Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges
Abstract
:1. Introduction
2. Cannabinoids Use in Late Life
2.1. Cannabinoid Systems and the Brain
2.2. Translational Research
2.2.1. Pre-Clinical Findings
2.2.2. In Vitro and In Vivo AD-Related Assays
2.2.3. In Vitro and In Vivo PD Related Assays
2.3. Clinical Findings
2.3.1. Parkinson’s Disease (PD)
2.3.2. Alzheimer’s Disease (AD) and Other Dementias
2.3.3. Management Challenges
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolita, L.; Spegman, A.; Tang, X.; Cronstein, B.N. Greater Number of Narcotic Analgesic Prescriptions for Osteoarthritis Is Associated with Falls and Fractures in Elderly Adults. J. Am. Geriatr. Soc. 2013, 61, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Tamblyn, R.; Abrahamowicz, M.; du Berger, R.; McLeod, P.; Bartlett, G. A 5-Year Prospective Assessment of the Risk Associated with Individual Benzodiazepines and Doses in New Elderly Users. J. Am. Geriatr. Soc. 2005, 53, 233–241. [Google Scholar] [CrossRef]
- Gill, S.S.; Bronskill, S.E.; Normand, S.-L.T.; Anderson, G.M.; Sykora, K.; Lam, K.; Bell, C.M.; Lee, P.E.; Fischer, H.D.; Herrmann, N.; et al. Antipsychotic Drug Use and Mortality in Older Adults with Dementia. Ann. Intern. Med. 2007, 146, 775–786. [Google Scholar] [CrossRef]
- Lin, S.M.; Borges, M.K.; de Siqueira, A.S.S.; Biella, M.M.; Jacob-Filho, W.; Cesari, M.; Voshaar, R.C.O.; Aprahamian, I. Serotonin Receptor Inhibitor Is Associated with Falls Independent of Frailty in Older Adults. Aging Ment. Health 2021, 25, 219–224. [Google Scholar] [CrossRef]
- Pisani, S.; McGoohan, K.; Velayudhan, L.; Bhattacharyya, S. Safety and Tolerability of Natural and Synthetic Cannabinoids in Older Adults: A Systematic Review and Meta-Analysis of Open-Label Trials and Observational Studies. Drugs Aging 2021, 38, 887–910. [Google Scholar] [CrossRef]
- Minerbi, A.; Häuser, W.; Fitzcharles, M.-A. Medical Cannabis for Older Patients. Drugs Aging 2019, 36, 39–51. [Google Scholar] [CrossRef]
- Weinstein, G.; Sznitman, S.R. The Implications of Late-Life Cannabis Use on Brain Health: A Mapping Review and Implications for Future Research. Ageing Res. Rev. 2020, 59, 101041. [Google Scholar] [CrossRef]
- Ginsburg, B.C.; Hensler, J.G. Age-Related Changes in CB1 Receptor Expression and Function and the Behavioral Effects of Cannabinoid Receptor Ligands. Pharmacol. Biochem. Behav. 2022, 213, 173339. [Google Scholar] [CrossRef]
- Alessandria, G.; Meli, R.; Infante, M.T.; Vestito, L.; Capello, E.; Bandini, F. Long-Term Assessment of the Cognitive Effects of Nabiximols in Patients with Multiple Sclerosis: A Pilot Study. Clin. Neurol. Neurosurg. 2020, 196, 105990. [Google Scholar] [CrossRef]
- Ueberall, M.A.; Essner, U.; Vila Silván, C.; Mueller-Schwefe, G.H.H. Comparison of the Effectiveness and Tolerability of Nabiximols (THC:CBD) Oromucosal Spray versus Oral Dronabinol (THC) as Add-on Treatment for Severe Neuropathic Pain in Real-World Clinical Practice: Retrospective Analysis of the German Pain e-Registry. J. Pain Res. 2022, 15, 267–286. [Google Scholar] [CrossRef]
- Abuhasira, R.; Schleider, L.B.-L.; Mechoulam, R.; Novack, V. Epidemiological Characteristics, Safety and Efficacy of Medical Cannabis in the Elderly. Eur. J. Intern. Med. 2018, 49, 44–50. [Google Scholar] [CrossRef]
