Evaluating the Alterations Induced by Virtual Reality in Cerebral Small-World Networks Using Graph Theory Analysis with Electroencephalography
Abstract
:1. Introduction
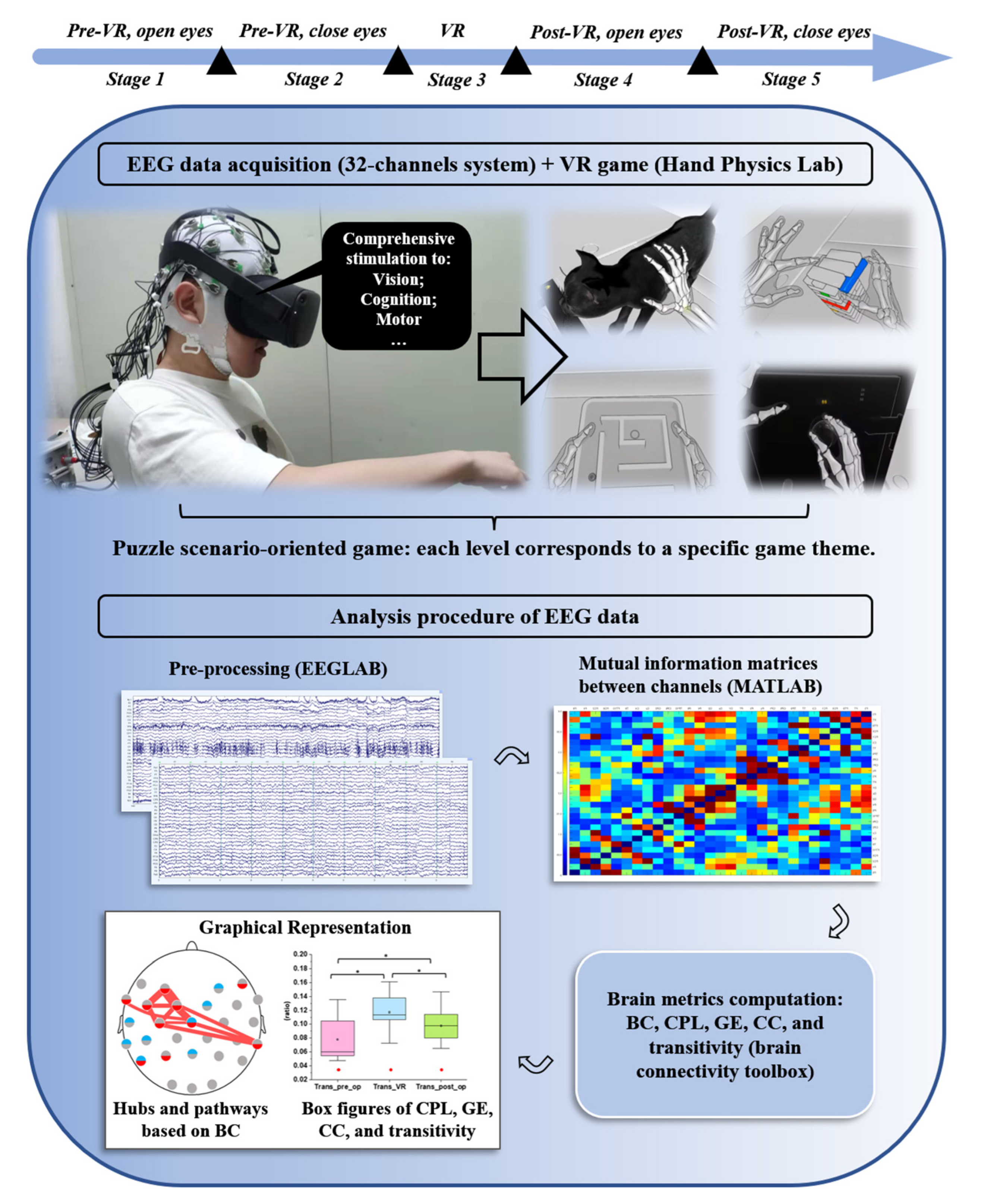
2. Materials and Methods
2.1. Participants
- Healthy participants who were proven to possess normal vision, hearing, and cognition by passing game levels 1–5
- Age between 20 to 35 years
- Right-handedness
- No drinking, coffee consumption, or smoking in the past 24 h
- No experience with VR or other three-dimensional games
- No addiction to two-dimensional games
2.2. EEG Recordings
- Stage one: Open-eyes without a task for 5 min
- Stage two: Close-eyes without a task for 5 min
- Stage three: Play the VR game for approximately 20 min
- Stage four: Open-eyes without a task for 5 min
- Stage five: Close-eyes without a task for 5 min
2.3. EEG Processing
2.3.1. EEGLAB (v2021.0) [26]
2.3.2. MATLAB (vR2021a) and the Brain Connectivity Toolbox (v2019_03_03) [29]
2.4. Statistical Analyses (IBM SPSS Statistics Version 26)
2.5. Network Measures and Small-World Network
3. Results
3.1. The MI Difference between VR and Pre-VR in Open Eyes, and Post-VR and Pre-VR in Open Eyes
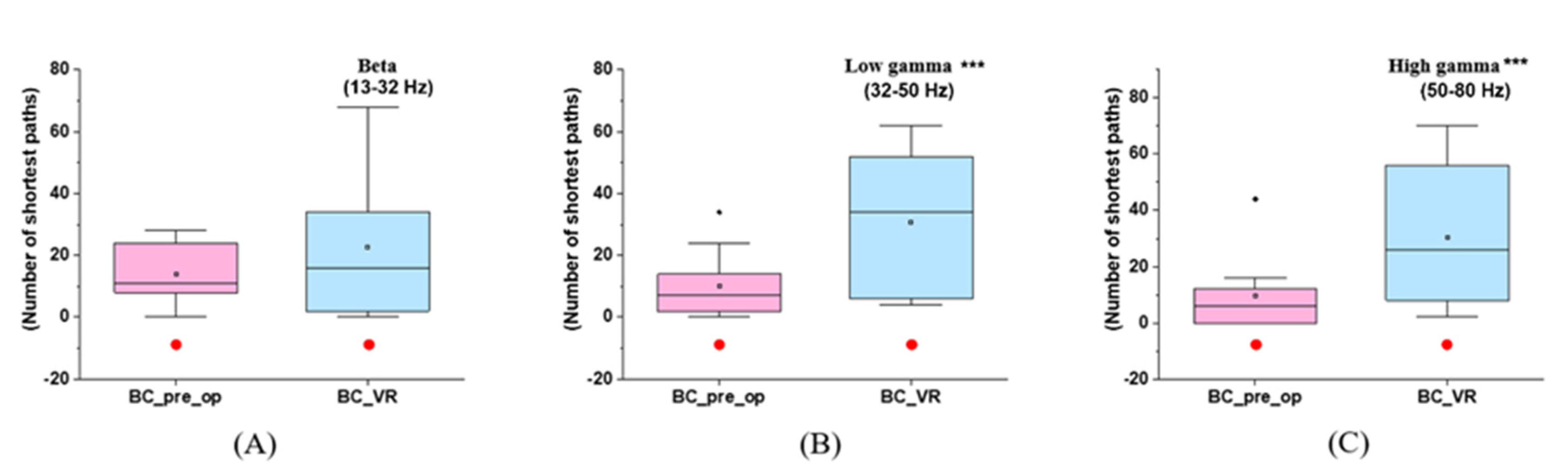
3.2. Alterations in BC Caused by Immersive VR Intervention
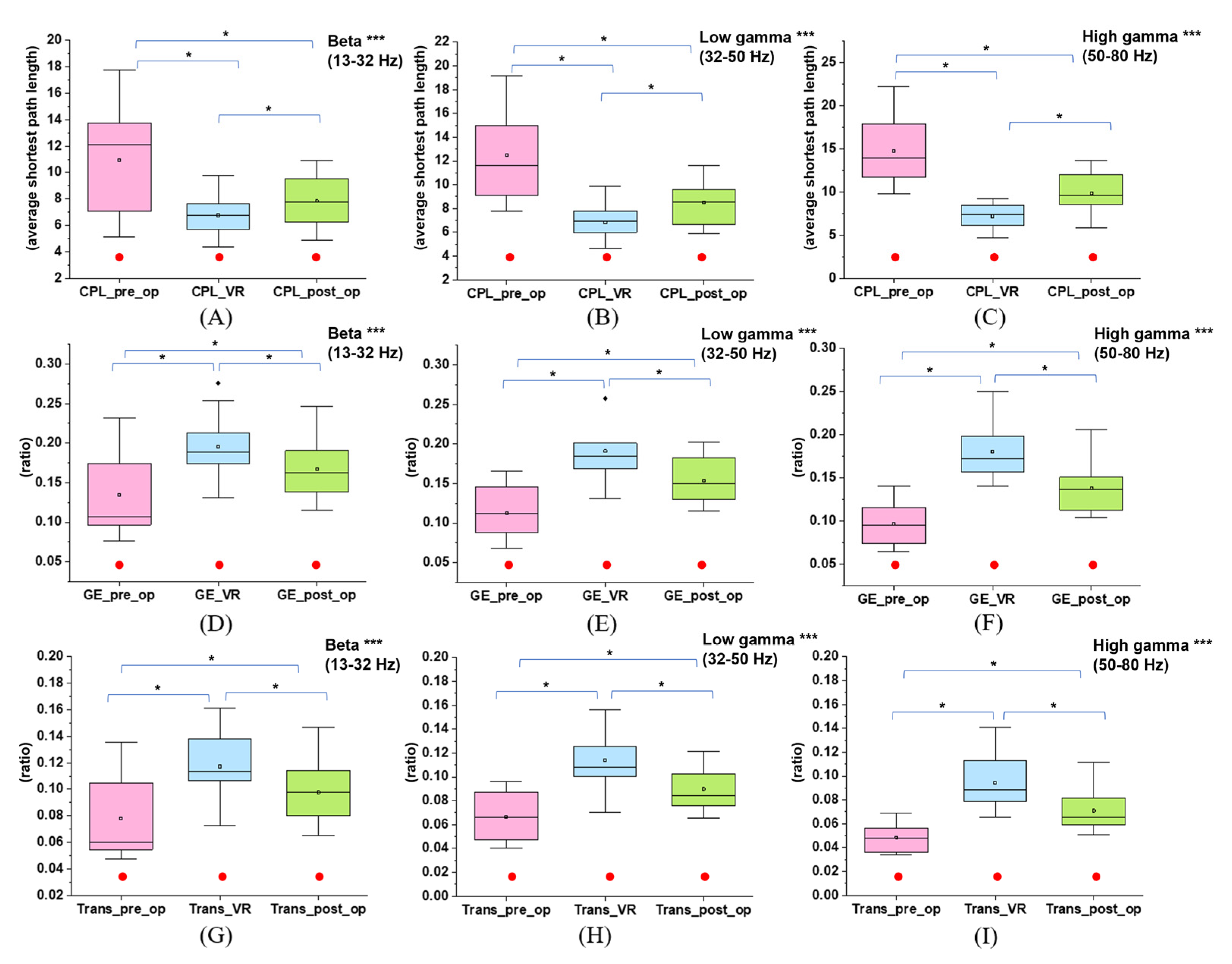
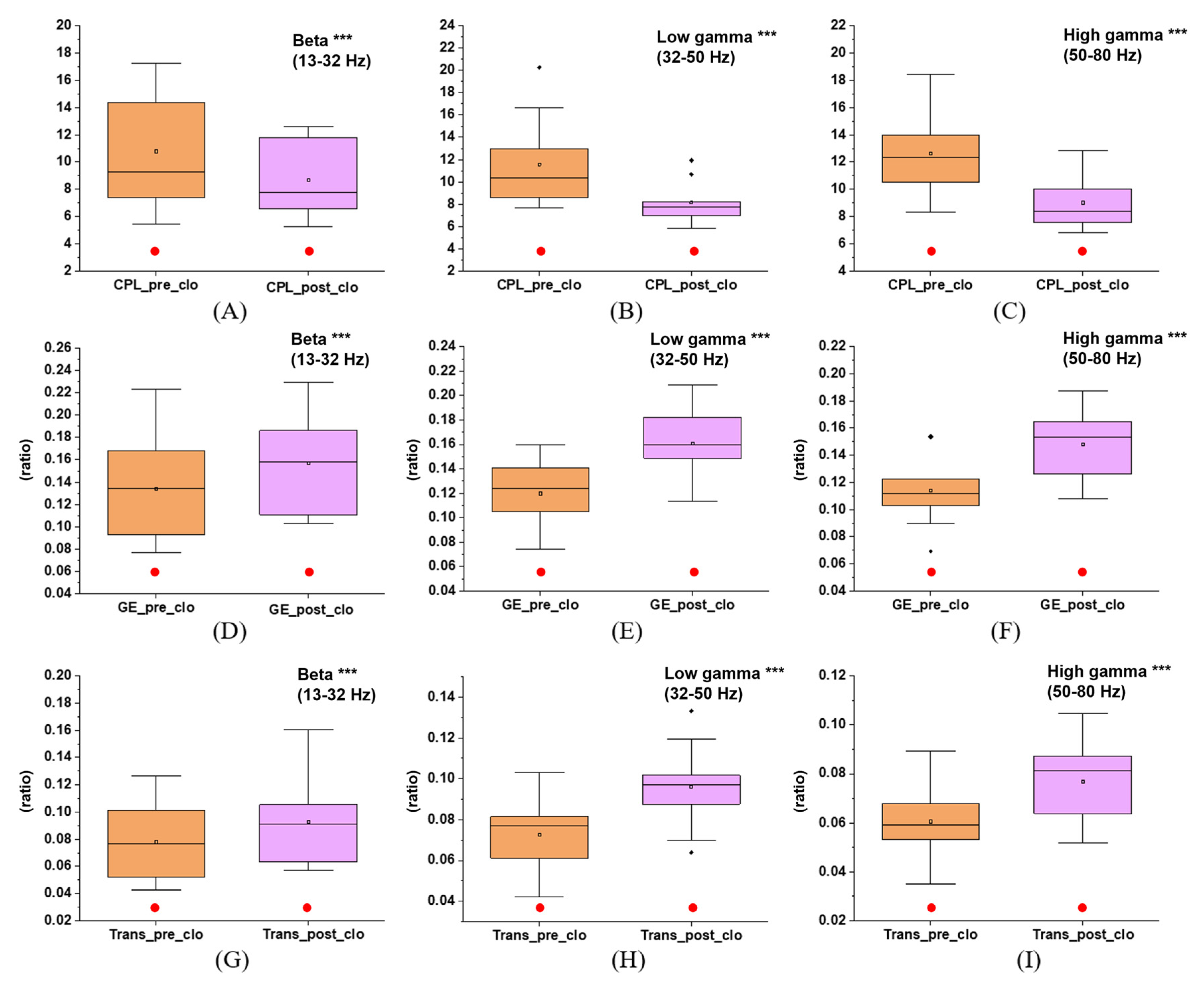
3.3. Effects of Immersive VR Interventions on Functional Integration and Segregation of the Brain Network
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clay, F.; Howett, D.; FitzGerald, J.; Fletcher, P.; Chan, D.; Price, A. Use of Immersive Virtual Reality in the Assessment and Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. 2020, 75, 23–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Huo, G.; Feng, Y.; Ma, Z. Application of virtual reality based on 3D-CTA in intracranial aneurysm surgery. J. Healthc. Eng. 2021, 2021, 9913949. