High Morphine Use Disorder Susceptibility Is Predicted by Impaired Learning Ability in Mice
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. CPP Behavioral Experiment
2.3. Open Field Test (OF)
2.4. Morris Water Maze (MWM)
2.5. Contextual Fear Conditioning (CFC)
2.6. Social Preference (SP) and Novel Object Recognition (NOR)
2.7. Tail Flick Latency
2.8. Western Blotting
2.9. Statistical Analyses
3. Results
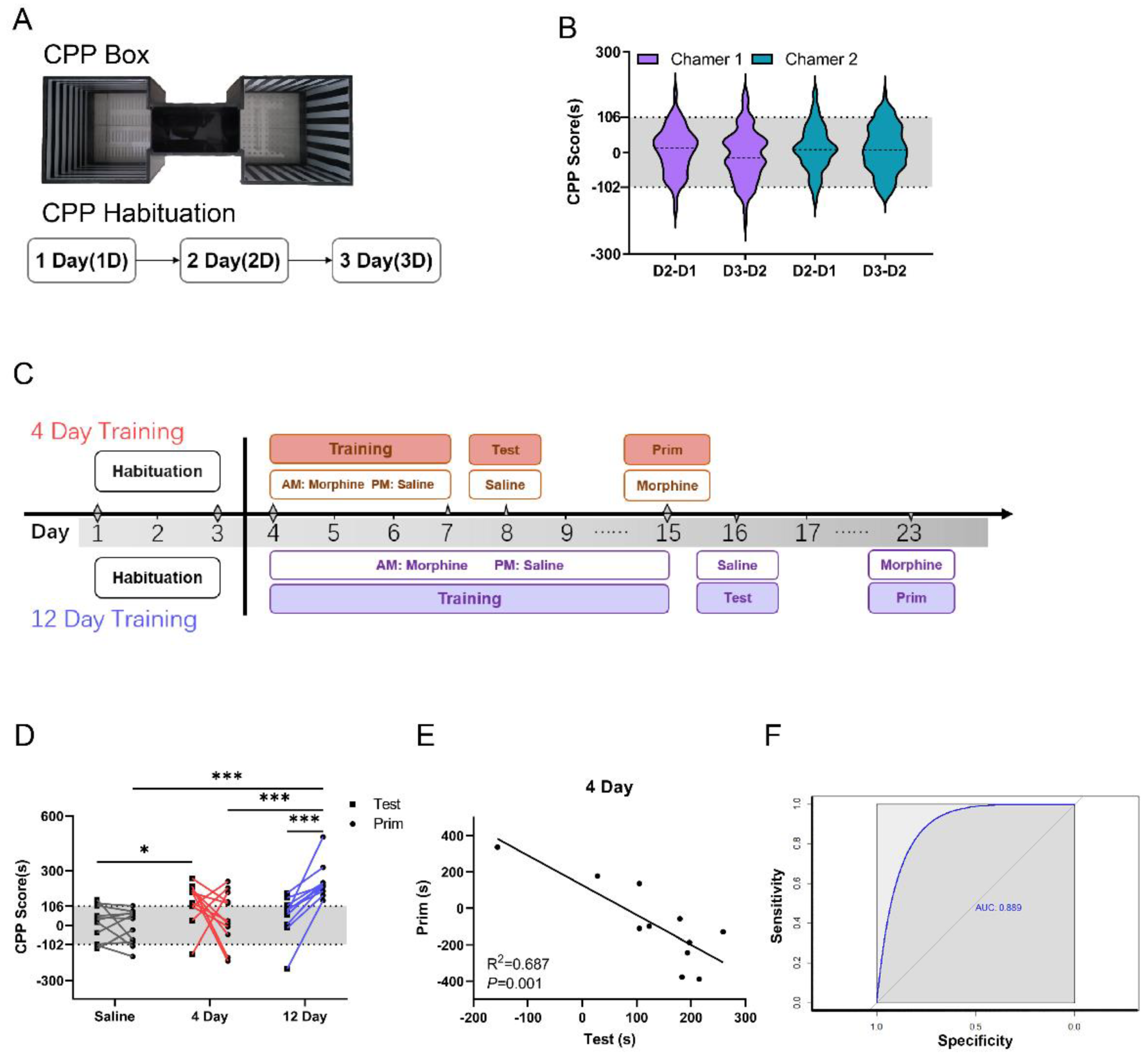
3.1. CPP Box Spontaneous Activity Score in C57 Mice
3.2. Learning Ability Is Negatively Correlated with SUD Susceptibility in C57 Mice
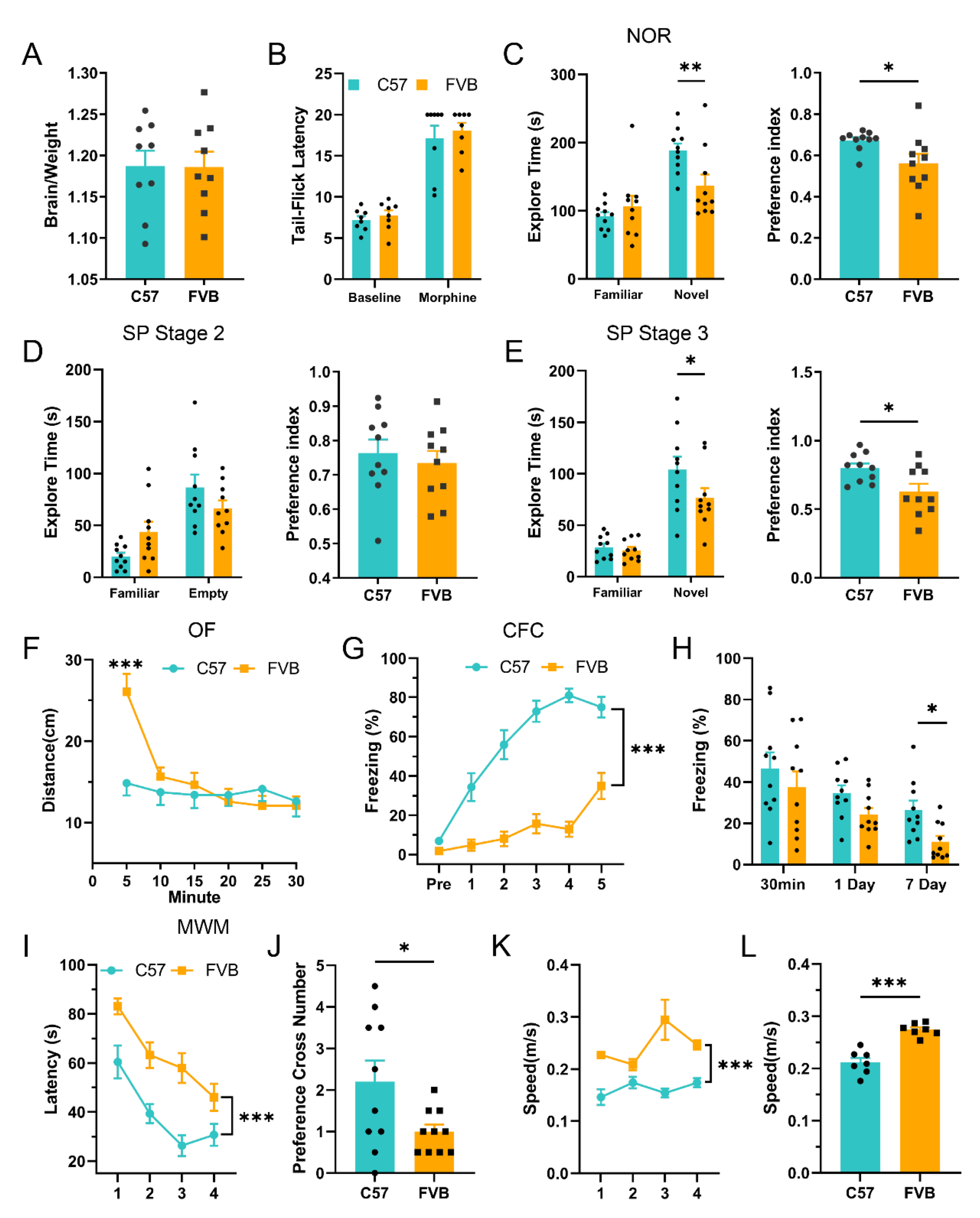
3.3. Poor Learning Ability in FVB Mice
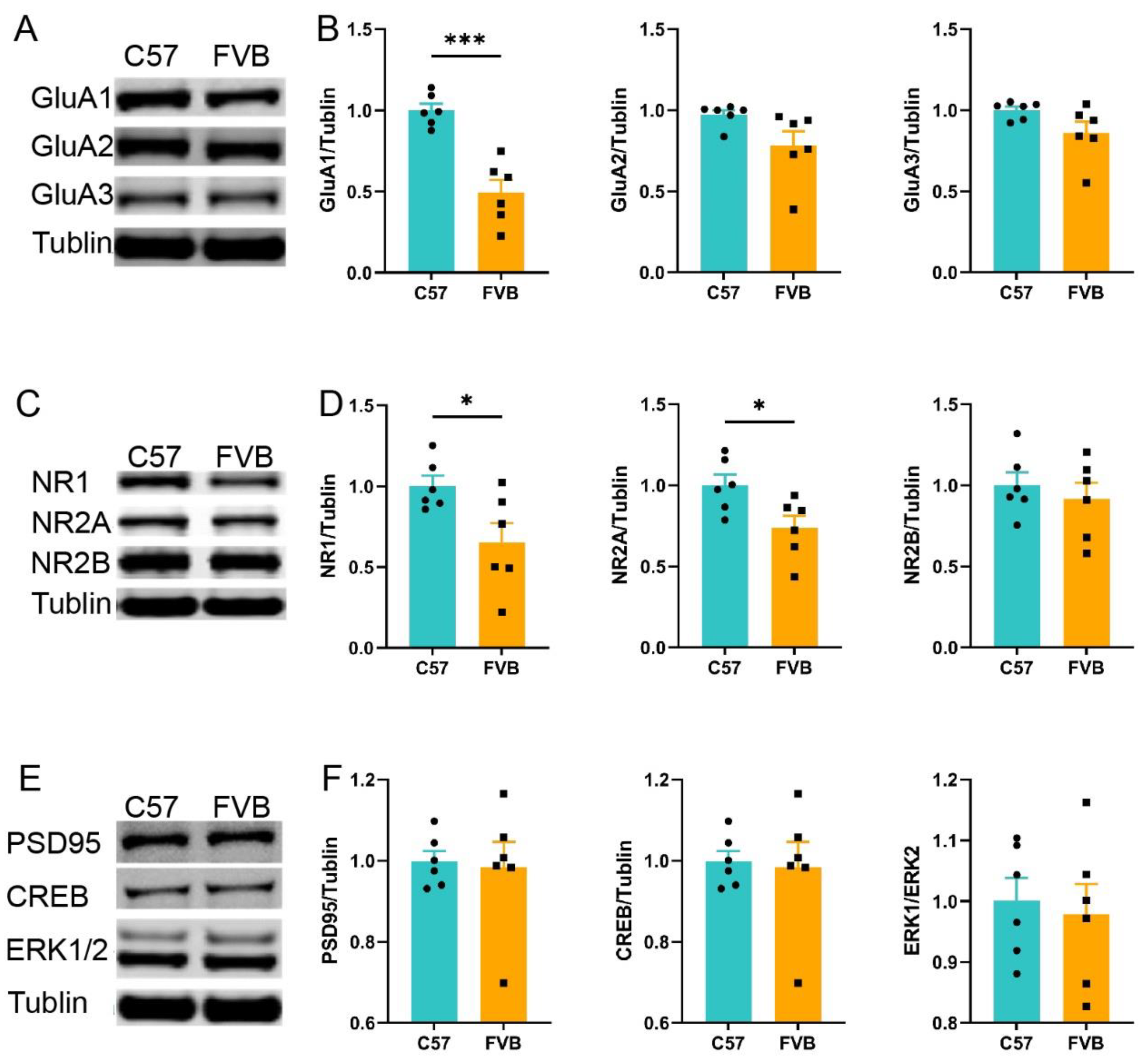
3.4. FVB Mice Have Higher SUD Susceptibility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajloo, N.; Sadeghi, H.; Nadinloei, K.B.; Habibi, Z. The Role of Meta-cognition in Students’ Addiction Potential Tendency. Int. J. High Risk Behav. Addict. 2014, 3, e9355. [Google Scholar] [CrossRef]
- Cadar, D.; Kaushal, A. Commentary on Daly & Egan (2017): Intelligence, education and addiction. Addiction 2017, 112, 660–661. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.J.; Demers, C.H.; Braud, J.; Briggs, R.; Adinoff, B.; Stein, E.A. Striatal-insula circuits in cocaine addiction: Implications for impulsivity and relapse risk. Am. J. Drug Alcohol Abus. 2013, 39, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, W.; Wang, H.; Wang, Y.; Zhang, Y.; Zhu, J.; Zheng, Y.; Zhang, D.; Wang, L.; Li, Y.; et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: An event-related functional magnetic resonance imaging study. Addict. Biol. 2014, 20, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Paterson, L.M.; Flechais, R.S.; Murphy, A.; Reed, L.J.; Abbott, S.; Boyapati, V.; Elliott, R.; Erritzoe, D.; Ersche, K.D.; Faluyi, Y.; et al. The Imperial College Cambridge Manchester (ICCAM) platform study: An experimental medicine platform for evaluating new drugs for relapse prevention in addiction. Part A: Study description. J. Psychopharmacol. 