- Bachhuber, M.; Arnsten, J.H.; Wurm, G. Use of Cannabis to Relieve Pain and Promote Sleep by Customers at an Adult Use Dispensary. J. Psychoact. Drugs 2019, 51, 400–404. [Google Scholar] [CrossRef]
- Han, B.H.; Sherman, S.; Mauro, P.M.; Martins, S.S.; Rotenberg, J.; Palamar, J.J. Demographic Trends among Older Cannabis Users in the United States, 2006–2013. Addiction 2017, 112, 516–525. [Google Scholar] [CrossRef]
- Stella, F.; Valiengo, L.C.L.; de Paula, V.J.R.; Lima, C.A.d.M.; Forlenza, O.V. Medical Cannabinoids for Treatment of Neuropsychiatric Symptoms in Dementia: A Systematic Review. Trends Psychiatry Psychother. 2021, 43, 243–255. [Google Scholar] [CrossRef]
- Yang, K.H.; Kaufmann, C.N.; Nafsu, R.; Lifset, E.T.; Nguyen, K.; Sexton, M.; Han, B.H.; Kim, A.; Moore, A.A. Cannabis: An Emerging Treatment for Common Symptoms in Older Adults. J. Am. Geriatr. Soc. 2021, 69, 91–97. [Google Scholar] [CrossRef]
- Baumbusch, J.; Sloan Yip, I. Exploring New Use of Cannabis among Older Adults. Clin. Gerontol. 2021, 44, 25–31. [Google Scholar] [CrossRef]
- Manning, L.; Bouchard, L. Medical Cannabis Use: Exploring the Perceptions and Experiences of Older Adults with Chronic Conditions. Clin. Gerontol. 2021, 44, 32–41. [Google Scholar] [CrossRef]
- Arora, K.; Qualls, S.H.; Bobitt, J.; Lum, H.D.; Milavetz, G.; Croker, J.; Kaskie, B. Measuring Attitudes toward Medical and Recreational Cannabis among Older Adults in Colorado. Gerontologist 2020, 60, e232–e241. [Google Scholar] [CrossRef] [Green Version]
- Bobitt, J.; Qualls, S.H.; Schuchman, M.; Wickersham, R.; Lum, H.D.; Arora, K.; Milavetz, G.; Kaskie, B. Qualitative Analysis of Cannabis Use Among Older Adults in Colorado. Drugs Aging 2019, 36, 655–666. [Google Scholar] [CrossRef]
- Han, B.H.; Funk-White, M.; Ko, R.; Al-Rousan, T.; Palamar, J.J. Decreasing Perceived Risk Associated with Regular Cannabis Use among Older Adults in the United States from 2015 to 2019. J. Am. Geriatr. Soc. 2021, 69, 2591–2597. [Google Scholar] [CrossRef]
- Ahn, K.; McKinney, M.K.; Cravatt, B.F. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem. Rev. 2008, 108, 1687–1707. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.A.; Ullrich, O. Endocannabinoids and the Brain Immune System: New Neurones at the Horizon? J. Neuroendocrinol. 2008, 20 (Suppl. 1), 15–19. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Springer: Cham, Switzerland, 2019; Volume 1162, pp. 1–12. [Google Scholar]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous Cannabinoid Receptor Ligands with Neuromodulatory Action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous Cannabinoids in the Brain and Peripheral Tissues: Regulation of Their Levels and Control of Food Intake. Int. J. Obes. 2006, 30 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [Green Version]
