Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Gaze Stabilization Exercises
2.4. Physical Therapy Treatments
2.5. Primary Outcome
2.6. Secondary Outcomes
2.7. Data and Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Primary Outcome
The Berg Balance Scale
3.3. Secondary Outcomes
3.3.1. Timed Up and Go Test
3.3.2. The Temporal and Spatial Characteristics of Gait
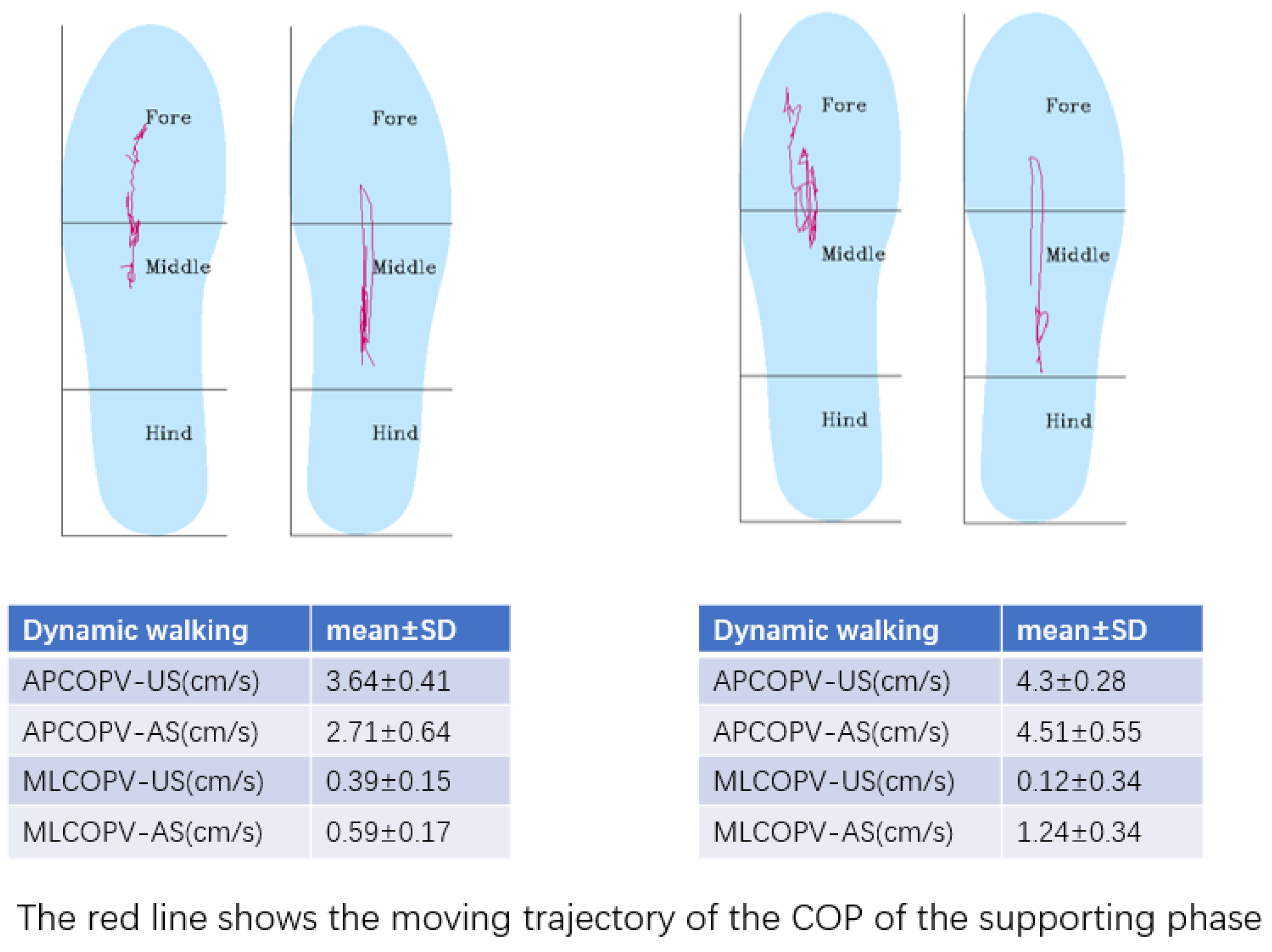
3.3.3. Plantar Pressure
4. Discussion
4.1. Balance and Fall Risk
4.2. Gait Performance
4.3. Plantar Pressure and Center of Pressure
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, A.; Paquette, C.; Fung, J. Stroke affects the coordination of gaze and posture during preplanned turns while walking. Neurorehabilit. Neural Repair 2007, 21, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, E.B. Contributions to the understanding of gait control. Dan. Med. J. 2014, 61, B4823. [Google Scholar]
- Lamontagne, A.; Fung, J. Gaze and postural reorientation in the control of locomotor steering after stroke. Neurorehabil. Neural Repair 2009, 23, 256–266. [Google Scholar] [CrossRef]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: From the American physical therapy association neurology section. J. Neurol. Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.; Lee, E. Effects of gaze stability exercises on cognitive function, dynamic postural ability, balance confidence, and subjective health status in old people with mild cognitive impairment. J. Exerc. Rehabil. 2019, 15, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, H.; Asai, Y.; Johnson, E.G.; Lohman, E.B.; Khoo, K.; Mizutani, Y.; Mizutani, T. Effect of oculo-motor and gaze stability exercises on postural stability and dynamic visual acuity in healthy young adults. Gait Posture 2011, 33, 600–603. [Google Scholar] [CrossRef]
- Lacour, M. Restoration of vestibular function: Basic aspects and practical advances for rehabilitation. Curr. Med. Res. Opin. 2006, 22, 1651–1659. [Google Scholar] [CrossRef]
- Deveze, A.; Bernard-Demanze, L.; Xavier, F.; Lavieille, J.P.; Elziere, M. Vestibular compensation and vestibular rehabilitation. Current concepts and new trends. Neurophysiol. Clin. 2014, 44, 49–57. [Google Scholar] [CrossRef]
- Allred, R.P.; Kim, S.Y.; Jones, T.A. Use it and/or lose it-experience effects on brain remodeling across time after stroke. Front. Hum. Neurosci. 2014, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Bernard-Demanze, L. Interaction between Vestibular Compensation Mechanisms and Vestibular Rehabilitation Therapy: 10 Recommendations for Optimal Functional Recovery. Front. Neurol. 2014, 5, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zobeiri, O.A.; Millar, J.L.; Schubert, M.C.; Cullen, K.E. Head movement kinematics are altered during gaze stability exercises in vestibular schwannoma patients. Sci. Rep. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Pimenta, C.; Correia, A.; Alves, M.; Virella, D. Effects of oculomotor and gaze stability exercises on balance after stroke: Clinical trial protocol. Porto Biomed. J. 2017, 2, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Fetter, M. Vestibulo-ocular reflex. Dev. Ophthalmol. 2007, 40, 35–51. [Google Scholar] [CrossRef]
