Anti-Correlated Myelin-Sensitive MRI Levels in Humans Consistent with a Subcortical to Sensorimotor Regulatory Process—Multi-Cohort Multi-Modal Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Cohorts and Image Sets
2.2. Image Processing
2.2.1. Preprocessing: Spatial Normalization
2.2.2. VBM
2.2.3. Preprocessing: Global Values
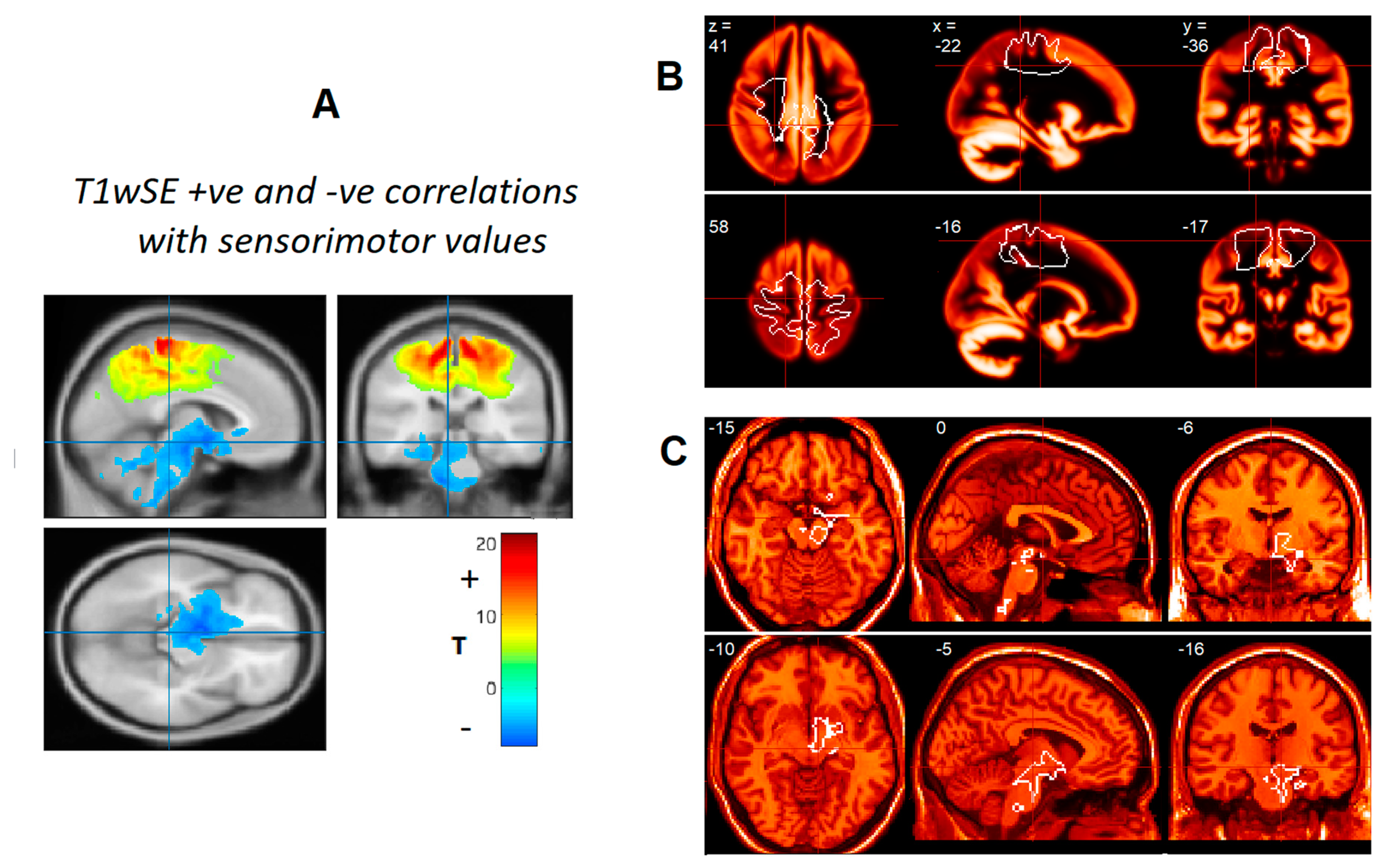
2.3. Regions of Interest (ROIs)
2.4. Removal of Group, Global and Age Variance before ROI Evaluation
2.5. Evaluation of Subcortical and Sensorimotor ROI Levels
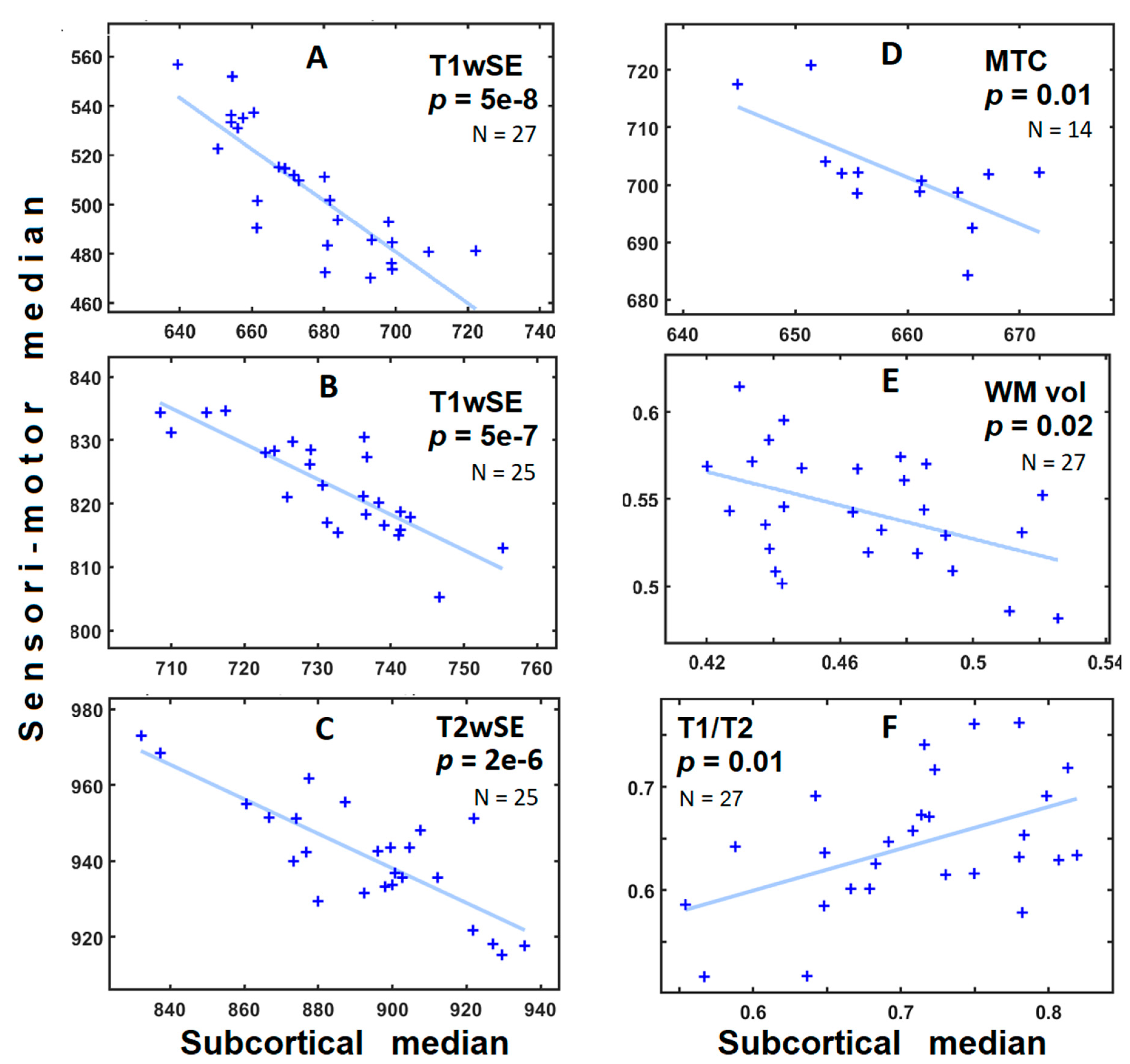
3. Results
4. Discussion
4.1. Myelin Regulation
- (a)
- (b)
- Fisher et al. [22] demonstrated that following the stimulation of different parts of the primary motor cortex (M1) and supplementary motor area (SMA) bilaterally, connections showed a high degree of convergence in reticulospinal neurons of the pontomedullary reticular formation allowing them to integrate information from across the motor areas of the cortex. The same neurons also receive converging sensory inputs from visual, auditory, cutaneous, proprioceptive, and vestibular systems—references in [22]. Thus, their output reflects both central and peripheral activity consistent with the afferent requirements of RAS excitatory neurons.