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The Endocannabinoid System in Anxiety, Fear Memory and Habituation. J. Psychopharmacol. 2012, 26, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- O’brien, C.P. Endocannabinoids: The Brain and Body’s Marijuana and Beyond-Preface; Onaivi, E.S., Sugiura, T., Di Marzo, V., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Uhl, G.R.; Ishiguro, H.; Onaivi, E.S.; Zhang, P.-W.; Akinshola, B.E.; Lin, Z.; Hope, B.; Leonard, C.M.; Liu, Q.-R. Molecular Neurobiological Methods in Marijuana-Cannabinoid Research. In Marijuana and Cannabinoid Research; Humana Press: Totowa, NJ, USA, 2006; Volume 123, pp. 1–17. [Google Scholar]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Ribeiro, R.T.; Mello, M.T.; Tufik, S.; Peres, M.F.P. Anandamide Is Related to Clinical and Cardiorespiratory Benefits of Aerobic Exercise Training in Migraine Patients: A Randomized Controlled Clinical Trial. Cannabis Cannabinoid Res. 2019, 4, 275–284. [Google Scholar] [CrossRef]
- Piscitelli, F.; Di Marzo, V. “Redundancy” of Endocannabinoid Inactivation: New Challenges and Opportunities for Pain Control. ACS Chem. Neurosci. 2012, 3, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid System and Mood Disorders: Priming a Target for New Therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the Players: 2-Arachidonoylglycerol Synthesis and Degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The Marijuana Component Cannabidiol Inhibits Beta-Amyloid-Induced Tau Protein Hyperphosphorylation through Wnt/Beta-Catenin Pathway Rescue in PC12 Cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef]
- Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 8920. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440. [Google Scholar] [CrossRef]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of Cellular Redox Homeostasis by the Endocannabinoid System. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef] [Green Version]
- Karl, T.; Garner, B.; Cheng, D. The Therapeutic Potential of the Phytocannabinoid Cannabidiol for Alzheimer’s Disease. Behav. Pharmacol. 2017, 28, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Coles, M.; Steiner-Lim, G.Z.; Karl, T. Therapeutic Properties of Multi-Cannabinoid Treatment Strategies for Alzheimer’s Disease. Front. Neurosci. 2022, 16, 962922. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- Jayant, S.; Sharma, B. Selective Modulator of Cannabinoid Receptor Type 2 Reduces Memory Impairment and Infarct Size During Cerebral Hypoperfusion and Vascular Dementia. Curr. Neurovasc. Res. 2016, 13, 289–302. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Khan, H.; Aschner, M.; Samini, F.; Pourbagher-Shahri, A.M.; Aramjoo, H.; Roshanravan, B.; Hoyte, C.; Mehrpour, O.; Samarghandian, S. Impact of Cannabis-Based Medicine on Alzheimer’s Disease by Focusing on the Amyloid β-Modifications: A Systematic Study. CNS Neurol. Disord. Drug Targets 2020, 19, 334–343. [Google Scholar] [CrossRef]
- Wu, J.; Chen, N.; Liu, Y.; Godlewski, G.; Kaplan, H.J.; Shrader, S.H.; Song, Z.-H.; Shao, H. Studies of Involvement of G-Protein Coupled Receptor-3 in Cannabidiol Effects on Inflammatory Responses of Mouse Primary Astrocytes and Microglia. PLoS ONE 2021, 16, e0251677. [Google Scholar] [CrossRef]
- Silvestro, S.; Schepici, G.; Bramanti, P.; Mazzon, E. Molecular Targets of Cannabidiol in Experimental Models of Neurological Disease. Molecules 2020, 25, 5186. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of Cannabidiol Interactions with Wnt/β-Catenin Pathway and PPARγ on Oxidative Stress and Neuroinflammation in Alzheimer’s Disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef] [Green Version]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoidmediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef] [Green Version]
