Abstract
Background: Huntington’s disease is an inherited autosomal dominant trait neuro-degenerative disorder caused by changes (mutations) of a gene called huntingtin (htt) that is located on the short arm (p) of chromosome 4, CAG expansion mutation. It is characterized by unusual movements, cognitive and psychiatric disorders. Objective: This review was undertaken to apprehend biological pathways of Huntington’s disease (HD) pathogenesis and its management by nature-derived products. Natural products can be lucrative for the management of HD as it shows protection against HD in pre-clinical trials. Advanced research is still required to assess the therapeutic effectiveness of the known organic products and their isolated compounds in HD experimental models. Summary: Degeneration of neurons in Huntington’s disease is distinguished by progressive loss of motor coordination and muscle function. This is due to the expansion of CAG trinucleotide in the first exon of the htt gene responsible for neuronal death and neuronal network degeneration in the brain. It is believed that the factors such as molecular genetics, oxidative stress, excitotoxicity, mitochondrial dysfunction, neuroglia dysfunction, protein aggregation, and altered UPS leads to HD. The defensive effect of the natural product provides therapeutic efficacy against HD. Recent reports on natural drugs have enlightened the protective role against HD via antioxidant, anti-inflammatory, antiapoptotic, and neurofunctional regulation.
1. Introduction
Huntington’s disease (HD) is an autosomal, escalating, and dominantly-inherited disease caused due to degeneration of neurons characterized by impairment of choreatic movements, and behavioral and psychiatric loss, principally in the cerebral cortex and striatum [1,2,3]. It claims its name from a physician, George Huntington, who first described the illness in 1872 [4]. In 1974, the first publication on HD was published [5]. HD is the earliest genetic condition to be linked to a specific chromosome site, and it has a special place in medical genetics research [6,7,8,9].
The biological pathways causing the disease are unknown and complicated, even though HD is distinguished by a well-defined genetic origin. Different mechanisms such as molecular genetics, leading to oxidative stress, metabolic dysfunction, and mitochondrial dysfunction, explain the pathological process of HD [10,11]. Thus, early diagnosis of clinical manifestation along with meticulous management becomes crucial.
HD is a neurological disease that develops slowly and is triggered due to sudden changes in the HD protein huntingtin (htt) [12,13]. An extension within the CAG repeat tract causes a mutation in htt, resulting in longer lengths of polyglutamine (polyQ) in the encoded protein. In unaffected populations, wild-type alleles contain up to 35 CAG repetitions, whereas 36 or more CAG repetitions specify HD allele [12,14,15,16]. The total amount of CAG repeats, as well as the phase of appearance of symptoms, is inversely connected, i.e., larger CAG repeat extensions are related to an initial phase of onset [17,18,19]. The protein encoded by the htt gene is a 348-kDa multidomain protein with a polymorphic glutamine/proline-rich domain at its amino terminus [20,21]. HD is currently the most extensively reported hereditary neurodegenerative disease that has diagnostic and prognostic genetic testing, with the probability of gene-targeted therapy in the nearby future [6]. HD was among the first diseases to be genetically tested before birth. Certainly, neuroimaging techniques have provided predictive and diagnostic genetic screening for ailment identification and its implications on sick persons and families through specialized facilities and genetic testing procedures [6,22,23].
Specific neurodevelopmental findings in HD include striatal degeneration and neuronal death, notably in the caudate nuclei, which target the cerebral cortex, pallidum, thalamus, brainstem, and cerebellum [24]. Neuroinflammation and microglial activation are hallmarks of the preliminary phase of HD [25]. In HD plasma, the levels of IL-6, matrix metallopeptidase 9, vascular endothelial growth factor (VEGF), and TGF-1 were significantly elevated, whilst the levels of IL-18 were markedly decreased. The prevalence of HD was reversely linked with plasma IL-6 [26]. HD is a severe autosomal-dominant late-onset neurological disorder that leads to chronic and incurable motor defects, leading to difficulties with coordination and mobility in addition to psychological-behavioral abnormalities. One of HD’s complications is visual deficit [27].