- (c)
4.2. Regulation of RAS–Sensorimotor Myelination
4.3. Generation of the Two ROIs
4.4. Advantage of GLM Methodology
4.5. T1/T2 Positive Correlation
4.6. Spin-Echo MRI Imaging
4.6.1. Myelin or Iron?
4.6.2. Spin-Echo Image Properties
4.6.3. T2SPACE
4.6.4. Improved Anatomical Support
4.6.5. Effect of Disease
4.7. T1wGRE (MPRAGE)
4.8. Artifacts from Scanner Differences
4.9. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnden, L.R.; Shan, Z.Y.; Staines, D.R.; Marshall-Gradisnik, S.; Finegan, K.; Ireland, T.; Bhuta, S. Hyperintense sensorimotor T1 spin echo MRI is associated with brainstem abnormality in chronic fatigue syndrome. NeuroImage Clin. 2018, 20, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Stüber, C.; Morawski, M.; Schäfer, A.; Labadie, C.; Wähnert, M.; Leuze, C.; Streicher, M.; Barapatre, N.; Reimann, K.; Geyer, S.; et al. Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. NeuroImage 2014, 93, 95–106. [Google Scholar] [CrossRef]
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M.; Boone, J.M.; Goldschmidt, E.J. The Essential Physics of Medical Imaging, 2nd ed.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Barnden, L.R.; Crouch, B.; Kwiatek, R.; Burnet, R.; Del Fante, P. Evidence in Chronic Fatigue Syndrome for severity-dependent upregulation of prefrontal myelination that is independent of anxiety and depression. NMR Biomed. 2015, 28, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Barnden, L.R.; Kwiatek, R.; Crouch, B.; Burnet, R.; Del Fante, P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. NeuroImage Clin. 2016, 11, 530–537. [Google Scholar] [CrossRef]
- Barnden, L.R.; Crouch, B.; Kwiatek, R.; Burnet, R.; Mernone, A.; Chryssidis, S.; Scroop, G.; Del Fante, P. A brain MRI study of chronic fatigue syndrome: Evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. 2011, 24, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, G.; Kamen, Y.; Evans, K.A.; Káradóttir, R.T. Unraveling Myelin Plasticity. Front. Cell. Neurosci. 2020, 14, 156. [Google Scholar] [CrossRef]
- Sled, J.G. Modelling and interpretation of magnetization transfer imaging in the brain. NeuroImage 2018, 182, 128–135. [Google Scholar] [CrossRef]
- Glasser, M.F.; Van Essen, D.C. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 2011, 31, 11597–11616. [Google Scholar] [CrossRef]
- Ganzetti, M.; Wenderoth, N.; Mantini, D. Mapping pathological changes in brain structure by combining T1- and T2-weighted MR imaging data. Neuroradiology 2015, 57, 917–928. [Google Scholar] [CrossRef]
- Thapaliya, K.; Marshall-Gradisnik, S.; Staines, D.; Barnden, L. Mapping of pathological change in chronic fatigue syndrome using the ratio of T1- and T2-weighted MRI scans. NeuroImage Clin. 2020, 28, 102366. [Google Scholar] [CrossRef] [PubMed]
- Mugler, J.P. Optimized three-dimensional fast-spin-echo MRI. J. Magn. Reson. Imaging 2014, 39, 745–767. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.; Frackowiak, R. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.F.; Pell, G.S.; Pardoe, H.; Jackson, G. Voxel-Based Iterative Sensitivity (VBIS): Methods and a validation of intensity scaling for T2-weighted imaging of hippocampal sclerosis. NeuroImage 2009, 44, 812–819. [Google Scholar] [CrossRef]
