [CrossRef]
- Rosenfeld, J.P.; Ward, A.; Frigo, V.; Drapekin, J.; Labkovsky, E. Evidence suggesting superiority of visual (verbal) vs. auditory test presentation modality in the P300-based, Complex Trial Protocol for concealed autobiographical memory detection. Int. J. Psychophysiol. 2015, 96, 16–22. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Eichele, H.; Eichele, T.; Bjelland, I.; Hovik, M.F.; Sorensen, L.; van Wageningen, H.; Worren, M.K.; Hugdahl, K.; Plessen, K.J. Performance Monitoring in Medication-Naive Children with Tourette Syndrome. Front. Neurosci. 2016, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Sauve, G.; Morand-Beaulieu, S.; O’Connor, K.P.; Blanchet, P.J.; Lavoie, M.E. P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders. Brain Sci. 2017, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.E., Jr.; Hietter, S.A.; Padamadan, H.; Bogner, J.E.; Andrews, J.M.; Weate, S. Auditory evoked potentials in Gilles de la Tourette syndrome. Clin. Electroencephalogr. 1992, 23, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kloft, L.; Riesel, A.; Kathmann, N. Inhibition-related differences between tic-free and tic-related obsessive-compulsive disorder: Evidence from the N2 and P3. Exp. Brain Res. 2019, 237, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Thibault, G.; Felezeu, M.; O’Connor, K.P.; Todorov, C.; Stip, E.; Lavoie, M.E. Influence of comorbid obsessive-compulsive symptoms on brain event-related potentials in Gilles de la Tourette syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 803–815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petruo, V.; Bodmer, B.; Brandt, V.C.; Baumung, L.; Roessner, V.; Munchau, A.; Beste, C. Altered perception-action binding modulates inhibitory control in Gilles de la Tourette syndrome. J. Child Psychol. Psychiatry 2019, 60, 953–962. [Google Scholar] [CrossRef]