Patients with HD have been shown to exhibit important visual system impairments, notably retinal thinning, thinning of the temporal retinal nerve fibre layer, deletion of retinal ganglion cells, vision impairment elicited possibilities, poor colour vision, and poor motion perception [28,29,30,31]. When the retina of transgenic HD R6/1 mice was examined under an electron microscope, it was discovered that the diseased retina of HD mice is loaded with peculiar organelles called myelinosomes. Myelinosomes, which contain mutant mHTT-exon 1, can be released from glial Müller cells and then integrated into neuronal cells via a membrane fusion mechanism, aiding in the spread of HD [32].
The CNS has demonstrated the activation of the neurotoxic branch of the Kynurenine Pathway. The serum of HD patients had lower tryptophan (TRP), greater amounts of Kynurenine (KYN), and higher KYN/TRP ratios, which suggested stronger Indolamine 2,3-dioxygenase activity [33,34]. The intensity of symptoms and the quantity of CAG repeats were inversely connected with the levels of TRP. At the same time, the inflammatory condition was positively correlated with the levels of Anthranilic acid, which may be a useful biomarker [35].
The objective of the therapy is to reduce symptoms and improve the standard of life [36]. For symptomatic care, there are numerous effective choices; however, both drug-based and non-drug-based therapy is used [37]. Nature, as the best combinatorial chemist and home to hundreds of plant species, could have a direct medicinal influence on the body [5,38]. Natural ingredients with antioxidants, anti-inflammation, anti-apoptosis, calcium antagonization, and neurofunctional regulatory properties have been proven to cure or treat neurodegenerative diseases [39].
A few of the significant phytochemicals having neuroprotective activity include flavonoids, celastrol, sesamol, and trehalose [10,40]. Drug-based therapy involves symptomatic treatment through various therapeutic drugs [41]. HD therapeutic development and advancement that modulates the mHTT level through genetic transfer is one of the most important strategies to ameliorate the disease [42].
Here, we review to accentuate the significance of the underlying biological procedure intricate in the pathological process of HD. Moreover, an effort has been taken to enlighten different diagnostic techniques and various natural drugs and their phytochemicals with valuable effects against HD. Valuable insights provided by this review on HD increase hope for more definitive therapeutic strategies.
2. Methodology
A Medline (PubMed), Cochrane Library, and Embase-based literature survey were performed using keywords of “neurodegenerative diseases, prevention, non-pharmacological therapies, and neurodegenerative diseases, phytochemicals, Huntington’s till July 2022.
Two independent authors screened all the titles and abstracts of the retrieved data, and disagreements were resolved by the consensus of a third author. Duplicated entries, retracted publications, studies on other diseases or conditions different from NDs or its subtypes, studies without statistical analysis, non-English written papers, publications that are not research studies (i.e., commentaries, letters, editorials, reviews, and meta-analysis), and any other article that did not fit within the scope of this review were excluded. Articles listed in the references were also reviewed in search of more data.
A total of 550 results were retrieved and screened with the above keywords. Of these, 249 publications were selected and eventually used for qualitative analysis.
3. Epidemiology
Between diverse geographic regions, the universality of HD spreads more than ten folds [43,44]. HD is a neuropsychiatric disorder with pervasiveness of 5–10 people per one lakh in the white-skinned European-origin community. In Japan, the incidence is around one-tenth of the white-skinned of European origin population [45,46]. There is no gender predominance, and its prevalence is 5–8 per 100,000 individuals worldwide. The highest frequency of HD is found in European countries. The universality of HD ranges from 4.1 to 8.4 per lakh people estimated in the USA [47]. In India, the universality of HD is greater and similar to that seen in Western Europe [48].
4. Clinical Assessment
HD is associated with dysfunction in motor, cognitive, and psychiatric functions, symptoms of which are tabulated in Table 1. People are suffering from HD show specific and characteristic cognitive difficulties [49]. Traditionally, this cognitive change has been stated to as dementia. Changes in behavior are a distinctive characteristic of HD, which is the most stressful facet of the circumstance for families and individuals coping with it [44,50]. Other less eminent but weakening characteristics of HD comprise unexpected weight loss, sleep, circadian rhythm problems, and autonomic nervous system (ANS) dysfunction. The average life span of the start of the disorder is 30 to 50 years old, with a range of 2 to 85 years, and the disease lasts 17 to 20 years on average [1,51].

Table 1.
Common symptoms of HD.