- Kozela, E.; Juknat, A.; Vogel, Z. Modulation of Astrocyte Activity by Cannabidiol, a Nonpsychoactive Cannabinoid. Int. J. Mol. Sci. 2017, 18, 1669. [Google Scholar] [CrossRef] [Green Version]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From Cannabis Sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5YAPP+ Cells through PPARγ Involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Ryan, D.; Drysdale, A.J.; Lafourcade, C.; Pertwee, R.G.; Platt, B. Cannabidiol Targets Mitochondria to Regulate Intracellular Ca2+ Levels. J. Neurosci. 2009, 29, 2053–2063. [Google Scholar] [CrossRef] [Green Version]
- Ester, A.; Andrés, -B.; Pol, I.F. Delineating the Efficacy of a Cannabis-Based Medicine at Advanced Stages of Dementia in a Murine Model. J. Alzheimer’s Dis. 2016, 54, 903–912. [Google Scholar]
- Ester, A.; Alexandre, S.P.; Esteban, V.L.; Rafael, M.; Isidro, F. Cannabis-Based Medicine Reduces Multiple Pathological Processes in AβPP/PS1 Mice. J. Alzheimer’s Dis. 2015, 43, 977–992. [Google Scholar]
- Franke, T.N.; Irwin, C.; Beindorff, N.; Bouter, Y.; Bouter, C. Effects of Tetrahydrocannabinol Treatment on Brain Metabolism and Neuron Loss in a Mouse Model of Sporadic Alzheimer’s Disease. Nuklearmedizin 2019, 58, 177. [Google Scholar]
- Van Vliet, S.A.; Vanwersch, R.A.; Jongsma, M.J.; Olivier, B.; Philippens, I.H. Therapeutic Effects of Δ9-Δ9-THC and Modafinil in a Marmoset Parkinson Model. Eur. Neuropsychopharmacol. 2008, 18, 383–389. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Cebeira, M.; de Ceballos, M.L.; Zeng, B.Y.; Jenner, P.; Ramos, J.A.; Fernández-Ruiz, J.J. Increased Cannabinoid CB1 Receptor Binding and Activation of GTP-Binding Proteins in the Basal Ganglia of Patients with Parkinson’s Syndrome and of MPTP-Treated Marmosets. Eur. J. Neurosci. 2001, 14, 1827–1832. [Google Scholar] [CrossRef]
- Burgaz, S.; García, C.; Gómez-Cañas, M.; Rolland, A.; Muñoz, E.; Fernández-Ruiz, J. Neuroprotection with the Cannabidiol Quinone Derivative VCE-004.8 (EHP-101) against 6-Hydroxydopamine in Cell and Murine Models of Parkinson’s Disease. Molecules 2021, 26, 3245. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Yang, G.; Hu, N.; Zhao, Z.; Zhao, L.; Li, S. Cannabidiol Alleviates the Damage to Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease Mice via Regulating Neuronal Apoptosis and Neuroinflammation. Neuroscience 2022, 498, 64–72. [Google Scholar] [CrossRef]
- Carlsson, A. Treatment of Parkinson’s with L-DOPA. The Early Discovery Phase, and a Comment on Current Problems. J. Neural Transm. 2002, 109, 777–787. [Google Scholar] [CrossRef]
- Marsden, C.D. Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 672–681. [Google Scholar] [CrossRef] [Green Version]
- Dos-Santos-Pereira, M.; da-Silva, C.A.; Guimarães, F.S.; Del-Bel, E. Co-Administration of Cannabidiol and Capsazepine Reduces L-DOPA-Induced Dyskinesia in Mice: Possible Mechanism of Action. Neurobiol. Dis. 2016, 94, 179–195. [Google Scholar] [CrossRef]
