Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Peripheral LS
2.2. sNfL Level Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patients
3.2. Effectiveness and Safety of CLAD Treatment
3.3. Lymphocyte Subsets
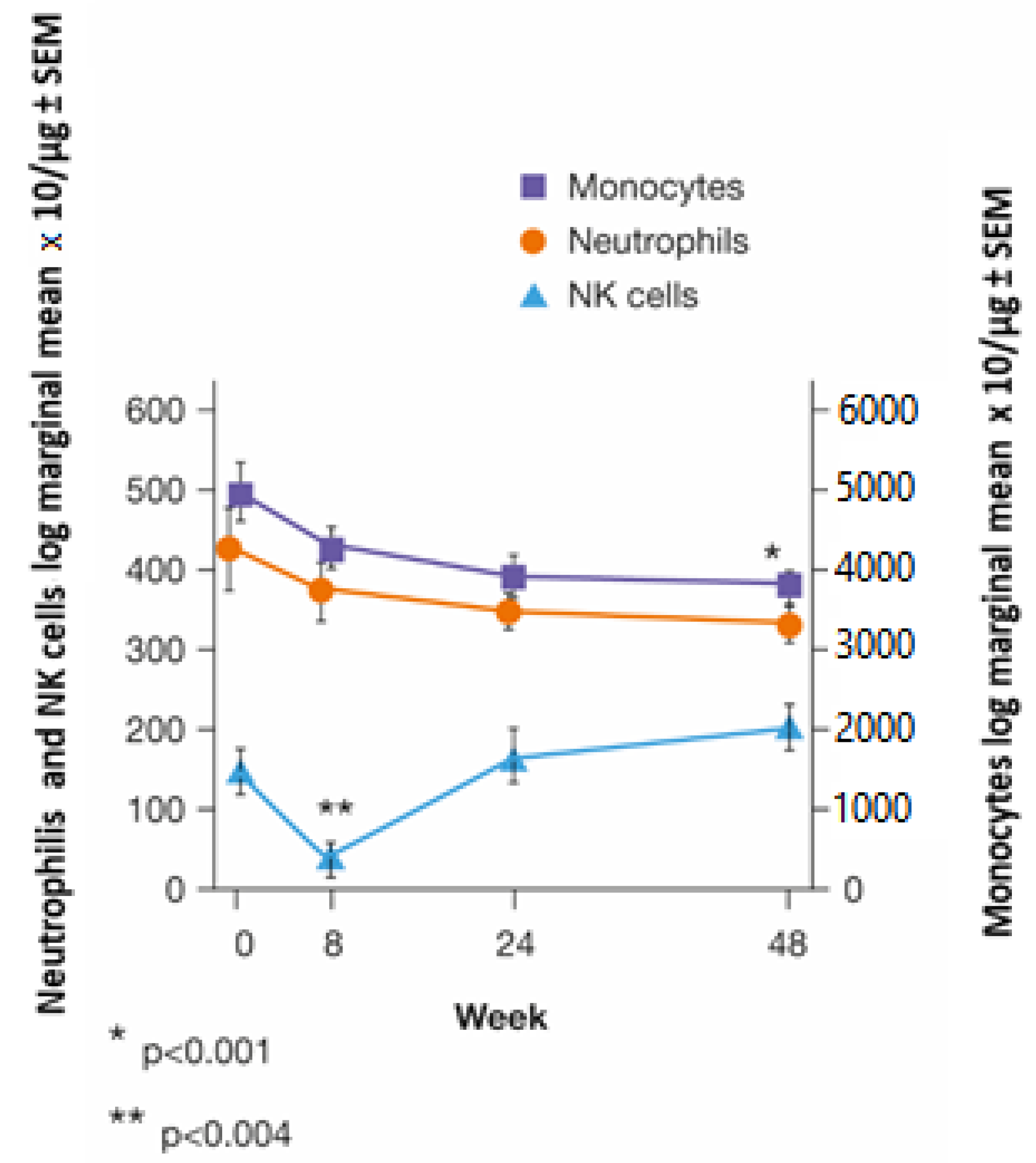
3.4. Innate Immunity
3.5. sNfL Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef]
- Willis, S.N.; Stathopoulos, P.; Chastre, A.; Compton, S.D.; Hafler, D.A.; O’Connor, K.C. Investigating the antigen specificity of multiple sclerosis central nervous system-derived immunoglobulins. Front. Immunol. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef] [PubMed]
- DiSano, K.D.; Gilli, F.; Pachner, A.R. Memory B cells in multiple sclerosis: Emerging players in disease pathogenesis. Front. Immunol. 2021, 12, 676686. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Amor, S.; Giovannoni, G.; Schmierer, K. Learning from other autoimmunities to understand targeting of B cells to control multiple sclerosis. Brain 2018, 141, 2834–2847. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Pryce, G.; Herrod, S.S.; Schmierer, K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult. Scler. Relat. Disord. 2019, 30, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Remmerswaal, E.B.; Schuurman, K.G.; Melief, J.; van Eden, C.G.; van Lier, R.A.W.; Huitinga, I.; Hamann, J. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol. 2013, 126, 525–535. [Google Scholar] [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, S. HIV-1 target cells in the CNS. J. Neurovirol. 2015, 21, 276–289. [Google Scholar] [CrossRef]
- Ho, E.L.; Ronquillo, R.; Altmeppen, H.; Spudich, S.S.; Price, R.W.; Sinclair, E. Cellular composition of cerebrospinal fluid in HIV-1 infected and uninfected subjects. PLoS ONE 2013, 8, e66188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, S.L.; Cree, B.A.C. Treatment of multiple sclerosis: A review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, P. Concepts of induction and escalation therapy in multiple sclerosis. J. Neurol. Sci. 2009, 277 (Suppl. S1), S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Lünemann, J.D.; Ruck, T.; Muraro, P.A.; Bar-Or, A.; Wiendl, H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 56–62, Erratum in Nat. Rev. Neurol. 2020, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Sellner, J.; Rommer, P.S. Immunological consequences of ‘immune reconstitution therapy’ in multiple sclerosis: A systematic review. Autoimmun. Rev. 2020, 19, 102492. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Ammoscato, F.; Giovannoni, G.; Baker, D.; Schmierer, K. Cladribine: Mechanisms and mysteries in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1266–1271. [Google Scholar] [CrossRef]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, G.; Sorensen, P.S.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [Green Version]
- Liliemark, J. The clinical pharmacokinetics of cladribine. Clin. Pharmacokinet. 1997, 32, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Karlsson, M.O.; Novakovic, A.M.; Terranova, N.; Fluck, M.; Munafo, A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin. Pharmacokinet. 2019, 58, 283–297, Erratum in Clin. Pharmacokinet. 2019, 58, 401. [Google Scholar] [CrossRef]
- Kearns, C.M.; Blakley, R.L.; Santana, V.M.; Crom, V.R. Pharmacokinetics of cladribine (2-chlorodeoxyadenosine) in children with acute leukemia. Cancer Res. 1994, 54, 1235–1239. [Google Scholar]