2015, 29, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.M.; Taylor, J.R. Neuroscience of learning and memory for addiction medicine: From habit formation to memory reconsolidation. Prog. Brain Res. 2016, 223, 91–113. [Google Scholar] [CrossRef]
- Leblanc, H.; Ramirez, S. Linking Social Cognition to Learning and Memory. J. Neurosci. 2020, 40, 8782–8798. [Google Scholar] [CrossRef]
- Bender, B.N.; Torregrossa, M.M. Molecular and circuit mechanisms regulating cocaine memory. Cell. Mol. Life Sci. 2020, 77, 3745–3768. [Google Scholar] [CrossRef]
- McKendrick, G.; Graziane, N.M. Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front. Behav. Neurosci. 2020, 14, 582147. [Google Scholar] [CrossRef]
- Xue, Y.-X.; Luo, Y.-X.; Wu, P.; Shi, H.-S.; Xue, L.-F.; Chen, C.; Zhu, W.-L.; Ding, Z.-B.; Bao, Y.-P.; Shi, J.; et al. A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse. Science 2012, 336, 241–245. [Google Scholar] [CrossRef]
- Aguilar, M.A.; Rodríguez-Arias, M.; Miñarro, J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res. Rev. 2009, 59, 253–277. [Google Scholar] [CrossRef] [PubMed]
- Deroche-Gamonet, V. The relevance of animal models of addiction. Addiction 2019, 115, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.N.; Kalivas, P.W.; Bobadilla, A.-C. Understanding Addiction Using Animal Models. Front. Behav. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef]
- Scherma, M.; Fattore, L.; Fratta, W.; Fadda, P. Conditioned Place Preference (CPP) in Rats: From Conditioning to Reinstatement Test. Methods Mol. Biol. 2020, 2201, 221–229. [Google Scholar] [CrossRef]
- Barzegari, A.A.; Shahabi, K. Effects of Isoniazid on Tolerance and Sensitization to the Rewarding Properties of Morphine: A Conditioned Place Preference Procedure Investigation in Mice. Basic Clin. Neurosci. J. 2020, 11, 481–490. [Google Scholar] [CrossRef]