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.L.; Moffitt, R.L.; Thomas, P.R.; Lovesday, K.; Watling, D.; Lombard, C.L.; Antonova, S.; Tremeer, M.A. A systematic review of the application of interactive virtual reality to sport. Virtual Real. 2018, 22, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Thornton, M.; Marshall, S.; McComas, J.; Finestone, H.; McCormick, A.; Sveistrup, H. Benefits of activity and virtual reality based balance exercise programmes for adults with traumatic brain injury: Perceptions of participants and their caregivers. Brain Inj. 2005, 19, 989–1000. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.J. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Sporns, O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci. 2014, 17, 652–660. [Google Scholar] [CrossRef]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Bassett, D.S. Brain graphs: Graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011, 7, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Graph theory methods: Applications in brain networks. Dialogues Clin. Neurosci. 2018, 20, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, W.; Chen, H.; Mantini, D.; Ding, J.R.; Xu, Q.; Wang, Z.; Yuan, C.; Chen, G.; Jiao, Q.; et al. Altered functional–structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 2011, 134, 2912–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephan, K.E.; Hilgetag, C.C.; Burns, G.A.; O’Neill, M.A.; Young, M.P.; Kötter, R. Computational analysis of functional connectivity between areas of primate cerebral cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayley, S. The neuroimmune-neuroplasticity interface and brain pathology. Front. Cell. Neurosci. 2014, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A data acquisition perspective. Neuroimage 2012, 62, 2222–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, R.; Naro, A.; Colucci, P.V.; Pranio, F.; Tardiolo, G.; Billeri, L.; Le Cause, M.; De Domenico, C.; Portaro, S.; Rao, G.; et al. Improvement of brain functional connectivity in autism spectrum disorder: An exploratory study on the potential use of virtual reality. J. Neural. Transm. 2021, 128, 371–380. [Google Scholar] [CrossRef]