- Rothenberger, A.; Banaschewski, T.; Heinrich, H.; Moll, G.H.; Schmidt, M.H.; van’t Klooster, B. Comorbidity in ADHD-children: Effects of coexisting conduct disorder or tic disorder on event-related brain potentials in an auditory selective-attention task. Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 101–110. [Google Scholar] [CrossRef]
- Hedges, L.V.; Higgins, J.; Rothstein, H. Introduction to Meta-Analysis; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Morand-Beaulieu, S.; Smith, S.D.; Ibrahim, K.; Wu, J.; Leckman, J.F.; Crowley, M.J.; Sukhodolsky, D.G. Electrophysiological signatures of inhibitory control in children with Tourette syndrome and attention-deficit/hyperactivity disorder. Cortex 2022, 147, 157–168. [Google Scholar] [CrossRef]
- Shephard, E.; Jackson, G.M.; Groom, M.J. Electrophysiological correlates of reinforcement learning in young people with Tourette syndrome with and without co-occurring ADHD symptoms. Int. J. Dev. Neurosci. 2016, 51, 17–27. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Rada, D.; Müller-Vahl, K.R.; Emrich, H.M.; Dengler, R.; Münte, T.F.; Dietrich, D. Tourette Syndrome and Obsessive-Compulsive Disorder: Event-related brain potentials show similar mechansims of frontal inhibition but dissimilar target evaluation processes. Behav. Neurol. 2003, 14, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D.; Dittmann-Balcar, A.; Schepker, R.; Eggers, C.; Zerbin, D. Auditory event-related potentials (ERPs) and mismatch negativity (MMN) in healthy children and those with attention-deficit or tourette/tic symptoms. Biol. Psychol. 1996, 43, 163–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Woerkom, T.; Roos, R.; Van Dijk, J. Altered attentional processing of background stimuli in Gilles de la Tourette syndrome: A study in auditory event-related potentials evoked in an oddball paradigm. Acta Neurol. Scand. 