5. Developmental Stages of HD
HD can be categorized into five stages (Table 2). Individuals with early-stage HD are functional. They continue to work, manage expenses, drive, and live independently. Individuals in the middle stage of HD begin to lose their power to work or drive [63]. They cannot handle their finances or conduct household tasks, but they can eat, dress, and keep themselves clean with assistance. It becomes difficult to handle patients suffering from HD as they are unable to sequence, consolidate, or prioritize information. As the stage moves into the early advanced and advanced stage of HD, individuals need complete assistance for daily living. Although they are bedridden and non-verbal, still people with HD seem to recollect some comprehension [64]. The total functional capacity is the most widely used rating scale based on functional abilities. A rating scale that rates the person’s level of independence based on occupation, the capability to handle finance, and the capability to perform household chores [59].

Table 2.
Stages of HD.
6. Huntington’s Pathogenesis: Mechanistic and Genetic Approach
HD is generated by a mutation in the htt gene, which codes for huntingtin, a ubiquitously expressed protein with 36 or more CAG repeats [12,14]. Despite the information that the genetic origin of HD is well documented, the various molecular modifications investigated in HD are broad and not utterly understood [12]. The expression of an enlarged PolyQ contributes to the impairment of wild-type protein [68]. Consequently, wild-type huntingtin loss or inactivation causes neurodegeneration. Different pathways involved in HD are illustrated below.
6.1. Transcriptional Dysregulation
One of the major players in the pathogenic process of HD is attributed to transcriptional dysregulation [69]. Mutant htt impairs transcription, according to DNA microarray studies [70,71]. This mutant htt interferes with transcription regulators such as p53, CREB–binding protein (CBP), and cAMP response element-binding (CREB) protein, all of which are significant for cellular functions and survival [72,73,74,75,76]. Dysregulation of CREB and Neuron restrictive silencer elements (NRSE) mediated transcription in HD and in normal person are described below.
6.1.1. cAMP Response Element-Binding (CREB) Protein Pathway
Normal Individual
In normal individuals (Figure 1A), the stimulation of certain transcription factors, such as CREB, which binds to DNA regions containing CRE in cellular promoters, is significant for neuronal survival [77]. Transcriptional activation due to CREB phosphorylation allows the recruitment of CBP [78]. CBP remodels chromatin further, allowing CREB to engage the TAFII130 component of TFIID [79]. Thus, the overall transcriptional machinery, including transcriptional factors TFIIA, B, D, E, F, and H, and TATA-binding protein (TBP), are activated. TFIIH phosphorylates RNA polymerase II (Pol II) in its carboxy-terminal domain (CTD) to commence transcription once it is suitable [12,80,81].
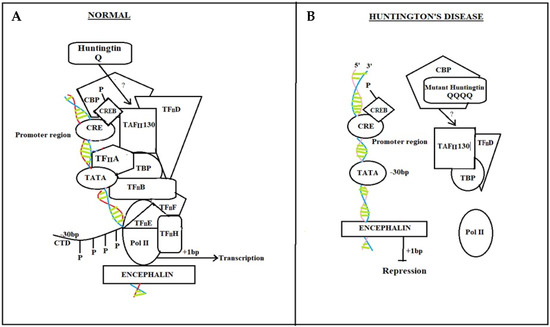
Figure 1.
CREB protein pathway in Normal individual and HD diseased patient. In normal individuals, CREB binding with CRE enables normal neuronal responses by activating a cascade of transcriptional factors (A) while in Huntington’s disease patients, due to mutant htt gene, CRE transcriptional cascade breaks, and there is no attachment of CBP and TAFII 130 with CRE. Pol II dispositioned (B).
HD Diseased Patient
Mutant htt interrupts CRE-mediated transcription in HD patients (Figure 1B) with direct interaction or sequestration of CBP and TAFII130 in the nucleus. CBP and TAFII 130 eventually lose their capability to attach to CRE sites in cellular promoters. Because the general transcription machinery, as well as Pol II, is not adequately positioned to the promoter, transcriptional activation is hampered [12,82,83].