- Fox, S.H.; Henry, B.; Hill, M.; Crossman, A.; Brotchie, J. Stimulation of Cannabinoid Receptors Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Nonhuman Primate Model of Parkinson’s Disease. Mov. Disord. 2002, 17, 1180–1187. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Mechoulam, R.; Offen, D. The CB1 Cannabinoid Receptor Agonist, HU-210, Reduces Levodopa-Induced Rotations in 6-Hydroxydopamine-Lesioned Rats. Pharmacol. Toxicol. 2003, 93, 66–70. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids Provide Neuroprotection against 6-Hydroxydopamine Toxicity in Vivo and in Vitro: Relevance to Parkinson’s Disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- Price, D.A.; Martinez, A.A.; Seillier, A.; Koek, W.; Acosta, Y.; Fernandez, E.; Strong, R.; Lutz, B.; Marsicano, G.; Roberts, J.L.; et al. 212-2, a Cannabinoid Receptor Agonist, Protects against Nigrostriatal Cell Loss in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson’s Disease. Eur. J. Neurosci. 2009, 29, 2177–2186. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Kluger, B.M.; Huang, A.P.; Miyasaki, J.M. Cannabinoids in Movement Disorders. Parkinsonism Relat. Disord. 2022, 102, 124–130. [Google Scholar] [CrossRef]
- Peball, M.; Seppi, K.; Krismer, F.; Knaus, H.-G.; Spielberger, S.; Heim, B.; Ellmerer, P.; Werkmann, M.; Poewe, W.; Djamshidian, A. Effects of Nabilone on Sleep Outcomes in Patients with Parkinson’s Disease: A Post-Hoc Analysis of NMS-Nab Study. Mov. Disord. Clin. Pract. 2022, 9, 751–758. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Wieghorst, A.; Roessler, K.K.; Hendricks, O.; Andersen, T.E. The Effect of Medical Cannabis on Cognitive Functions: A Systematic Review. Syst. Rev. 2022, 11, 210. [Google Scholar] [CrossRef]
- Urbi, B.; Corbett, J.; Hughes, I.; Owusu, M.A.; Thorning, S.; Broadley, S.A.; Sabet, A.; Heshmat, S. Effects of Cannabis in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Parkinson’s Dis. 2022, 12, 495–508. [Google Scholar] [CrossRef]
- Oikonomou, P.; Jost, W.H. Randomized Controlled Trials on the Use of Cannabis-Based Medicines in Movement Disorders: A Systematic Review. J. Neural Transm. 2022, 129, 1247–1256. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Pinto, J.P.; Chagas, M.H.N.; Rodrigues, G.G.R.; Dursun, S.M.; Tumas, V. Cannabidiol for the Treatment of Psychosis in Parkinson’s Disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef]
- Chagas, M.H.N.; Eckeli, A.L.; Zuardi, A.W.; Pena-Pereira, M.A.; Sobreira-Neto, M.A.; Sobreira, E.T.; Camilo, M.R.; Bergamaschi, M.M.; Schenck, C.H.; Hallak, J.E.C.; et al. Cannabidiol Can Improve Complex Sleep-Related Behaviours Associated with Rapid Eye Movement Sleep Behaviour Disorder in Parkinson’s Disease Patients: A Case Series. J. Clin. Pharm. Ther. 2014, 39, 564–566. [Google Scholar] [CrossRef]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.C.; Crippa, J.A.S. Effects of Cannabidiol in the Treatment of Patients with Parkinson’s Disease: An Exploratory Double-Blind Trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef]
- Xiong, Y.; Lim, C.-S. Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease. Brain Sci. 2021, 11, 1211. [Google Scholar] [CrossRef]