- Mitsutake, T.; Sakamoto, M.; Ueta, K.; Oka, S.; Horikawa, E. Poor gait performance is influenced with decreased vestibulo-ocular reflex in poststroke patients. Neuroreport 2017, 28, 745–748. [Google Scholar] [CrossRef]
- Boyle, R. Activity of medial vestibulospinal tract cells during rotation and ocular movement in the alert squirrel monkey. J. Neurophysiol. 1993, 70, 2176–2180. [Google Scholar] [CrossRef]
- McCall, A.A.; Miller, D.M.; Yates, B.J. Descending Influences on Vestibulospinal and Vestibulosympathetic Reflexes. Front. Neurol. 2017, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Cecen, S.; Niazi, I.K.; Nedergaard, R.W.; Cade, A.; Allen, K.; Holt, K.; Haavik, H.; Turker, K.S. Posture modulates the sensitivity of the H-reflex. Exp. Brain Res. 2018, 236, 829–835. [Google Scholar] [CrossRef]
- Yeo, S.S.; Kwon, J.W.; Cho, I.H. Associations between Age-Related Changes in the Core Vestibular Projection Pathway and Balance Ability: A Diffusion Tensor Imaging Study. Behav. Neurol. 2020, 2020, 2825108. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Klein, C.S.; Suresh, N.L.; Rymer, W.Z. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: Evidence for a vestibulospinal role. Clin. Neurophysiol. 2014, 125, 2070–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.D.; Heusel-Gillig, L.; Tusa, R.J.; Herdman, S.J. Efficacy of gaze stability exercises in older adults with dizziness. J. Neurol. Phys. Ther. 2010, 34, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyd, B.J.; Fangman, A.; Peterson, D.S.; Gappmaier, E.; Schubert, M.C.; Thackery, A.; Dibble, L. Rehabilitation to improve gaze and postural stability in people with multiple sclerosis: Study protocol for a prospective randomized clinical trial. BMC Neurol. 2019, 19, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed-Jones, R.J.; Powell, D.W. The effects of gaze stabilization on gait parameters in individuals with Parkinson’s disease. Neurosci. Lett. 2017, 655, 156–159. [Google Scholar] [CrossRef]
- Correia, A.; Pimenta, C.; Alves, M.; Virella, D. Better balance: A randomised controlled trial of oculomotor and gaze stability exercises to reduce risk of falling after stroke. Clin. Rehabil. 2021, 35, 213–221. [Google Scholar] [CrossRef]
- Mitsutake, T.; Sakamoto, M.; Ueta, K.; Horikawa, E. Transient Effects of Gaze Stability Exercises on Postural Stability in Patients With Posterior Circulation Stroke. J. Mot. Behav. 2018, 50, 467–472. [Google Scholar] [CrossRef]
- Hiengkaew, V.; Jitaree, K.; Chaiyawat, P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-min walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch. Phys. Med. Rehabil. 2012, 93, 1201–1208. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Simel, D.L. Is this patient having a stroke. JAMA 2005, 293, 2391–2402. [Google Scholar] [CrossRef]
- Mathias, B.; Michael, F. Duu’s Topical Diagnosis in Neurology, 4th ed.; Thieme: New York, NY, USA, 2005; pp. 137–143. ISBN 978-158-890-215-3. [Google Scholar]
- Ricci, R.; Chatterjee, A. Context and crossover in unilateral neglect. Neuropsychologia 2001, 39, 1138–1143. [Google Scholar] [CrossRef]