- Stefano Filho, C.A.; Ignacio Serrano, J.; Attux, R.; Castellano, G.; Rocon, E.; del Castillo, M.D. Reorganization of Resting-State EEG Functional Connectivity Patterns in Children with Cerebral Palsy Following a Motor Imagery Virtual-Reality Intervention. Appl. Sci. 2021, 11, 2372. [Google Scholar] [CrossRef]
- Lin, C.T.; Chung, I.F.; Ko, L.W.; Chen, Y.C.; Liang, S.F.; Duann, J.R. EEG-based assessment of driver cognitive responses in a dynamic virtual-reality driving environment. IEEE Trans. Biomed. Eng. 2007, 54, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Raz, G.; Gurevitch, G.; Vaknin, T.; Aazamy, A.; Gefen, I.; Grunstein, S.; Azouri, G.; Goldway, N. Electroencephalographic evidence for the involvement of mirror-neuron and error-monitoring related processes in virtual body ownership. Neuroimage 2020, 207, 116351. [Google Scholar] [CrossRef] [PubMed]
- Pavone, E.F.; Tieri, G.; Rizza, G.; Tidoni, E.; Grisoni, L.; Aglioti, S.M. Embodying Others in Immersive Virtual Reality: Electro-Cortical Signatures of Monitoring the Errors in the Actions of an Avatar Seen from a First-Person Perspective. J. Neurosci. 2016, 36, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Corchs, S.; Chioma, G.; Dondi, R.; Gasparini, F.; Manzoni, S.; Markowska-Kaczmar, U.; Mauri, G.; Zoppis, I.; Morreale, A. Computational methods for resting-state EEG of patients with disorders of consciousness. Front. Neurosci. 2019, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, X.; Li, X. A review of resting-state electroencephalography analysis in disorders of consciousness. Front. Neurol. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Cassani, R.; Falk, T.H.; Fraga, F.J.; Kanda, P.A.; Anghinah, R. The effects of automated artifact removal algorithms on electroencephalography-based Alzheimer’s disease diagnosis. Front. Aging Neurosci. 2014, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffar, S.; Petr, D.W. Artifact Extraction from EEG Data Using Independent Component Analysis; Technical Report ITTC-FY2003-TR-03050-02; Information Telecommunication and Technology Center, University of Kansas: Lawrence, KS, USA, 2002. [Google Scholar]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Molina, A.J.; Soriano, M.F.; Iglesias-Parro, S. Mutual Information of Multiple Rhythms for EEG Signals. Front. Neurosci. 