- Seyedaghamiri, F.; Heysieattalab, S.; Hosseinmardi, N.; Janahmadi, M.; Elahi-Mahani, A.; Salari, F.; Golpayegani, M.; Khoshbouei, H. Hippocampal glial cells modulate morphine-induced behavioral responses. Physiol. Behav. 2018, 191, 37–46. [Google Scholar] [CrossRef]
- Han, H.; Dong, Z.; Jia, Y.; Mao, R.; Zhou, Q.; Yang, Y.; Wang, L.; Xu, L.; Cao, J. Opioid addiction and withdrawal differentially drive long-term depression of inhibitory synaptic transmission in the hippocampus. Sci. Rep. 2015, 5, 9666. [Google Scholar] [CrossRef]
- Dong, Z.; Cao, J.; Xu, L. Opiate withdrawal modifies synaptic plasticity in subicular–nucleus accumbens pathway in vivo. Neuroscience 2007, 144, 845–854. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Garcia-Fernandez, G.; Dom, G. Cognition and addiction. Dialogues Clin. Neurosci. 2019, 21, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Cushman, S.; Byrne, J.H. Special issue covering the relationship between mechanisms of addiction and learning. Learn. Mem. 2018, 25. [Google Scholar]
- Chiamulera, C.; Piva, A.; Abraham, W.C. Glutamate receptors and metaplasticity in addiction. Curr. Opin. Pharmacol. 2020, 56, 39–45. [Google Scholar] [CrossRef]
- Polli, F.S.; Ipsen, T.H.; Caballero-Puntiverio, M.; Østerbøg, T.B.; Aznar, S.; Andreasen, J.T.; Kohlmeier, K.A. Cellular and Molecular Changes in Hippocampal Glutamate Signaling and Alterations in Learning, Attention, and Impulsivity Following Prenatal Nicotine Exposure. Mol. Neurobiol. 2020, 57, 2002–2020. [Google Scholar] [CrossRef]
- Heinsbroek, J.A.; De Vries, T.J.; Peters, J. Glutamatergic Systems and Memory Mechanisms Underlying Opioid Addiction. Cold Spring Harb. Perspect. Med. 2020, 11, a039602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, K.; Li, Y.-H.; Han, T.-Z. NR2A-containing NMDA receptors are required for L-LTP induction and depotentiation in CA1 region of hippocampal slices. Eur. J. Neurosci. 