- Sheppard, S.M.; Sebastian, R. Diagnosing and managing post-stroke aphasia. Expert Rev. Neurother. 2021, 21, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Lajoie, Y.; Gallagher, S.P. Predicting falls within the elderly community: Comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch. Gerontol. Geriatr. 2004, 38, 11–26. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar] [CrossRef]
- Yu, X.M.; Jin, X.M.; Lu, Y.; Gao, Y.; Xu, H.C.; Xue, X.; Fang, L.; Hu, J. Effects of Body Weight Support-Tai Chi Footwork Training on Balance Control and Walking Function in Stroke Survivors with Hemiplegia: A Pilot Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2020, 2020, 9218078. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, J.S.; Lai, S.M.; Duncan, P.W.; Studenski, S. Falls in community-dwelling stroke survivors: An accumulated impairments model. J. Rehabil. Res. Dev. 2002, 39, 385–394. [Google Scholar]
- Schmid, A.A.; Kapoor, J.R.; Dallas, M.; Bravata, D.M. Association between stroke severity and fall risk among stroke patients. Neuroepidemiology 2010, 34, 158–162. [Google Scholar] [CrossRef]
- Jefferis, B.J.; Iliffe, S.; Kendrick, D.; Kerse, N.; Trost, S.; Lennon, L.T.; Ash, S.; Sartini, C.; Morris, R.W.; Wannamethee, S.G.; et al. How are falls and fear of falling associated with objectively measured physical activity in a cohort of community-dwelling older men? BMC Geriatr. 2014, 14, 114. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H. The effects of eyeball exercise on balance ability and falls efficacy of the elderly who have experienced a fall: A single-blind, randomized controlled trial. Arch. Gerontol. Geriatr. 2017, 68, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Al-Eisa, E.S.; Anwer, S.; Sarkar, B. Reliability, validity, and responsiveness of three scales for measuring balance in patients with chronic stroke. BMC Neurol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Downs, S.; Marquez, J.; Chiarelli, P. The Berg Balance Scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: A systematic review. J. Physiother. 2013, 59, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Auvinet, B.; Touzard, C.; Montestruc, F.; Delafond, A.; Goeb, V. Gait disorders in the elderly and dual task gait analysis: A new approach for identifying motor phenotypes. J. Neuroeng. Rehabil. 2017, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S.M.; Rehab, N.I.; Aly, S.M.A. Effect of aquatic versus land motor dual task training on balance and gait of patients with chronic stroke: A randomized controlled trial. NeuroRehabilitation 2019, 44, 485–492. [Google Scholar] [CrossRef]
- Kamono, A.; Ogihara, N. Weight-shift ability significantly correlates with walking velocity in post-acute stroke patients. Proc. Inst. Mech. Eng. Part H 2018, 232, 361–370. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef]



| Baseline Characteristics | Control Group | GSEs Group | t/χ2 | p-Value |
|---|---|---|---|---|
| Age (year) | 54.45 ± 13.94 | 60.4 ± 12.32 | 1.430 | 0.161 |
| Gender (n, male/female) | 15/5 | 15/5 | 0.000 | 1.00 |
| Height (m) | 1.69 ± 0.07 | 1.68 ± 0.08 | −0.381 | 0.705 |
| Weight (kg) | 71.11 ± 9.51 | 68.75 ± 11.47 | −0.929 | 0.359 |