2020, 14, 574796. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Zhuravlev, M.O.; Runnova, A.E.; Protasov, P.; Maksimenko, V.A.; Frolov, N.S.; Pisarchik, A.N.; Hramov, A.E. Betweenness centrality in multiplex brain network during mental task evaluation. Phys. Rev. E 2018, 98, 062413. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Pan, Y.; Lan, W.; Zhang, R.; Wu, F.X.; Wang, J. Complex brain network analysis and its applications to brain disorders: A survey. Complexity 2017, 2, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Kim, E.S.; Noh, B.H.; Liang, J.G.; Lee, D.; Hur, Y.J.; Kim, N.Y.; Kim, H.D. Alteration in brain connectivity in patients with Dravet syndrome after vagus nerve stimulation (VNS): Exploration of its effectiveness using graph theory analysis with electroencephalography. J. Neural Eng. 2020, 17, 036014. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ’small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeon, H.J.; Lim, E.C.; Koo, J.W.; Lee, H.J.; Kim, H.J.; Lee, J.S.; Song, C.G.; Hong, S.K. Feasibility of eye tracking assisted vestibular rehabilitation strategy using immersive virtual reality. Clin. Exp. Otorhinolaryngol. 2019, 12, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auld, M.L.; Boyd, R.; Moseley, G.L.; Ware, R.; Johnston, L.M. Tactile function in children with unilateral cerebral palsy compared to typically developing children. Disabil. Rehabil. 2012, 34, 1488–1494. [Google Scholar] [CrossRef]
- Harada, T.; Saito, D.N.; Kashikura, K.I.; Sato, T.; Yonekura, Y.; Honda, M.; Sadato, N. Asymmetrical neural substrates of tactile discrimination in humans: A functional magnetic resonance imaging study. J. Neurosci. 2004, 24, 7524–7530. [Google Scholar] [CrossRef] [Green Version]
- Porac, C.; Coren, S. The dominant eye. Psychol. Bull. 1976, 83, 880–897. [Google Scholar] [CrossRef]