2009, 29, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Appleby, V.J.; Correa, S.A.; Duckworth, J.K.; Nash, J.E.; Noel, J.; Fitzjohn, S.M.; Collingridge, G.L.; Molnar, E. LTP in hippocampal neurons is associated with a CaMKII-mediated increase in GluA1 surface expression. J. Neurochem. 2011, 116, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Albrecht, D. Glutamate receptor GluA1 subunit is implicated in capsaicin induced modulation of amygdala LTP but not LTD. Learn. Mem. 2017, 25, 1–7. [Google Scholar] [CrossRef]
- Brigman, J.L.; Feyder, M.; Saksida, L.M.; Bussey, T.J.; Mishina, M.; Holmes, A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn. Mem. 2008, 15, 50–54. [Google Scholar] [CrossRef][Green Version]
- Lu, G.; Zhou, Q.-X.; Kang, S.; Li, Q.-L.; Zhao, L.-C.; Chen, J.D.; Sun, J.-F.; Cao, J.; Wang, Y.-J.; Chen, X.-Y.; et al. Chronic Morphine Treatment Impaired Hippocampal Long-Term Potentiation and Spatial Memory via Accumulation of Extracellular Adenosine Acting on Adenosine A1 Receptors. J. Neurosci. 2010, 30, 5058–5070. [Google Scholar] [CrossRef]
- Adinoff, B.; Gu, H.; Merrick, C.; McHugh, M.; Jeon-Slaughter, H.; Lu, H.; Yang, Y.; Stein, E.A. Basal Hippocampal Activity and Its Functional Connectivity Predicts Cocaine Relapse. Biol. Psychiatry 2015, 78, 496–504. [Google Scholar] [CrossRef]
- Lehmann, V.E.; Kenny, P.J. Hippocampal plasticity may drive cocaine relapse. Proc. Natl. Acad. Sci. USA 2020, 117, 30003–30005. [Google Scholar] [CrossRef]
- Alvandi, M.S.; Bourmpoula, M.; Homberg, J.R.; Fathollahi, Y. Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats. Addict. Biol. 2017, 22, 1883–1894. [Google Scholar] [CrossRef]
- Carr, G.D.; White, N.M. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 1983, 33, 2551–2557. [Google Scholar] [CrossRef]
- Etemad, L.; Farkhari, H.; Alavi, M.S.; Roohbakhsh, A. The Effect of Dihydromyricetin, a Natural Flavonoid, on Morphine-induced Conditioned Place Preference and Physical Dependence in Mice. Drug Res. 2020, 70, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Rezayof, A.; Nazari-Serenjeh, F.; Zarrindast, M.-R.; Sepehri, H.; Delphi, L. Morphine-induced place preference: Involvement of cholinergic receptors of the ventral tegmental area. Eur. J. Pharmacol. 2007, 562, 92–102. [Google Scholar] [CrossRef]