- Baker, D.; Jacobs, B.M.; Gnanapavan, S.; Schmierer, K.; Giovannoni, G. Plasma cell and B cell-targeted treatments for use in advanced multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 35, 19–25. [Google Scholar] [CrossRef]
- Ruggieri, M.; Gargano, C.D.; Ferretta, A.; Manni, A.; Capacchione, A.; Frigeri, A.; Iaffaldano, P.; Trojano, M.; Paolicelli, D. Effect of cladribine on neuronal apoptosis: New insight of in vitro study in multiple sclerosis therapy. Brain Sci. 2020, 10, 548. [Google Scholar] [CrossRef]
- Kopadze, T.; Döbert, M.; Leussink, V.I.; Dehmel, T.; Kieseier, B.C. Caldribine impedes in vitro migration of mononuclear cells: A possible implication for treating multilple sclerosis. Eur. J. Neurol. 2009, 16, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Cai, L.; Huang, J. Neurofilament light chain as a biological marker for multiple sclerosis: A meta-analysis study. Neuropsychiatr. Dis. Treat. 2018, 14, 2241–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Stuve, O.; Soerensen, P.S.; Leist, T.; Giovannoni, G.; Hyvert, Y.; Damian, D.; Dangond, F.; Boschert, U. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: An extended analysis of surface markers. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419854986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, D.; Marta, M.; Pryce, G.; Giovannoni, G.; Schmierer, K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017, 16, 41–50. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef]
- Wiendl, H.; Schmierer, K.; Hodgkinson, S.; Derfuss, T.; Chan, A.; Sellebjerg, F.; Achiron, A.; Montalban, X.; Prat, A.; De Stefano, N.; et al. Characterization of peripheral immune cell dynamics and repopulation patterns in the first 12 months of cladribine tablets treatment: MAGNIFY-MS study (2235). Neurology 2021, 96 (Suppl. S15). [Google Scholar]
- Costantino, C.M.; Baecher-Allan, C.M.; Hafler, D.A. Human regulatory T cells and autoimmunity. Eur. J. Immunol. 2008, 38, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Mur, E.; Pfeiffer, K.P.; Thaler, J.; Konwalinka, G. The safety profile of low-dose cladribine in refractory rheumatoid arthritis. A pilot trial. Scand. J. Rheumatol. 1997, 26, 376–379. [Google Scholar] [CrossRef]
- Katz, S.I.; Parker, D.; Turk, J.L. B-cell suppression of delayed hypersensitivity reactions. Nature 1974, 251, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Zalewski, L.; Albor, C.; Schmierer, K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e360. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Sánchez, N.; Cruz-Chamorro, I.; Díaz-Sánchez, M.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci. Rep. 2019, 9, 2302. [Google Scholar] [CrossRef] [Green Version]
- Peelen, E.; Damoiseaux, J.; Smolders, J.; Knippenberg, S.; Menheere, P.; Tervaert, J.W.C.; Hupperts, R.; Thewissen, M. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J. Neuroimmunol. 2011, 240–241, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storek, J.; Zhao, Z.; Lin, E.; Berger, T.; McSweeney, P.A.; Nash, R.A.; Akatsuka, Y.; Metcalf, M.D.; Lu, H.; Kalina, T.; et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases). Clin. Immunol. 2004, 113, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, P.; Comi, G.; Cook, S.; Giovannoni, G.; Rammohan, K.; Sorensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Viglietta, V.; et al. Effects of cladribine tablets on peripheral lymphocyte subtypes implicated in multiple sclerosis immunopathogenesis: Surface marker analysis for a subset of patients from the 96-week, phase III, double-blind, placebocontrolled CLARITY study. Mult. Scler. 2009, 15, S248–S249. [Google Scholar]