| BMI (kg/m2) | 25.20 ± 2.72 | 23.69 ± 2.95 | 1.682 | 0.101 |
| Disease duration (month) | 3.35 ± 1.87 | 3.95 ± 2.3 | −0.904 | 0.372 |
| ADL (score) | 69.35 ± 11.26 | 67.65 ± 13.61 | −0.937 | 0.355 |
| FMA-LE (score) | 23.26 ± 2.64 | 22.55 ± 3.02 | −0.731 | 0.469 |
| Affected side (n, left/right) | 9/11 | 6/14 | 0.960 | 0.327 |
| Stroke type (n, infarction/hemorrhage) | 12/8 | 14/6 | 0.440 | 0.714 |
| Lesion locations (n, basal ganglia region/cerebral hemisphere) | 12/8 | 13/7 | 0.107 | 0.744 |
| Pretraining | Post-Training | |||||
|---|---|---|---|---|---|---|
| Control Group (Mean ± SD) | GSEs Group (Mean ± SD) | p-Value | Control Group (Mean ± SD) | GSEs Group (Mean ± SD) | p-Value (Effect Size) | |
| BBS (score) | 44.25 ± 6.94 | 43.35 ± 4.89 | 0.135 | 48.35 ± 6.65 a | 49.05 ± 4.70 a | <0.001 (0.339) |
| TUGT (s) | 28.60 ± 11.80 | 28.99 ± 12.31 | 0.419 | 23.03 ± 8.79 a | 22.83 ± 11.14 a | <0.001 m (0.255) |
| ST-US (s) | 1.55 ± 0.65 | 1.67 ± 0.81 | 0.595 | 1.40 ± 0.54 a | 1.42 ± 0.65 a | 0.307 (0.027) |
| ST-AS (s) | 1.50 ± 0.66 | 1.51 ± 0.69 | 0.959 | 1.30 ± 0.48 a | 1.31 ± 0.48 a | 0.970 (0.001) |
| SW-US (s) | 0.37 ± 0.96 | 0.34 ± 0.55 | 0.129 | 0.41 ± 0.12 a | 0.36 ± 0.12 | 0.690 (0.004) |
| SW-AS (s) | 0.50 ± 1.35 | 0.53 ± 2.77 | 0.785 | 0.53 ± 0.15 | 0.45 ± 0.14 a | 0.041 (0.105) |
| ST-ASI | 9.42 ± 9.06 | 10.94 ± 8.42 | 0.586 | 11.56 ± 8.15 | 12.53 ± 9.75 | 0.862 (0.001) |
| SW-ASI | 32.96 ± 23.94 | 39.98 ± 31.10 | 0.160 | 32.65 ± 25.91 | 30.57 ± 33.08 a | 0.025 (0.126) |
| Pretraining | Post-Training | |||||
|---|---|---|---|---|---|---|
| Control Group (Mean ± SD) | GSEs Group (Mean ± SD) | p-Value | Control Group (Mean ± SD) | GSEs Group (Mean ± SD) | p-Value (Effect Size) | |
| APCOPV-US (cm/s) | 10.76 ± 5.78 | 9.10 ± 5.91 | 0.374 | 11.13 ± 4.46 a | 12.03 ± 6.83 a | 0.018 (0.139) |
| APCOPV-AS (cm/s) | 8.84 ± 4.95 | 8.30 ± 4.30 | 0.715 | 10.80 ± 4.27 | 11.82 ± 5.46 a | 0.013 m (0.155) |
| EEA-EO (mm2) | 177.82 ± 256.47 | 205.02 ± 403.66 | 0.801 | 143.01 ± 111.49 | 153.15 ± 200.73 | 0.358 m (0.021) |
| EEA-EC (mm2) | 234.57 ± 228.92 | 274.62 ± 184.22 | 0.546 | 146.28 ± 136.65 a | 73.21 ± 72.40 a | 0.014 m (0.152) |
| PPF-EO (%) | 44.20 ± 18.12 | 47.22 ± 18.06 | 0.601 | 48.71 ± 18.48 | 60.28 ± 17.86 a | 0.037 (0.110) |
| PPF-EC (%) | 43.87 ± 17.85 | 42.20 ± 12.99 | 0.737 | 48.19 ± 18.75 | 55.04 ± 13.77 a | 0.043 (0.103) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Lu, J.; Xiao, Y.; Liu, X.; Wang, Y.; Xu, G. Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial. Brain Sci. 2022, 12, 1694. https://doi.org/10.3390/brainsci12121694
Zhao R, Lu J, Xiao Y, Liu X, Wang Y, Xu G. Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial. Brain Sciences. 2022; 12(12):1694. https://doi.org/10.3390/brainsci12121694
Chicago/Turabian StyleZhao, Ruoxin, Jun Lu, Yue Xiao, Xinrong Liu, Yu Wang, and Guangxu Xu. 2022. "Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial" Brain Sciences 12, no. 12: 1694. https://doi.org/10.3390/brainsci12121694
APA StyleZhao, R., Lu, J., Xiao, Y., Liu, X., Wang, Y., & Xu, G. (2022). Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial. Brain Sciences, 12(12), 1694. https://doi.org/10.3390/brainsci12121694