- Wang, W.; Ju, Y.-Y.; Zhou, Q.-X.; Tang, J.-X.; Li, M.; Zhang, L.; Kang, S.; Chen, Z.-G.; Wang, Y.-J.; Ji, H.; et al. The Small GTPase Rac1 Contributes to Extinction of Aversive Memories of Drug Withdrawal by Facilitating GABAA Receptor Endocytosis in the vmPFC. J. Neurosci. 2017, 37, 7096–7110. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; Gonzalez, H.M.; Leger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Field, M.; Kersbergen, I. Are animal models of addiction useful? Addiction 2020, 115, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kraus, L.; Seitz, N.-N.; Schulte, B.; Cremer-Schaeffer, P.; Braun, B.; Verthein, U.; Pfeiffer-Gerschel, T. Estimation of the Number of People With Opioid Addiction in Germany. Dtsch. Arztebl. Int. 2019, 116, 137–143. [Google Scholar] [CrossRef]
- Guenzel, N.; McChargue, D. Addiction Relapse Prevention. In StatPearls; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Batty, G.D.; Deary, I.J.; Macintyre, S. Childhood IQ in relation to risk factors for premature mortality in middle-aged persons: The Aberdeen Children of the 1950s study. J. Epidemiology Community Heal. 2007, 61, 241–247. [Google Scholar] [CrossRef]
- Corley, J.; Gow, A.; Starr, J.M.; Deary, I.J. Smoking, childhood IQ, and cognitive function in old age. J. Psychosom. Res. 2012, 73, 132–138. [Google Scholar] [CrossRef]
- Venniro, M.; Caprioli, D.; Shaham, Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res. 2016, 224, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Stewart, J. Cocaine-induced conditioned place preference: Reinstatement by priming injections of cocaine after extinction. Behav. Brain Res. 2000, 115, 39–47. [Google Scholar] [CrossRef]