- Arbib, M.A. Towards a Computational Comparative Neuroprimatology: Framing the language-ready brain. Phys. Life Rev. 2016, 16, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Ronconi, L.; Gori, S.; Federici, A.; Devita, M.; Carna, S.; Sali, M.E.; Molteni, M.; Casartelli, L.; Facoetti, A. Weak surround suppression of the attentional focus characterizes visual selection in the ventral stream in autism. Neuroimage Clin. 2018, 18, 912–922. [Google Scholar] [CrossRef]
- Popov, T.; Jensen, O.; Schoffelen, J.M. Dorsal and ventral cortices are coupled by cross-frequency interactions during working memory. Neuroimage 2018, 178, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Youssofzadeh, V.; Prasad, G.; Fagan, A.J.; Reilly, R.B.; Martens, S.; Meaney, J.F.; Wong-Lin, K. Signal Propagation in the Human Visual Pathways: An Effective Connectivity Analysis. J. Neurosci. 2015, 35, 13501–13510. [Google Scholar] [CrossRef] [Green Version]
- Hütt, M.T.; Kaiser, M.; Hilgetag, C.C. Perspective: Network-guided pattern formation of neural dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Ungerleider, L.G.; Mishkin, M. The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends Cogn. Sci. 2013, 17, 26–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzkopf, D.S.; Robertson, D.J.; Song, C.; Barnes, G.R.; Rees, G. The frequency of visually induced γ-band oscillations depends on the size of early human visual cortex. J. Neurosci. 2012, 32, 1507–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betti, V.; Della Penna, S.; de Pasquale, F.; Corbetta, M. Spontaneous beta band rhythms in the predictive coding of natural stimuli. Neuroscience 2021, 27, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Zalesky, A.; Bullmore, E. Centrality and hubs. Fundamentals of Brain Network Analysis; Academic Press: Cambridge, MA, USA, 2016; pp. 137–161. [Google Scholar]
- Salvador, R.; Suckling, J.; Coleman, M.R.; Pickard, J.D.; Menon, D.; Bullmore, E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 2005, 15, 1332–1342. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Hilgetag, C.C.; Zhou, C. Trade-off between multiple constraints enables simultaneous formation of modules and hubs in neural systems. PLoS Comput. Biol. 2013, 9, e1002937. [Google Scholar] [CrossRef]