- Lazari, A.; Lipp, I. Can MRI measure myelin? Systematic review, qualitative assessment, and meta-analysis of studies validating microstructural imaging with myelin histology. NeuroImage 2021, 230, 117744. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Kezunovic, N.; Hyde, J.; Simon, C.; Beck, P.; Urbano, F.J. Coherence and frequency in the reticular activating system (RAS). Sleep Med. Rev. 2013, 17, 227–238. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; D’Onofrio, S.; Mahaffey, S. Bottom-up Gamma: The Pedunculopontine Nucleus and Reticular Activating System. Transl. Brain Rhythm. 2016, 1, 49–53. [Google Scholar] [CrossRef]
- Salami, M.; Itami, C.; Tsumoto, T.; Kimura, F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 6174–6179. [Google Scholar] [CrossRef]
- Kimura, F.; Itami, C. Myelination and isochronicity in neural networks. Front. Neuroanat. 2009, 3, 12. [Google Scholar] [CrossRef]
- Fisher, K.M.; Zaaimi, B.; Edgley, S.A.; Baker, S.N. Extensive Cortical Convergence to Primate Reticulospinal Pathways. J. Neurosci. 2021, 41, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Giulietti, G.; Dowell, N.; Spanò, B.; Harrison, N.; Bozzali, M.; Cercignani, M. Introducing axonal myelination in connectomics: A preliminary analysis of g-ratio distribution in healthy subjects. NeuroImage 2018, 182, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Micheva, K.D.; Wolman, D.; Mensh, B.D.; Pax, E.; Buchanan, J.; Smith, S.J.; Bock, D.D. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 2016, 5, e15784. [Google Scholar] [CrossRef] [PubMed]
- Micheva, K.D.; Kiraly, M.; Perez, M.M.; Madison, D.V. Conduction Velocity along the Local Axons of Parvalbumin Interneurons Correlates with the Degree of Axonal Myelination. Cereb. Cortex 2021, 31, 3374–3392. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Lutti, A.; Helms, G.; Frackowiak, R.; Ashburner, J. Multiparametric brainstem segmentation using a modified multivariate mixture of Gaussians. NeuroImage Clin. 2013, 2, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Saxe, R.; Brett, M.; Kanwisher, N. Divide and conquer: A defense of functional localizers. NeuroImage 2006, 30, 1088–1096. [Google Scholar] [CrossRef]
- Barnden, L.; Marshall, G.D.; Staines, D.; Crouch, B.; Shan, Z.Y. Sensitivity of structural brain MRI in clinical cross-sectional studies. In Proceedings of the ISMRM and SMRT Annual Meeting and Exhibition, Online, 15–20 May 2021. [Google Scholar]
- Keil, B.; Blau, J.N.; Biber, S.; Hoecht, P.; Tountcheva, V.; Setsompop, K.; Triantafyllou, C.; Wald, L. A 64-channel 3T array coil for accelerated brain MRI. Magn. Reson. Med. 2013, 70, 248–258. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Iron levels in the human brain: A post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Alonso-Ortiz, E.; Levesque, I.R.; Pike, G.B. Impact of magnetic susceptibility anisotropy at 3 T and 7 T on T2*-based myelin water fraction imaging. NeuroImage 2018, 182, 370–378. [Google Scholar] [CrossRef]