1994, 90, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Van Woerkom, T.C.; Fortgens, C.; Rompel-Martens, C.M.; Van de Wetering, B.J. Auditory event-related potentials in adult patients with Gilles de la Tourette’s syndrome in the oddball paradigm. Electroencephalogr. Clin. Neurophysiol. 1988, 71, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Van de Watering, B.; Martens, C.; Fortgens, C.; Slaets, J.; Van Woerkom, T. Late components of the auditory evoked potentials in Gilles de la Tourette syndrome. Clin. Neurol. Neurosurg. 1985, 87, 181–186. [Google Scholar] [CrossRef]
- Borja, A.; Ponde, M. P300: Avaliação do potencial evocado cognitivo em crianças com e sem TDAH. Rev. Ciênc. Méd. Biol. 2009, 8, 198–205. [Google Scholar] [CrossRef][Green Version]
- Earls, H.A.; Curran, T.; Mittal, V. A Meta-analytic Review of Auditory Event-Related Potential Components as Endophenotypes for Schizophrenia: Perspectives From First-Degree Relatives. Schizophr. Bull. 2016, 42, 1504–1516. [Google Scholar] [CrossRef]
- Oades, R.D. Differential measures of ‘sustained attention’in children with attention-deficit/hyperactivity or tic disorders: Relations to monoamine metabolism. Psychiat. Res. 2000, 93, 165–178. [Google Scholar] [CrossRef]
- Polich, J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroen. Clin. Neuro. 1987, 68, 311–320. [Google Scholar] [CrossRef]
- Shephard, E.; Jackson, G.M.; Groom, M.J. The effects of co-occurring ADHD symptoms on electrophysiological correlates of cognitive control in young people with Tourette syndrome. J. Neuropsychol. 2016, 10, 223–238. [Google Scholar] [CrossRef]
- Brandt, V.C.; Stock, A.K.; Munchau, A.; Beste, C. Evidence for enhanced multi-component behaviour in Tourette syndrome—An EEG study. Sci. Rep. 2017, 7, 7722. [Google Scholar] [CrossRef] [PubMed]