- Nitzan, K.; Ellenbogen, L.; Bentulila, Z.; David, D.; Franko, M.; Break, E.P.; Zoharetz, M.; Shamir, A.; Sarne, Y.; Doron, R. An Ultra-Low Dose of ∆9-Tetrahydrocannabinol Alleviates Alzheimer’s Disease-Related Cognitive Impairments and Modulates TrkB Receptor Expression in a 5XFAD Mouse Model. Int. J. Mol. Sci. 2022, 23, 9449. [Google Scholar] [CrossRef]
- Bosnjak Kuharic, D.; Markovic, D.; Brkovic, T.; Jeric Kegalj, M.; Rubic, Z.; Vuica Vukasovic, A.; Jeroncic, A.; Puljak, L. Cannabinoids for the Treatment of Dementia. Cochrane Database Syst. Rev. 2021, 9, CD012820. [Google Scholar]
- Hoch, E.; Niemann, D.; von Keller, R.; Schneider, M.; Friemel, C.M.; Preuss, U.W.; Hasan, A.; Pogarell, O. How Effective and Safe Is Medical Cannabis as a Treatment of Mental Disorders? A Systematic Review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Bajtel, Á.; Kiss, T.; Tóth, B.; Kiss, S.; Hegyi, P.; Vörhendi, N.; Csupor-Löffler, B.; Gede, N.; Hohmann, J.; Csupor, D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals 2022, 15, 100. [Google Scholar] [CrossRef]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of Dronabinol on Anorexia and Disturbed Behavior in Patients with Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Abuhasira, R.; Ron, A.; Sikorin, I.; Novack, V. Medical Cannabis for Older Patients-Treatment Protocol and Initial Results. J. Clin. Med. 2019, 8, 1819. [Google Scholar] [CrossRef] [Green Version]
- MacCallum, C.A.; Russo, E.B. Practical Considerations in Medical Cannabis Administration and Dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Lo, L.A.; Boivin, M. “Is Medical Cannabis Safe for My Patients?” A Practical Review of Cannabis Safety Considerations. Eur. J. Intern. Med. 2021, 89, 10–18. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Cannabidiol Dosing in Clinical Populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef]
- Van den Elsen, G.A.H.; Ahmed, A.I.A.; Lammers, M.; Kramers, C.; Verkes, R.J.; van der Marck, M.A.; Rikkert, M.G.M.O. Efficacy and Safety of Medical Cannabinoids in Older Subjects: A Systematic Review. Ageing Res. Rev. 2014, 14, 56–64. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; de Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus Recommendations on Dosing and Administration of Medical Cannabis to Treat Chronic Pain: Results of a Modified Delphi Process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- Velayudhan, L.; McGoohan, K.; Bhattacharyya, S. Safety and Tolerability of Natural and Synthetic Cannabinoids in Adults Aged over 50 Years: A Systematic Review and Meta-Analysis. PLoS Med. 2021, 18, e1003524. [Google Scholar] [CrossRef]
- Ahmed, A.I.A.; van den Elsen, G.A.H.; Colbers, A.; van der Marck, M.A.; Burger, D.M.; Feuth, T.B.; Rikkert, M.G.M.O.; Kramers, C. Safety and Pharmacokinetics of Oral Delta-9-Tetrahydrocannabinol in Healthy Older Subjects: A Randomized Controlled Trial. Eur. Neuropsychopharmacol. 2014, 24, 1475–1482. [Google Scholar] [CrossRef]
- Yamaori, S.; Koeda, K.; Kushihara, M.; Hada, Y.; Yamamoto, I.; Watanabe, K. Comparison in the in Vitro Inhibitory Effects of Major Phytocannabinoids and Polycyclic Aromatic Hydrocarbons Contained in Marijuana Smoke on Cytochrome P450 2C9 Activity. Drug Metab. Pharmacokinet. 2012, 27, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Grayson, L.; Vines, B.; Nichol, K.; Szaflarski, J.P. UAB CBD Program An Interaction between Warfarin and Cannabidiol, a Case Report. Epilepsy Behav. Case Rep. 2018, 9, 10–11. [Google Scholar] [CrossRef]