- Hametner, S.; Endmayr, V.; Deistung, A.; Palmrich, P.; Prihoda, M.; Haimburger, E.; Menard, C.; Feng, X.; Haider, T.; Leisser, M.; et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—A biochemical and histological validation study. NeuroImage 2018, 179, 117–133. [Google Scholar] [CrossRef]
| R1 (=1/T1) | R2* (=1/T2*) | |||
|---|---|---|---|---|
| Myelin | Iron | Myelin | Iron | |
| GM | 64% | 36% | 19% | 81% |
| WM | 90% | 10% | 56% | 44% |
| MRI | Cohort | N | Tesla | TR, TE, Flip Angle | nAv | Voxel Size | Scan Time |
|---|---|---|---|---|---|---|---|
| Image-Set | (ms/ms/degrees) | X Y Z mm | min:s | ||||
| T1wSE | 2016 | 27 | 3.0 | 600/6.4/90 | 2 | 0.86 0.86 3.0 | 8:52 |
| O3D FSE | 2016 | 27 | 3.0 | 3200/563/variable | 1 | 0.88 0.88 0.90 | 5:44 |
| T1wGRE | 2016 | 27 | 3.0 | 2400/1.81/8 | 1 | 1.0 1.0 1.0 | 3:13 |
| T1wSE | 2012 | 13 | 1.5 | 600/15/90 | 2 | 0.82 0.82 3.0 | 9:10 |
| MTC * | 2012 | 14 | 1.5 | 600/15/90 * | 2 | 0.82 0.82 3.0 | 6:08 |
| T1wSE | 2006 | 25 | 1.5 | 600/15/90 | 2 | 0.82 0.82 3.0 | 9:10 |
| T2wSE | 2006 | 25 | 1.5 | 4000/80/90 | 1 | 0.86 0.86 3.0 | 4:24 |
| MRI Modality | Cohort | N | p | R2 | Slope |
|---|---|---|---|---|---|
| T1wSE | 2016 | 27 | 5 × 10−8 | 0.69 | −1.0 |
| T1wSE | 2012 | 13 | 0.002 | 0.38 | −0.60 |
| T1wSE | 2006 | 25 | 5 × 10−7 | 0.66 | −0.56 |
| T2wSE | 2006 | 25 | 2 × 10−6 | 0.62 | −0.46 |
| MTC | 2012 | 14 | 0.01 | 0.39 | −0.81 |
| T1GRE | 2016 | 27 | 0.99 | 0.04 | +0.001 |
| T2SPACE | 2016 | 27 | 0.04 | 0.12 | −0.18 |
| WM volume | 2016 | 27 | 0.02 | 0.18 | −0.48 |
| T1/T2 | 2016 | 27 | 0.01 | 0.20 | +0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnden, L.; Crouch, B.; Kwiatek, R.; Shan, Z.; Thapaliya, K.; Staines, D.; Bhuta, S.; Del Fante, P.; Burnet, R. Anti-Correlated Myelin-Sensitive MRI Levels in Humans Consistent with a Subcortical to Sensorimotor Regulatory Process—Multi-Cohort Multi-Modal Evidence. Brain Sci. 2022, 12, 1693. https://doi.org/10.3390/brainsci12121693
Barnden L, Crouch B, Kwiatek R, Shan Z, Thapaliya K, Staines D, Bhuta S, Del Fante P, Burnet R. Anti-Correlated Myelin-Sensitive MRI Levels in Humans Consistent with a Subcortical to Sensorimotor Regulatory Process—Multi-Cohort Multi-Modal Evidence. Brain Sciences. 2022; 12(12):1693. https://doi.org/10.3390/brainsci12121693
Chicago/Turabian StyleBarnden, Leighton, Benjamin Crouch, Richard Kwiatek, Zack Shan, Kiran Thapaliya, Donald Staines, Sandeep Bhuta, Peter Del Fante, and Richard Burnet. 2022. "Anti-Correlated Myelin-Sensitive MRI Levels in Humans Consistent with a Subcortical to Sensorimotor Regulatory Process—Multi-Cohort Multi-Modal Evidence" Brain Sciences 12, no. 12: 1693. https://doi.org/10.3390/brainsci12121693
APA StyleBarnden, L., Crouch, B., Kwiatek, R., Shan, Z., Thapaliya, K., Staines, D., Bhuta, S., Del Fante, P., & Burnet, R. (2022). Anti-Correlated Myelin-Sensitive MRI Levels in Humans Consistent with a Subcortical to Sensorimotor Regulatory Process—Multi-Cohort Multi-Modal Evidence. Brain Sciences, 12(12), 1693. https://doi.org/10.3390/brainsci12121693