- Liang, J.G.; Kim, N.Y.; Ko, A.; Kim, H.D.; Lee, D. Changes in functional brain network topology after successful and unsuccessful corpus callosotomy for Lennox-Gastaut Syndrome. Sci. Rep. 2018, 8, 3414. [Google Scholar] [CrossRef]
- Hilgetag, C.C.; Goulas, A. Is the brain really a small-world network. Brain Struct. Funct. 2016, 221, 2361–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratwicke, J.; Jahanshahi, M.; Foltynie, T. Parkinson’s disease dementia: A neural networks perspective. Brain 2015, 138, 1454–1476. [Google Scholar] [CrossRef] [PubMed]
- Ellard, K.K.; Zimmerman, J.P.; Kaur, N.; Van Dijk, K.R.A.; Roffman, J.L.; Nierenberg, A.A.; Dougherty, D.D.; Deckersbach, T.; Camprodon, J.A. Functional Connectivity Between Anterior Insula and Key Nodes of Frontoparietal Executive Control and Salience Networks Distinguish Bipolar Depression from Unipolar Depression and Healthy Control Subjects. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 473–484. [Google Scholar] [CrossRef]
- Alderson, T.H.; Bokde, A.L.W.; Kelso, J.A.S.; Maguire, L.; Coyle, D. Metastable neural dynamics in Alzheimer’s disease are disrupted by lesions to the structural connectome. Neuroimage 2018, 183, 438–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Diessen, E.; Numan, T.; van Dellen, E.; van der Kooi, A.W.; Boersma, M.; Hofman, D.; van Lutterveld, R.; van Dijk, B.W.; van Straaten, E.C.; Hillebrand, A.; et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin. Neurophysiol. 2015, 126, 1468–1481. [Google Scholar] [CrossRef]
- Wang, Z.J.; Lee, P.W.H.; McKeown, M.J. A novel segmentation, mutual information network framework for EEG analysis of motor tasks. Biomed. Eng. Online 2009, 8, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, A.Y.; Fingelkurts, A.A.; Fingelkurts, A.A.; Borisov, S.V.; Darkhovsky, B.S. Nonstationary nature of the brain activity as revealed by EEG/MEG: Methodological, practical and conceptual challenges. Signal. Process. 2005, 85, 2190–2212. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Matsuhashi, M.; Mima, T.; Fukuyama, H.; Takahashi, R.; Hanakawa, T.; Ikeda, A. Comparison of phase synchronization measures for identifying stimulus-induced functional connectivity in human magnetoencephalographic and simulated data. Front. Neurosci. 2020, 14, 648. [Google Scholar] [CrossRef]
