Smokers’ Self-Report and Behavioral Reactivity to Combined Personal Smoking Cues (Proximal + Environment + People): A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Overview
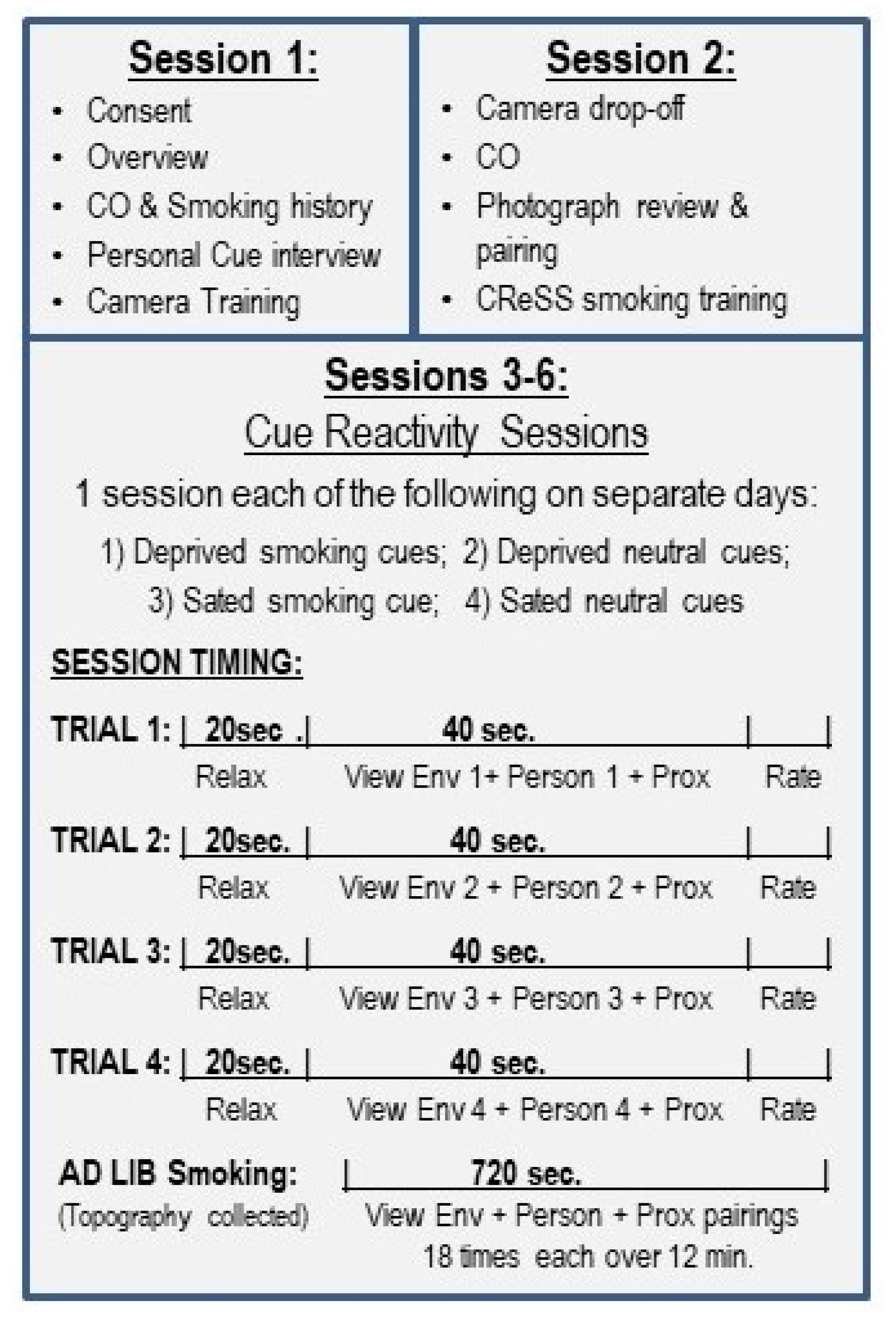
2.3. Session 1
2.4. Session 2
2.5. Sessions 3–6
A prompt will appear on the screen instructing you to sit back in the chair and relax. Following that, pictures of people will appear on the computer screen to the left in front of you, objects are on the table to the right in front of you, and pictures of environments will be displayed on the wall across from you. You are to focus on the people and items within the environments you see. Keep focusing on these things until the computer screen changes and prompts you to answer questions based on how you felt while focusing on that scenario.
When the pictures reappear, you may smoke as much or as little as you like. You do not have to smoke if you choose not to. If you smoke, you must light the cigarette and use the cigarette holder the same way you did earlier.