6.1.2. NRSE Mediated Pathway
Normal Individual
In a normal individual (Figure 2A), the wild type htt regulates the action of the genes that contain (NRSE) by modifying cytoplasmic hiring to the nucleus of NRSE binding transcription factors, which are critical survival factors for striatal neurons [84]. The transcription factor REST-NRSF (repressor-element-1 transcription factor-neuron restrictive silencer factor) attaches to NRSEs in neuronal gene promoters such as the BDNF gene [85]. Wild type htt survives BDNF synthesis by networking with REST-NRSF in the cytoplasm, alleviating its capacity to attach to NRSE sites in the nucleus. Activators that attach to the BDNF promoter regions and afterward engage the transcription process and pol II boost BDNF transcription in these settings [12,86,87].
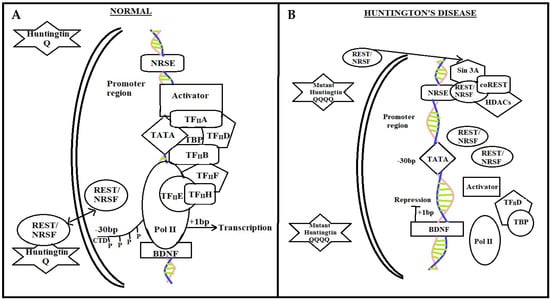
Figure 2.
NRSE mediated pathway in a normal individual and diseased patient. In normal individuals, transcription factor REST–NRSF binds to NRSEs in neuronal gene promoters such as in the brain-derived neurotrophic factor (BDNF) gene. By interacting with REST-NRSF in the cytoplasm and lowering its availability in the nucleus to bind to NRSE sites, wild-type htt maintains BDNF synthesis, which is a crucial survival factor for the striatal neurons that die in HD. In these circumstances, activators can bind to the BDNF promoter regions and then recruit the general transcriptional machinery and Pol II, promoting the transcription of BDNF. While in HD, REST-NRSF levels in the nucleus rise as a result of mutant htt’s failure to connect with REST-NRSF in the cytoplasm. In these circumstances, REST-NRSF binds to the NRSE with vigor and stimulates the recruitment of Sin3A-histone-deacetylase complexes (HDACs), which contain histone deacetylase activity for remodeling chromatin into a closed architecture and squelching BDNF transcription. REST stands for repressor-element-1 transcription factor. NRSE stands for neuron-restrictive silencer element.
Diseased HD Patient
In HD individuals, mutant htt decline to interlink with REST–NRSF, resulting in elevated REST–NRSF levels in the nucleus (Figure 2B). REST–NRSF attaches to the NRSE with vigor in these conditions, promoting the incorporation of Sin3A–histone deacetylase complexes (HDACs) with histone deacetylase activity for chromatin remodeling framework [88,89,90]. Consequently, the expression of NRSE, NRSF, BDNF, and REST are suppressed [12].
6.2. Ubiquitin-Protease System
6.2.1. Chaperones and the Proteasome Dysfunction
Through numerous sequential actions, molecular chaperones are used in the precise folding of newly produced proteins into correct conformation, and this procedure can be fruitless [91]. Heat shock proteins play an important role in protein folding and quality control. In the context of polyglutamine diseases, such as HD, heat shock protein 70 (Hsp70; Hspa1a/b), Hsp40 (Dnajb1), and Hsp90 (Hsp90aa1 and Hsp90ab1) have been the subject of several studies. Elevation of Hsp70 levels has been found to be neuroprotective in several animal models [92]. For instance, Hsp70 overexpression suppressed neuropathology and improved motor function in a spinocerebellar ataxia mouse model. Pharmacological and genetic Hsp90 inhibition induces mHtt degradation. Furthermore, Hsp70 and Hsp40 attenuated the assembly of polyglutamine proteins into amyloid-like fibrils [93]. As a result, proteins must either be refolded into their proper configuration, or the ubiquitin-proteasome system (UPS) degrades them [94]. Hsp70 (Heat-shock protein 70) and Hsp40 (Heat-shock protein 40) are two prominent types of molecular chaperones that help in the folding of polypeptides and hence prevent misfolded proteins development. Furthermore, in other polyQ proteins, the mutant htt has been shown to be linked with the Hsp70 and Hsp40 chaperone families and also colocalize with aggregates [95]. Chaperone sequestration into aggregates reduces the quantity of soluble chaperones in the cell, which helps to alleviate aberrant protein folding [96].