- Giovannoni, G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017, 14, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.J.; Martin, S.J.; Burton, D.R.; Roitt, I.M. Roitt’s Essential Immunology, 13th ed.; Wiley-Blackwell: Oxford, UK, 2017; ISBN 13:978-1118415771. [Google Scholar]
- Allen-Philbey, K.; De Trane, S.; Schmierer, K. Subcutaneous cladribine to treat multiple sclerosis: Experience in 208 patients. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211057661. [Google Scholar] [CrossRef]



| Characteristic | Entire Cohort (n = 74) |
|---|---|
| Age, mean ± SD (range) | 35.2 ± 11.3 (18–56) |
| Women, n (%) | 53 (72%) |
| Disease duration, mean ± SD years (range) | 9.4 ± 8.8 (0.12–44.0) |
| Previous treatment duration, mean ± SD years | 10.8 ± 8.9 |
| Previous therapy, n (%) | |
| First line | 41 (55%) |
| Second line | 20 (27%) |
| Naïve | 13 (18%) |
| EDSS, median (IQR) | 2.5 (1–7) |
| ARR (in the previous year), mean ± SD | 0.68 ± 0.76 |
| Patients with Gd-enhanced lesions, n (%) (in the previous year) | 43 (62%) |
| Patients with new T2 lesions, n (%) (in the previous year) | 33 (52%) |
| Follow-Up | NEDAYes | NEDANo |
|---|---|---|
| 12 months | ||
| (51 patients) * | n = 27 | n = 24 |
| Previous treatment: | ||
| Naïve, n (%) | 3 (11%) | 3 (12%) |
| First line, n (%) | 17 (63%) | 11 (46%) |
| Second line, n (%) | 7 (26%) | 10 (42%) |
| 18 months | ||
| (17 patients) * | n = 11 | n = 6 |
| Previous treatment: | ||
| Naïve, n (%) | 2 (19%) | 1 (17%) |
| First line, n (%) | 5 (45%) | 5 (83%) |
| Second line, n (%) | 4 (36%) | - |
| 24 months | ||
| (8 patients) * | n = 5 (62.5) | n = 3 (37.5) |
| First line, n (%) | 3 (60%) | 2 (67%) |
| Second line, n (%) | 2 (40%) | 1 (33%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolicelli, D.; Ruggieri, M.; Manni, A.; Gargano, C.D.; Carleo, G.; Palazzo, C.; Iaffaldano, A.; Bollo, L.; Guerra, T.; Saracino, A.; et al. Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sci. 2022, 12, 1595. https://doi.org/10.3390/brainsci12121595
Paolicelli D, Ruggieri M, Manni A, Gargano CD, Carleo G, Palazzo C, Iaffaldano A, Bollo L, Guerra T, Saracino A, et al. Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sciences. 2022; 12(12):1595. https://doi.org/10.3390/brainsci12121595
Chicago/Turabian StylePaolicelli, Damiano, Maddalena Ruggieri, Alessia Manni, Concetta D. Gargano, Graziana Carleo, Claudia Palazzo, Antonio Iaffaldano, Luca Bollo, Tommaso Guerra, Annalisa Saracino, and et al. 2022. "Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients" Brain Sciences 12, no. 12: 1595. https://doi.org/10.3390/brainsci12121595
APA StylePaolicelli, D., Ruggieri, M., Manni, A., Gargano, C. D., Carleo, G., Palazzo, C., Iaffaldano, A., Bollo, L., Guerra, T., Saracino, A., Frigeri, A., Iaffaldano, P., & Trojano, M. (2022). Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sciences, 12(12), 1595. https://doi.org/10.3390/brainsci12121595