- Šverko, Z.; Vrankić, M.; Vlahinić, S.; Rogelj, P. Complex Pearson Correlation Coefficient for EEG Connectivity Analysis. Sensors 2022, 22, 1477. [Google Scholar] [CrossRef]
- Forlim, C.G.; Bittner, L.; Mostajeran, F.; Steinicke, F.; Gallinat, J.; Kühn, S. Stereoscopic Rendering via Goggles Elicits Higher Functional Connectivity During Virtual Reality Gaming. Front. Hum. Neurosci. 2019, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Yockey R, D. SPSS Demystified: A Step-By-Step Guide to Successful Data Analysis; Pearson College Division: London, UK, 2008; p. 2008.00365. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Hwang, H.-S.; Zhu, B.-H.; Chen, J.; Enkhzaya, G.; Wang, Z.-J.; Kim, E.-S.; Kim, N.-Y. Evaluating the Alterations Induced by Virtual Reality in Cerebral Small-World Networks Using Graph Theory Analysis with Electroencephalography. Brain Sci. 2022, 12, 1630. https://doi.org/10.3390/brainsci12121630
Yang S, Hwang H-S, Zhu B-H, Chen J, Enkhzaya G, Wang Z-J, Kim E-S, Kim N-Y. Evaluating the Alterations Induced by Virtual Reality in Cerebral Small-World Networks Using Graph Theory Analysis with Electroencephalography. Brain Sciences. 2022; 12(12):1630. https://doi.org/10.3390/brainsci12121630
Chicago/Turabian StyleYang, Shan, Hyeon-Sik Hwang, Bao-Hua Zhu, Jian Chen, Ganbold Enkhzaya, Zhi-Ji Wang, Eun-Seong Kim, and Nam-Young Kim. 2022. "Evaluating the Alterations Induced by Virtual Reality in Cerebral Small-World Networks Using Graph Theory Analysis with Electroencephalography" Brain Sciences 12, no. 12: 1630. https://doi.org/10.3390/brainsci12121630
APA StyleYang, S., Hwang, H.-S., Zhu, B.-H., Chen, J., Enkhzaya, G., Wang, Z.-J., Kim, E.-S., & Kim, N.-Y. (2022). Evaluating the Alterations Induced by Virtual Reality in Cerebral Small-World Networks Using Graph Theory Analysis with Electroencephalography. Brain Sciences, 12(12), 1630. https://doi.org/10.3390/brainsci12121630