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, E.K.; Monti, P.M. The cue reactivity paradigm in addiction research. In The Wiley-Blackwell Handbook of Addiction Psychopharmacology; Wiley: Hoboken, NJ, USA, 2013; pp. 381–410. [Google Scholar] [CrossRef]
- Betts, J.M.; Dowd, A.N.; Forney, M.; Hetelekides, E.; Tiffany, S.T. A meta-analysis of cue reactivity in tobacco cigarette smokers. Nicotine Tob. Res. 2021, 23, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.L.; Tiffany, S.T. Meta-analysis of cue-reactivity in addiction research. Addiction 1999, 94, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.A.; Vella, E.J.; Joyce, C.J.; Salkeld, R.P.; Perkins, K.A.; Parzynski, C.S. Examining the relationship between cue-induced craving and actual smoking. Exp. Clin. Psychopharmacol. 2015, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.G.; Shiffman, S. The relevance and treatment of cue-induced cravings in tobacco dependence. J. Subst. Abuse Treat. 2009, 36, 235–243. [Google Scholar] [CrossRef]
- Conklin, C.A. Environments as cues to smoke: Implications for human extinction-based research and treatment. Exp. Clin. Psychopharmacol. 2006, 14, 12. [Google Scholar] [CrossRef]
- AhnAllen, C.G.; Tidey, J.W. Personalized smoking environment cue reactivity in smokers with schizophrenia and controls: A pilot study. Psychiatry Res. 2011, 188, 286–288. [Google Scholar] [CrossRef]
- Conklin, C.A.; Robin, N.; Perkins, K.A.; Salkeld, R.P.; McClernon, F.J. Proximal versus distal cues to smoke: The effects of environments on smokers’ cue-reactivity. Exp. Clin. Psychopharmacol. 2008, 16, 207–214. [Google Scholar] [CrossRef]
- Conklin, C.A.; Salkeld, R.P.; Perkins, K.A.; Robin, N. Do people serve as cues to smoke? Nicotine Tob. Res. 2013, 15, 2081–2087. [Google Scholar] [CrossRef]
- García-Rodríguez, O.; Pericot-Valverde, I.; Gutiérrez-Maldonado, J.; Ferrer-García, M.; Secades-Villa, R. Validation of smoking-related virtual environments for cue exposure therapy. Addict. Behav. 2012, 37, 703–708. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lim, Y.; Wiederhold, B.K.; Graham, S.J. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl. Psychophysiol. Biofeedback 2005, 30, 195–204. [Google Scholar] [CrossRef]
- McClernon, F.J.; Conklin, C.A.; Kozink, R.V.; Adcock, R.A.; Sweitzer, M.M.; Addicott, M.A.; Chou, Y.; Chen, N.; Hallyburton, M.B.; DeVito, A.M. Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug Context Effects in Nicotine Dependence. Neuropsychopharmacology 2015, 41, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.G.; Oliver, J.A.; Hallyburton, M.B.; Sweitzer, M.M.; Conklin, C.A.; McClernon, F.J. Smoking environment cues reduce ability to resist smoking as measured by a delay to smoking task. Addict. Behav. 2017, 67, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Brandon, K.O.; Vinci, C.; Kleinjan, M.; Hernandez, L.M.; Sawyer, L.E.; Sutton, S.K.; Brandon, T.H. Testing augmented reality for Eliciting cue-Provoked Urges to smoke: Toward moving cue-exposure into the real world. Nicotine Tob. Res. 2021, 23, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Vollstaedt-Klein, S.; Nees, F.; Wieland, A.; Karl, D.; Diener, C.; Smolka, M.; Flor, H. Contexts enhance ratings of craving and psychophysiological responses of cue-reactivity in tobacco use disorder. medRxiv 2022. [Google Scholar] [CrossRef]
- Conklin, C.A.; Perkins, K.A.; Robin, N.; McClernon, F.J.; Salkeld, R.P. Bringing the real world into the laboratory: Personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010, 111, 58–63. [Google Scholar] [CrossRef]
- Conklin, C.A.; McClernon, F.J.; Vella, E.J.; Joyce, C.J.; Salkeld, R.P.; Parzynski, C.S.; Bennett, L. Combined smoking cues enhance reactivity and predict immediate subsequent smoking. Nicotine Tob. Res. 2019, 21, 241–248. [Google Scholar] [CrossRef]
- Yang, M.-J.; Brandon, K.O.; Sutton, S.K.; Kleinjan, M.; Hernandez, L.M.; Sawyer, L.E.; Brandon, T.H.; Vinci, C. Augmented reality for extinction of cue-provoked urges to smoke: Proof of concept. Psychol. Addict. Behav. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Shiffman, S.; Dunbar, M.; Kirchner, T.; Li, X.; Tindle, H.; Anderson, S.; Scholl, S. Smoker reactivity to cues: Effects on craving and on smoking behavior. J. Abnorm. Psychol. 2013, 122, 264. [Google Scholar] [CrossRef]