- Eichele, H.; Eichele, T.; Marquardt, L.; Adolfsdottir, S.; Hugdahl, K.; Sorensen, L.; Plessen, K.J. Development of Performance and ERPs in a Flanker Task in Children and Adolescents with Tourette Syndrome-A Follow-Up Study. Front. Neurosci. 2017, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Huettel, S.A.; McCarthy, G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia 2004, 42, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Muller-Vahl, K.R.; Kaufmann, J.; Grosskreutz, J.; Dengler, R.; Emrich, H.M.; Peschel, T. Prefrontal and anterior cingulate cortex abnormalities in Tourette Syndrome: Evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.M.; Sullivan, E.V.; Marsh, L.; White, P.M.; Lim, K.O.; Pfefferbaum, A. The relationship between P300 amplitude and regional gray matter volumes depends upon the attentional system engaged. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Crossley, N.; Woolley, J.; Carletti, F.; Perez-Iglesias, R.; Broome, M.; Johns, L.; Tabraham, P.; Bramon, E.; McGuire, P. White matter alterations related to P300 abnormalities in individuals at high risk for psychosis: An MRI-EEG study. J. Psychiatry Neurosci. 2011, 36, 239–248. [Google Scholar] [CrossRef]
- Helms, H.C.C.; Nielsen, C.U.; Waagepetersen, H.S.; Brodin, B. Glutamate Transporters in the Blood-Brain Barrier. Adv. Neurobiol. 2017, 16, 297–314. [Google Scholar] [CrossRef]
- Kalanithi, P.S.; Zheng, W.; Kataoka, Y.; DiFiglia, M.; Grantz, H.; Saper, C.B.; Schwartz, M.L.; Leckman, J.F.; Vaccarino, F.M. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 13307–13312. [Google Scholar] [CrossRef]
- Anderson, G.M.; Pollak, E.S.; Chatterjee, D.; Leckman, J.F.; Riddle, M.A.; Cohen, D.J. Postomortem analysis of subcortical monoamines and amino acids in Tourette syndrome. Adv. Neurol. 1992, 58, 123–133. [Google Scholar]
- Hall, M.H.; Jensen, J.E.; Du, F.; Smoller, J.W.; O’Connor, L.; Spencer, K.M.; Ongur, D. Frontal P3 event-related potential is related to brain glutamine/glutamate ratio measured In Vivo. Neuroimage 2015, 111, 186–191. [Google Scholar] [CrossRef]