- Eisener-Dorman, A.F.; Grabowski-Boase, L.; Tarantino, L.M. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav. Brain Funct. 2011, 7, 29. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Metten, P.; Finn, D.A.; Rhodes, J.S.; Bergeson, S.E.; Harris, R.A.; Crabbe, J.C. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol. Clin. Exp. Res. 2005, 29, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Ozburn, A.R.; Harris, R.A.; Blednov, Y.A. Behavioral differences between C57BL/6J x FVB/NJ and C57BL/6J x NZB/B1NJ F1 hybrid mice: Relation to control of ethanol intake. Behav. Genet. 2010, 40, 551–563. [Google Scholar] [CrossRef]
- Kauer, J.A.; Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007, 8, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Barker, G.; Warburton, E.C. Object-in-Place Associative Recognition Memory Depends on Glutamate Receptor Neurotransmission Within Two Defined Hippocampal-Cortical Circuits: A Critical Role for AMPA and NMDA Receptors in the Hippocampus, Perirhinal, and Prefrontal Cortices. Cereb. Cortex 2013, 25, 472–481. [Google Scholar] [CrossRef]
- Marcondes, L.A.; Nachtigall, E.G.; Zanluchi, A.; Myskiw, J.D.C.; Izquierdo, I.; Furini, C.R.G. Involvement of medial prefrontal cortex NMDA and AMPA/kainate glutamate receptors in social recognition memory consolidation. Neurobiol. Learn. Mem. 2020, 168, 107153. [Google Scholar] [CrossRef]
- Reimers, J.M.; Milovanovic, M.; Wolf, M.E. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011, 1367, 223–233. [Google Scholar] [CrossRef]
- Ding, R.; Li, Y.; Du, A.; Yu, H.; He, B.; Shen, R.; Zhou, J.; Li, L.; Cui, W.; Zhang, G.; et al. Changes in hippocampal AMPA receptors and cognitive impairments in chronic ketamine addiction models: Another understanding of ketamine CNS toxicity. Sci. Rep. 2016, 6, 38771. [Google Scholar] [CrossRef]
- Lane, D.; Reed, B.; Kreek, M.; Pickel, V. Differential glutamate AMPA-receptor plasticity in subpopulations of VTA neurons in the presence or absence of residual cocaine: Implications for the development of addiction. Neuropharmacology 2011, 61, 1129–1140. [Google Scholar] [CrossRef]