- Conklin, C.A.; Tiffany, S.T. Applying extinction research and theory to cue-exposure addiction treatments. Addict. Abingdon Engl. 2002, 97, 155–167. [Google Scholar] [CrossRef]
- Conklin, C.A.; Tiffany, S.T. Cue-Exposure Treatment: Time for Change. Addiction 2002, 97, 1219–1221. [Google Scholar] [CrossRef]
- Martin, J.E.; Calfas, K.J.; Patten, C.A.; Polarek, M.; Hofstetter, C.R.; Noto, J.; Beach, D. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: One-year results of Project SCRAP-Tobacco. J. Consult. Clin. Psychol. 1997, 65, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Goedeker, K.C.; Tiffany, S.T. The impact of cigarette deprivation and cigarette availability on cue–reactivity in smokers. Addiction 2010, 105, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.L.; Robinson, J.D.; Lam, C.Y.; Wetter, D.W.; Tsan, J.Y.; Day, S.X.; Cinciripini, P.M. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob. Res. 2006, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Control, C.; CDC. Vital signs: Current cigarette smoking among adults aged ≥ 18 years–United States, 2005–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1207–1212. [Google Scholar]
- Perkins, K.A.; Karelitz, J.L.; Jao, N.C. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob. Res. 2013, 15, 978–982. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Carter, B.L.; Tiffany, S.T. Cue-reactivity and the future of addiction research. Addict. Abingdon Engl. 1999, 94, 349–351. [Google Scholar]
- Baker, T.B.; Morse, E.; Sherman, J.E. The Nebraska Symposium on Motivation: Alcohol Use and Abuse; University of Nebraska Press: Lincoln, NE, USA, 1987. [Google Scholar]
- Powell, J. Conditioned responses to drug-related stimuli: Is context crucial. Addiction 1995, 90, 1089–1095. [Google Scholar]
- Mackintosh, N.J. The Psychology of Animal Learning; Academic Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Hutchison, K.E.; Monti, P.M.; Rohsenow, D.J.; Swift, R.M.; Colby, S.M.; Gnys, M.; Niaura, R.S.; Sirota, A.D. Effects of naltrexone with nicotine replacement on smoking cue reactivity: Preliminary results. Psychopharmacology 1999, 142, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.J.; Shiffman, S.; Sayette, M.A.; Paty, J.A.; Gwaltney, C.J.; Balabanis, M.H. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J. Consult. Clin. Psychol. 2004, 72, 1136. [Google Scholar] [CrossRef]
- Franklin, T.; Wang, Z.; Suh, J.J.; Hazan, R.; Cruz, J.; Li, Y.; Goldman, M.; Detre, J.A.; O’Brien, C.P.; Childress, A.R. Effects of Varenicline on Smoking Cue–Triggered Neural and Craving Responses. Arch. Gen. Psychiatry 2011, 68, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Kroczek, A.M.; Häußinger, F.B.; Rohe, T.; Schneider, S.; Plewnia, C.; Batra, A.; Fallgatter, A.J.; Ehlis, A.-C. Effects of transcranial direct current stimulation on craving, heart-rate variability and prefrontal hemodynamics during smoking cue exposure. Drug Alcohol Depend. 2016, 168, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mondino, M.; Luck, D.; Grot, S.; Januel, D.; Suaud-Chagny, M.F.; Poulet, E.; Brunelin, J. Effects of repeated transcranial direct current stimulation on smoking, craving and brain reactivity to smoking cues. Sci. Rep. 2018, 8, 8724. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.O.; Rinck, M.; Rosenfield, D.; Davis, M.L.; Fisher, J.R.; Becker, E.S.; Powers, M.B.; Smits, J.A. Reducing approach bias to achieve smoking cessation: A pilot randomized placebo-controlled trial. Cogn. Ther. Res. 2017, 41, 662–670. [Google Scholar] [CrossRef]
- Wiers, R.W.; Eberl, C.; Rinck, M.; Becker, E.S.; Lindenmeyer, J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol. Sci. 2011, 22, 490–497. [Google Scholar] [CrossRef]
- Carpenter, M.J.; Saladin, M.E.; DeSantis, S.; Gray, K.M.; LaRowe, S.D.; Upadhyaya, H.P. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addict. Behav. 2009, 34, 536–541. [Google Scholar] [CrossRef]
- Payne, T.J.; Smith, P.O.; Adams, S.G.; Diefenbach, L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict. Behav. 2006, 31, 702–710. [Google Scholar] [CrossRef]