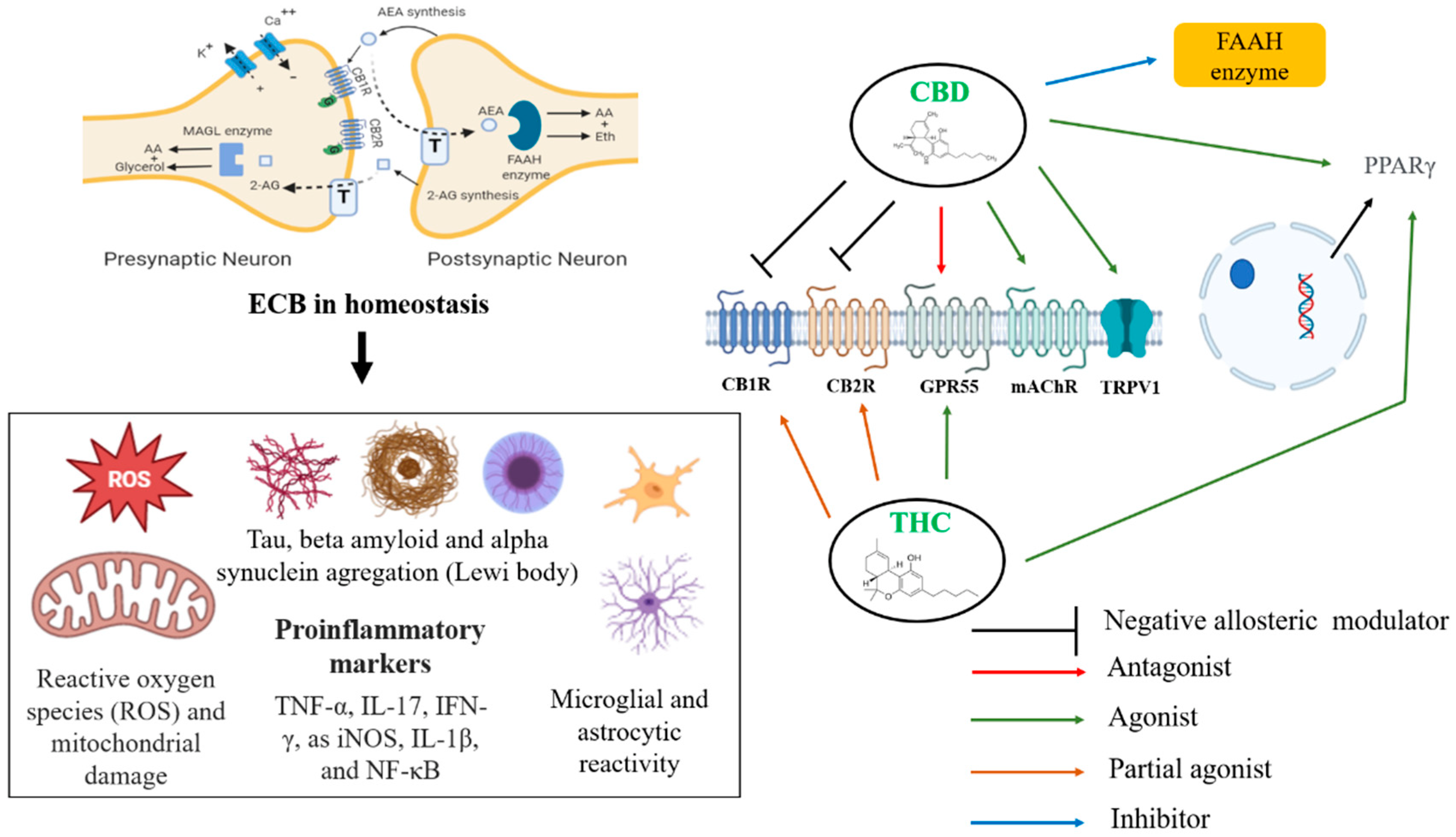
| Receptors | Action | Pharmacology Propriety |
|---|---|---|
| CB1 | Direct antagonist and negative allosteric modulation antagonist | Attenuation of learning deficit, memory, and psychotic effects of THC |
| CB2 | Antagonist & reverse agonist | Anti-inflammatory |
| GPR55 | Antagonist | Antitumor |
| 5HT1A | Agonist | Analgesia and anxiolytic |
| mAChR | Agonist | Cognition improvement |
| TRPV1 | Agonist | Anti-inflammatory and analgesia |
| PPARγ | Agonist | Antioxidant and anti-inflammatory |
| Neuropsychiatric Disorder | Potential (Off Label) Indication | Suggested Dose Regimen |
|---|---|---|
| Parkinson’s disease | Resistant tremor or dyskinesia | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. Maximum dose: 20 mg twice a week. |
| Resistant anxiety | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. May split the dose in two or three intakes. Maximum dose: 90 mg twice a week (CBD monotherapy). 1 mg of THC can be initiated with CBD or after 20 mg of CBD without a positive effect. Increase THC to a maximum of 20 mg combined to a maximum of 40 mg of CBD. | |
| Agitation due psychosis partially treated with quetiapine or clozapine | ||
| Persisted sleeping disturbance albeit treated with two first-line treatment | Starting dose: CBD (<0.3% THC) 5 mg at night. Increase 5 mg every 3 days. Maximum dose: 20 mg | |
| Alzheimer’s disease | Persisting agitation or aggression besides non-pharmacologic and first-line drug treatment implemented | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. May split the dose in two or three intakes. Maximum dose: 20 mg twice a week. 1 mg of THC can be initiated with CBD or after 20 mg of CBD without a positive effect. Increase THC to a maximum of 20 mg |
| Major adverse event with first-line drug treatment for agitation, anxiety, or aggression | ||
| Persisting anorexia albeit traditional treatment for dementia and exclusion of secondary causes | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. Maximum dose: 10 mg twice daily |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.C.; Joaquim, H.P.G.; Pedrazzi, J.F.C.; Pain, A.d.O.; Duque, G.; Aprahamian, I. Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges. Brain Sci. 2022, 12, 1596. https://doi.org/10.3390/brainsci12121596
Costa AC, Joaquim HPG, Pedrazzi JFC, Pain AdO, Duque G, Aprahamian I. Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges. Brain Sciences. 2022; 12(12):1596. https://doi.org/10.3390/brainsci12121596
Chicago/Turabian StyleCosta, Alana C., Helena P. G. Joaquim, João F. C. Pedrazzi, Andreia de O. Pain, Gustavo Duque, and Ivan Aprahamian. 2022. "Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges" Brain Sciences 12, no. 12: 1596. https://doi.org/10.3390/brainsci12121596
APA StyleCosta, A. C., Joaquim, H. P. G., Pedrazzi, J. F. C., Pain, A. d. O., Duque, G., & Aprahamian, I. (2022). Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges. Brain Sciences, 12(12), 1596. https://doi.org/10.3390/brainsci12121596