- Carr, D.B.; Kalivas, P.W. Confused about NMDA and Addiction? Targeted Knockouts Provide Answers and New Questions. Neuron 2008, 59, 353–355. [Google Scholar] [CrossRef][Green Version]
- Roche, K.W. The expanding role of PSD-95: A new link to addiction. Trends Neurosci. 2004, 27, 699–700. [Google Scholar] [CrossRef]
- Wolf, M.E. Regulation of AMPA Receptor Trafficking in the Nucleus Accumbens by Dopamine and Cocaine. Neurotox. Res. 2010, 18, 393–409. [Google Scholar] [CrossRef]
- Aitta-Aho, T.; Möykkynen, T.P.; Panhelainen, A.E.; Vekovischeva, O.Y.; Bäckström, P.; Korpi, E.R. Importance of GluA1 Subunit-Containing AMPA Glutamate Receptors for Morphine State-Dependency. PLoS ONE 2012, 7, e38325. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.-F.; Zhao, Y.-B.; Yang, Y.-X.; Ma, C.; Li, M.; Li, X.; Ma, G.-R.; Zhu, L.-S.; Xu, L.; Zhou, Q.-X. High Morphine Use Disorder Susceptibility Is Predicted by Impaired Learning Ability in Mice. Brain Sci. 2022, 12, 1650. https://doi.org/10.3390/brainsci12121650
Hou X-F, Zhao Y-B, Yang Y-X, Ma C, Li M, Li X, Ma G-R, Zhu L-S, Xu L, Zhou Q-X. High Morphine Use Disorder Susceptibility Is Predicted by Impaired Learning Ability in Mice. Brain Sciences. 2022; 12(12):1650. https://doi.org/10.3390/brainsci12121650
Chicago/Turabian StyleHou, Xue-Fei, Ya-Bo Zhao, Yue-Xiong Yang, Chen Ma, Meng Li, Xin Li, Guo-Rui Ma, Li-Su Zhu, Lin Xu, and Qi-Xin Zhou. 2022. "High Morphine Use Disorder Susceptibility Is Predicted by Impaired Learning Ability in Mice" Brain Sciences 12, no. 12: 1650. https://doi.org/10.3390/brainsci12121650
APA StyleHou, X.-F., Zhao, Y.-B., Yang, Y.-X., Ma, C., Li, M., Li, X., Ma, G.-R., Zhu, L.-S., Xu, L., & Zhou, Q.-X. (2022). High Morphine Use Disorder Susceptibility Is Predicted by Impaired Learning Ability in Mice. Brain Sciences, 12(12), 1650. https://doi.org/10.3390/brainsci12121650