6.2.2. Ubiquitin-Proteasome System Impairment
The stages involved in the UPS system failing and causing cellular pathogenesis in HD (Figure 3) are listed below [12].
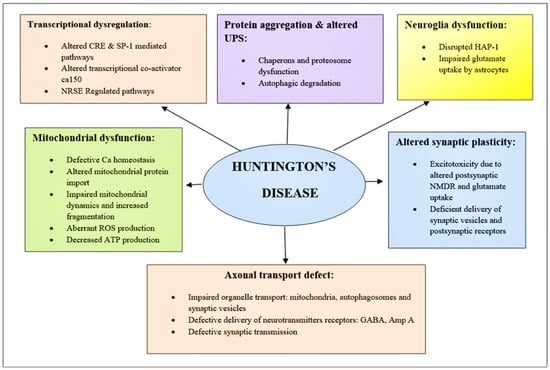
Figure 3.
Schematic of diverging pathways leading to the pathogenesis of HD. Here is the mechanism of pathogenesis, paying particular attention to those that are related to promising therapeutic targets. BDNF, Brain-derived neurotrophic factor; ROS, reactive oxygen species; NMDAR, N-methyl-D-aspartate receptor; UPS, Ubiquitin-protease System; NRSE, Neuron restrictive silencer elements; CRE, cAMP response element.
- (1)
- Hsp70 and Hsp40, two molecular chaperones, cause newly produced htt to pleat into a native structure [97]. The cytoplasmic functions of wild-type htt include vesicle transport, clathrin-mediated endocytosis, cytoskeletal anchoring, postsynaptic signaling, and neuronal transport. On the other hand, this htt might be carried into the nucleus and aid in transcriptional control.
- (2)
- Chaperones aid in the identification of aberrant proteins, promoting refolding or Ub (ubiquitination) and obliteration by the 26S proteasome [98]. Mutations produce conformational anomalies and improper folding of htt in HD patients, resulting in a buildup of misfolded htt in the cytoplasm if chaperones are not precise.
- (3)
- However, mutant htt is cleaved by proteases, resulting in the formation of amino-terminal components that form β-sheet structures [99].
- (4)
- As a result, cleaved N-terminal fragments or mutant full-length htt cause toxicity, forming soluble monomers, oligomers, or massive insoluble aggregates. Mutant forms of htt in cytoplasm disrupt the UPS, allowing misfolded proteins to accumulate [100].
- (5)
- Vesicle transport and clathrin-mediated endocytosis are disrupted by these noxious proteins. Furthermore, mutant htt promotes pro-apoptotic proteins through mitochondrial malfunction, causing cellular noxiousness and other negative implications [101].
- (6)
- For defence, the cell gathers hazardous pieces into ubiquitinated cytoplasmic perinuclear aggregates [102].
- (7)
- Mutant htt are translocated into the nucleus, resulting in nuclear inclusions that can interrupt transcription and the UPS (Figure 4) [103].
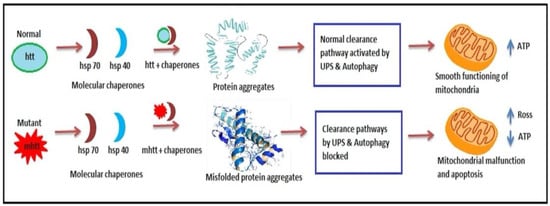 Figure 4. Ubiquitin-proteasome impairment in HD. In Normal individuals, Hsp70 and Hsp40 aid in transcriptional functions resulting in protein formation and aggregation and smooth functioning of mitochondria. However, in HD diseased patient mutant mhtt causes misfolding of protein aggregates and disrupts clearance pathways leading to mitochondrial dysfunction.
Figure 4. Ubiquitin-proteasome impairment in HD. In Normal individuals, Hsp70 and Hsp40 aid in transcriptional functions resulting in protein formation and aggregation and smooth functioning of mitochondria. However, in HD diseased patient mutant mhtt causes misfolding of protein aggregates and disrupts clearance pathways leading to mitochondrial dysfunction.