- Watson, T.D.; Petrakis, I.L.; Edgecombe, J.; Perrino, A.; Krystal, J.H.; Mathalon, D.H. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int. J. Neuropsychopharmacol. 2009, 12, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Draper, A.; Stephenson, M.C.; Jackson, G.M.; Pepes, S.; Morgan, P.S.; Morris, P.G.; Jackson, S.R. Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr. Biol. 2014, 24, 2343–2347. [Google Scholar] [CrossRef]
- Mahone, E.M.; Puts, N.A.; Edden, R.A.E.; Ryan, M.; Singer, H.S. GABA and glutamate in children with Tourette syndrome: A (1) H MR spectroscopy study at 7T. Psychiatry Res. Neuroimaging 2018, 273, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wang, P.P.; Liu, S.; Zhang, X. P300 amplitude and latency in autism spectrum disorder: A meta-analysis. Eur. Child Adolesc. Psychiatry 2017, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.W.P.; Gelade, K.; van Mourik, R.; Maras, A.; Oosterlaan, J. An ERP source imaging study of the oddball task in children with Attention Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2016, 127, 1351–1357. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; Lavoie, M.E. Cognitive and motor event-related potentials in Tourette syndrome and tic disorders: A systematic review. Clin. Neurophysiol. 2019, 130, 1041–1057. [Google Scholar] [CrossRef]

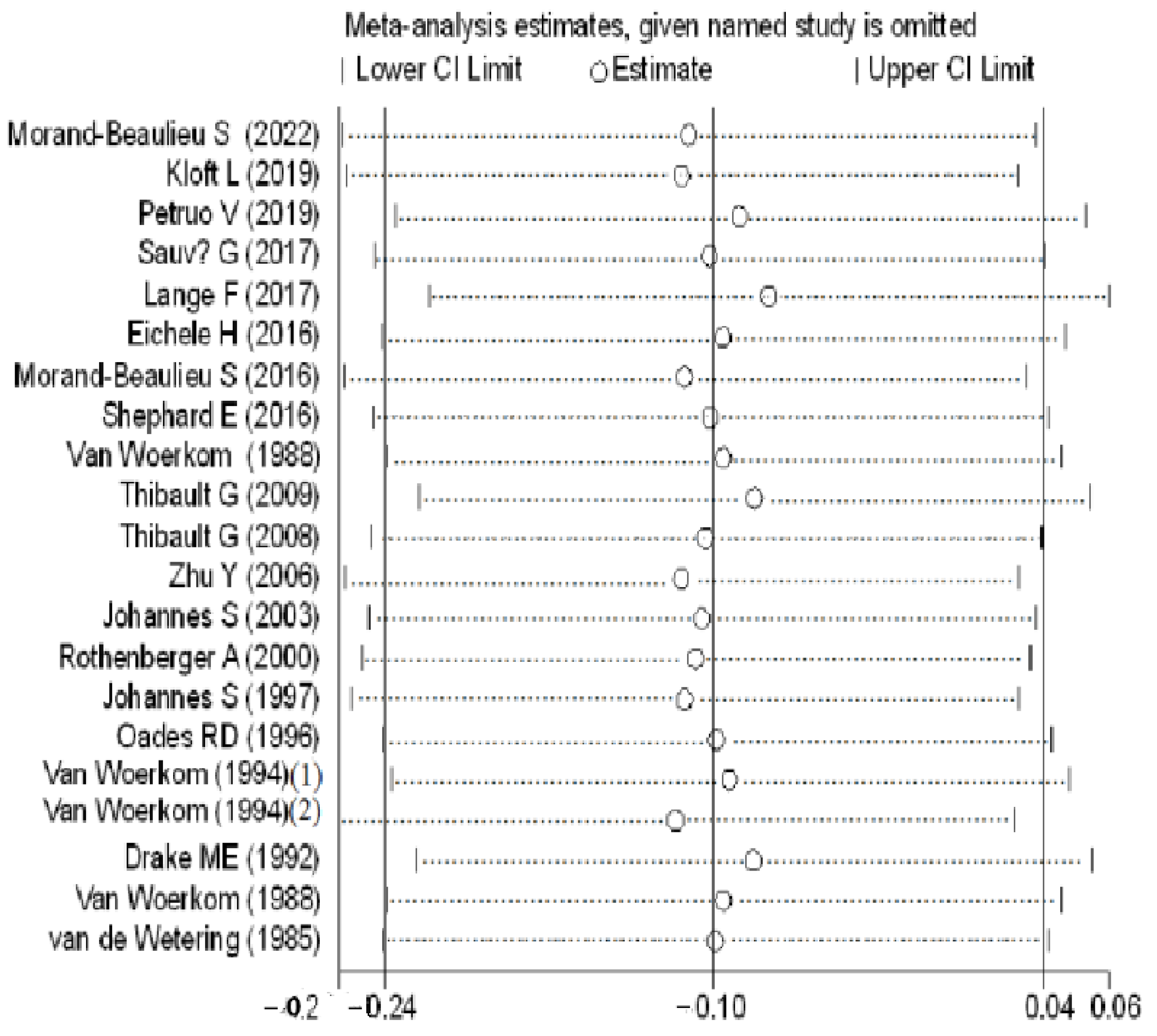
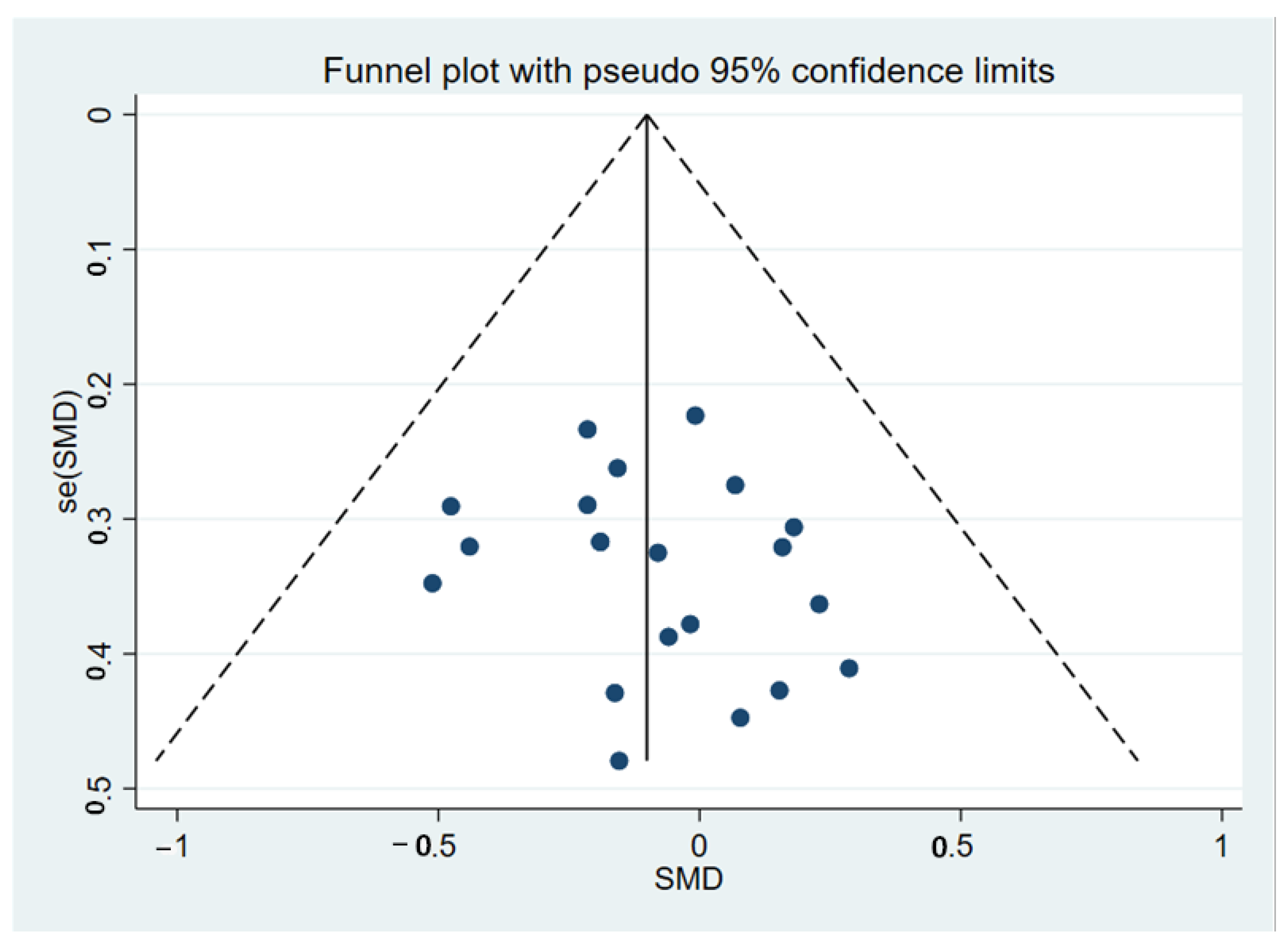
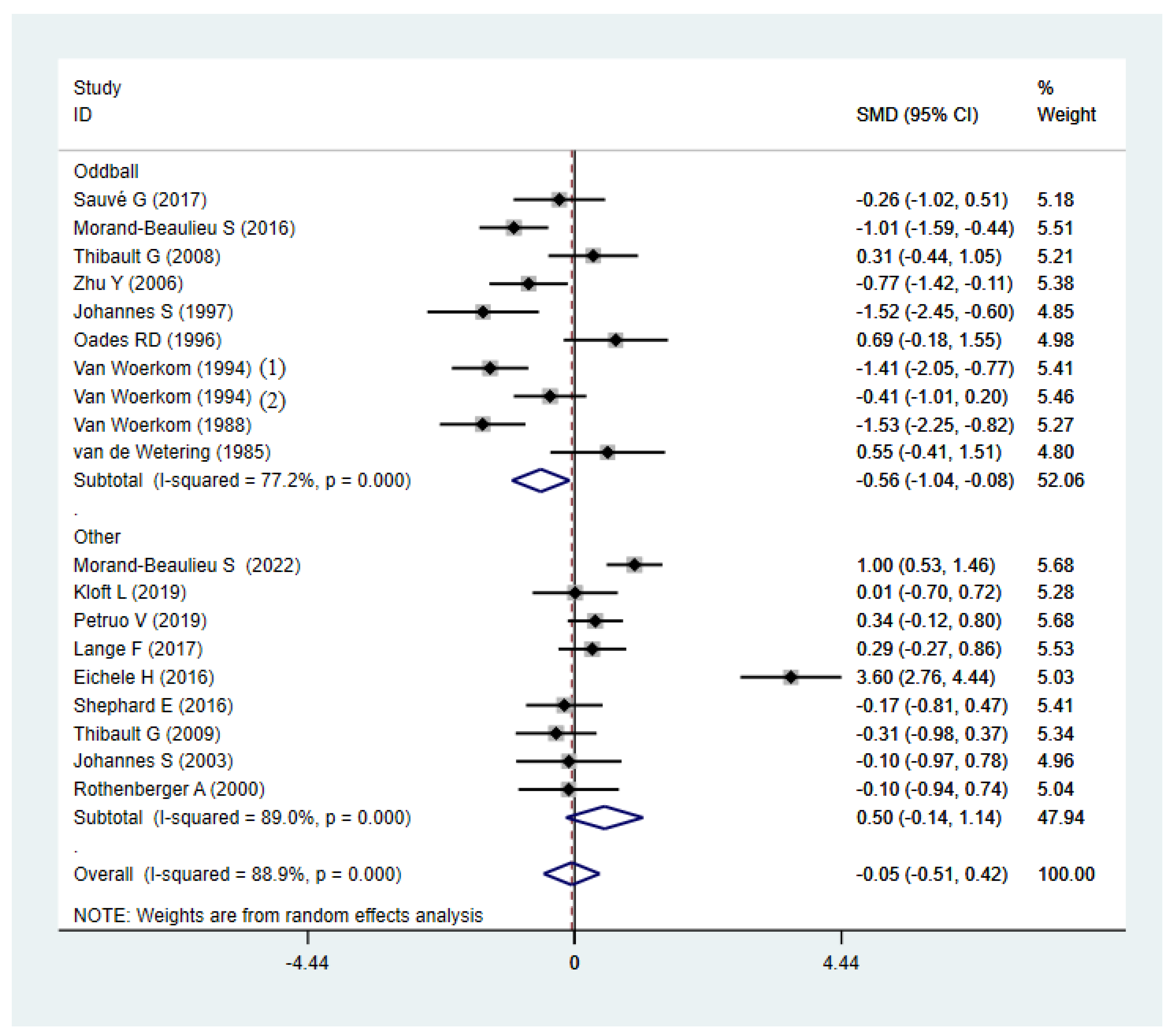

| Amplitude | Latency | |||||||
|---|---|---|---|---|---|---|---|---|
| k | p | I2(%) | SMD (95%CI) | k | p | I2 (%) | SMD (95%CI) | |
| Age group | ||||||||
| Adult | 11 | 0.000 | 77.10% | −0.46 (−0.91, −0.01) | 11 | 0.848 | 0.00% | −0.12 (−0.32, 0.09) |
| Child | 8 | 0.000 | 92.30% | 0.60 (−0.21, 1.41) | 9 | 0.961 | 0.00% | −0.04 (−0.24, 0.16) |