- Conklin, C.A.; Parzynski, C.S.; Salkeld, R.P.; Perkins, K.A.; Fonte, C.A. Cue reactivity as a predictor of successful abstinence initiation among adult smokers. Exp. Clin. Psychopharmacol. 2012, 20, 473. [Google Scholar] [CrossRef][Green Version]
- Sayette, M.A.; Tiffany, S.T. Peak provoked craving: An alternative to smoking cue-reactivity. Addiction 2013, 108, 1019–1025. [Google Scholar] [CrossRef]
- Kang, O.-S.; Chang, D.-S.; Jahng, G.-H.; Kim, S.-Y.; Kim, H.; Kim, J.-W.; Chung, S.-Y.; Yang, S.-I.; Park, H.-J.; Lee, H. Individual differences in smoking-related cue reactivity in smokers: An eye-tracking and fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 285–293. [Google Scholar] [CrossRef]
- Rahmani, N.; Chung, J.; Eizenman, M.; Jiang, P.; Zhang, H.; Selby, P.; Zawertailo, L. Differences in attentional bias to smoking-related, affective, and sensation-seeking cues between smokers and non-smokers: An eye-tracking study. Psychopharmacology 2022, 239, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Duka, T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacol. Biochem. Behav. 2004, 78, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Doran, N. Sex differences in smoking cue reactivity: Craving, negative affect, and preference for immediate smoking. Am. J. Addict. 2014, 23, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.J.; Saladin, M.E.; LaRowe, S.D.; McClure, E.A.; Simonian, S.; Upadhyaya, H.P.; Gray, K.M. Craving, cue reactivity, and stimulus control among early-stage young smokers: Effects of smoking intensity and gender. Nicotine Tob. Res. 2014, 16, 208–215. [Google Scholar] [CrossRef]
- Perkins, K.A.; Gerlach, D.; Vender, J.; Meeker, J.; Hutchison, S.; Grobe, J. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob. Res. 2001, 3, 141–150. [Google Scholar] [CrossRef]
- McClernon, F.J.; Kozink, R.V.; Rose, J.E. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 2008, 33, 2148–2157. [Google Scholar] [CrossRef]
- Wetherill, R.R.; Young, K.A.; Jagannathan, K.; Shin, J.; O’Brien, C.P.; Childress, A.R.; Franklin, T.R. The impact of sex on brain responses to smoking cues: A perfusion fMRI study. Biol. Sex Differ. 2013, 4, 9. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Cartocci, G.; Modica, E.; Rossi, D.; Martinez-Levy, A.C.; Cherubino, P.; Tamborra, L.; Babiloni, F. Smoke signals: A study of the neurophysiological reaction of smokers and non-smokers to smoking cues inserted into antismoking public service announcements. Int. J. Psychophysiol. 2021, 167, 22–29. [Google Scholar] [CrossRef]
- Niaura, R.; Shadel, W.G.; Abrams, D.B.; Monti, P.M.; Rohsenow, D.J.; Sirota, A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addict. Behav. 1998, 23, 209–224. [Google Scholar] [CrossRef]


| Dependent Variable | Cue Type | Condition | |
|---|---|---|---|
| Sated | Deprived | ||
| Mean (SD) | Mean (SD) | ||
| Cue-Provoked Craving | Nonsmoking Cues | 9 (14) | 53 (34) |
| Smoking Cues | 30 (29) | 78 (27) | |
| Latency to Light (s) | Nonsmoking Cues | 578 (264) | 77 (177) |
| Smoking Cues | 381 (352) | 24 (37) | |
| Puff Volume (mL) | Nonsmoking Cues | 130.3 (251.6) | 649.2 (473.9) |
| Smoking Cues | 394.3 (440.8) | 873.3 (447.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conklin, C.A.; Coffman, B.A.; McClernon, F.J.; Joyce, C. Smokers’ Self-Report and Behavioral Reactivity to Combined Personal Smoking Cues (Proximal + Environment + People): A Pilot Study. Brain Sci. 2022, 12, 1547. https://doi.org/10.3390/brainsci12111547
Conklin CA, Coffman BA, McClernon FJ, Joyce C. Smokers’ Self-Report and Behavioral Reactivity to Combined Personal Smoking Cues (Proximal + Environment + People): A Pilot Study. Brain Sciences. 2022; 12(11):1547. https://doi.org/10.3390/brainsci12111547
Chicago/Turabian StyleConklin, Cynthia A., Brian A. Coffman, F. Joseph McClernon, and Christopher Joyce. 2022. "Smokers’ Self-Report and Behavioral Reactivity to Combined Personal Smoking Cues (Proximal + Environment + People): A Pilot Study" Brain Sciences 12, no. 11: 1547. https://doi.org/10.3390/brainsci12111547
APA StyleConklin, C. A., Coffman, B. A., McClernon, F. J., & Joyce, C. (2022). Smokers’ Self-Report and Behavioral Reactivity to Combined Personal Smoking Cues (Proximal + Environment + People): A Pilot Study. Brain Sciences, 12(11), 1547. https://doi.org/10.3390/brainsci12111547