| Paradigm | ||||||||
| Oddball | 10 | 0.000 | 77.20% | −0.56 (−1.04, −0.08) | 11 | 0.949 | 0.00% | −0.05 (−0.25, 0.15) |
| Other | 9 | 0.000 | 89.00% | 0.50 (−0.14, 1.14) | 9 | 0.788 | 0.00% | −0.14 (−0.34, 0.06) |
| Modality | ||||||||
| Visual | 11 | 0.000 | 90.90% | 0.17 (−0.51, 0.84) | 11 | 0.852 | 0.00% | −0.08 (−0.27, 0.11) |
| Auditory | 8 | 0.000 | 85.80% | −0.26 (−0.91, 0.39) | 9 | 0.903 | 0.00% | −0.12 (−0.33, 0.10) |
| Comorbidity | ||||||||
| None | 7 | 0.000 | 83.70% | −0.16 (−0.75, 0.43) | 7 | 0.878 | 0.00% | −0.04 (−0.27, 0.18) |
| Yes | 6 | 0.000 | 93.40% | 0.38 (−0.8, 1.55) | 7 | 0.728 | 0.00% | −0.09 (−0.33, 0.15) |
| Unknown | 6 | 0.000 | 88.60% | −0.20 (−1.06, 0.65) | 6 | 0.785 | 0.00% | −0.18 (−0.45, 0.09) |
| Reaction method | ||||||||
| Keypress | 16 | 0.000 | 90.50% | −0.16 (−0.69, 0.37) | 17 | 0.906 | 0.00% | −0.07 (−0.22, 0.07) |
| Counts | 4 | 0.000 | 84.60% | −0.46 (−1.39, 0.47) | 4 | 0.935 | 0.00% | −0.11 (−0.44, 0.22) |
| None | 3 | 0.000 | 93.10% | 0.10 (−1.48, 1.68) | 3 | 0.493 | 0.00% | −0.17 (−0.54, 0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yang, H.; Deng, Y.; Yu, T.; Luo, R. P3b Amplitude and Latency in Tic Disorders: A Meta-Analysis. Brain Sci. 2022, 12, 1712. https://doi.org/10.3390/brainsci12121712
Yang Y, Yang H, Deng Y, Yu T, Luo R. P3b Amplitude and Latency in Tic Disorders: A Meta-Analysis. Brain Sciences. 2022; 12(12):1712. https://doi.org/10.3390/brainsci12121712
Chicago/Turabian StyleYang, Yue, Hua Yang, Yao Deng, Tao Yu, and Rong Luo. 2022. "P3b Amplitude and Latency in Tic Disorders: A Meta-Analysis" Brain Sciences 12, no. 12: 1712. https://doi.org/10.3390/brainsci12121712
APA StyleYang, Y., Yang, H., Deng, Y., Yu, T., & Luo, R. (2022). P3b Amplitude and Latency in Tic Disorders: A Meta-Analysis. Brain Sciences, 12(12), 1712. https://doi.org/10.3390/brainsci12121712