6.2.3. Altered Synaptic Plasticity
Early pathogenic processes in HD include synaptic and neuronal anomalies [104]. Reduced transcription of significant genes in signaling and neurotransmission disrupts neuronal homeostasis [105]. This causes imperfection accompanied by their axons in the transmission of organelles and proteins. Pathogenic htt also prevents organelle transportation across the axon [106,107]. Htt accelerates vesicle trafficking by acting as a scaffold connecting microtubules, cargoes, and motor proteins including dyneins and kinesins [108,109]. Huntingtin-associated protein1 (HAP1) mediates this interface, which tends to be impaired in HD disease [110].
6.2.4. Mitochondrial Dysfunction
Mitochondria serve as locations for oxidative phosphorylation and cellular respiration, both of which result in the production of ATP. They are also important for keeping the cytosol’s calcium concentration low. A mitochondrial defect has been discovered in HD patients, which results in lower mitochondrial oxygen consumption, glucose metabolism, and cAMP levels in the cerebrospinal fluid [111,112,113]. Oxidative stress is also responsible for the neurodegenerative procedure of HD. Since mitochondria are the primary communicator of ROS (reactive oxygen species) in neurons, oxidative stress is related to mitochondrial dysfunction in HD. With an enhancement in ROS or RNS (reactive nitrogen species) production, susceptible neurons in the patient’s brain suffering from HD may be unable to handle it well. Increase in levels of ROS or RNS in membranes may boost an intracellular cascade of oxidative stress by triggering lipid peroxidation and oxidizing DNA and proteins [114,115]. A substantial rise of 8-hydroxydeoxyguanosine, an oxidized DNA marker, and a higher surge of malondialdehyde (MDA), lipid peroxidation marker is perceived in the brain of HD. Oxidative stress also promotes mutant htt-dependent cell death by mimicking proteasomal malfunction and htt aggregation. Elevation of free radical outcomes in the impairment of mitochondrial function, metabolic dysfunction, impairment of energy production, and excitotoxicity [116,117,118,119,120,121].
6.3. Neuroglia Dysfunction
6.3.1. Astrocytes and Microglial Dysfunction
Htt is known to be much more abundant in neurons as compared to non-neuronal glial cells. Glial cells are majorly responsible for HD progression and pathogenesis [122,123]. Astrocytes, a kind of glia, protect neurons from excitotoxicity by providing support and allowing extracellular glutamate absorption. In the occurrence of HD disease, however, an N-terminal htt with 160Q is expressed exclusively in astrocytes [124]. Consequently, HD astrocytes contribute to neurological symptoms as well as other problems, such as reduced chemokine CCL5 or BDNF discharge [125].
Microglial and astrocytic contributes to neuronal death in HD. Surveilling microglia are activated by stimulating molecules through NF-κB signaling, upregulation of PU1, and CCAT binding. Activated microglia and reactive astrocytes produce ROS and neurotoxic molecules (such as quinolinic acid), which can induce molecular processes leading to neuronal death. Stimulatory molecules also induce reactive astrogliosis that leads to the upregulation of pro-inflammatory cytokine production, glutamate excitotoxicity, and hyperexcitability of neurons. Activated microglia can adopt different states, commonly, this polarization has been categorized as M1 and M2 states, and microglial cells can alternate between the two states. M1 microglia role in the inflammatory response and are thought to be the major initiators of both innate and adaptive immunity in the brain [126]. These cells have a phagocytic function and will release cytotoxic factors such as nitric oxide (NO), ROS, and quinolinic acid to confer toxicity to invading pathogens [126,127]. M2 microglia also carry out phagocytosis but contrary to the role of M1 microglia, M2 microglia exhibit an anti-inflammatory role [126]. This is through the release of anti-inflammatory mediators such as interleukin 4 (IL-4), interleukin 13 (IL-13), IL-10, and transforming growth factor beta (TGF-β) to suppress inflammatory responses [128].
6.3.2. Release of Pro-Inflammatory Cytokines and Chemokines
The secretion of pro-inflammatory cytokines by astrocytes is associated with neuroinflammation and neurodegeneration in HD [41]. Microglial generation of pro-inflammatory cytokines is induced by htt expression in immune cells [129]. Mutant htt affects inflammatory responses in the peripheral immune system by inhibiting NF-κB signalling, implying that neuro-inflammation is both a reactive and proactive mechanism in disease development [130]. IL-6 is upregulated in the plasma of HD patients [131]. IL-6 stimulates the expression of another acute phase protein: -macroglobulin (M). M is upregulated in plasma of HD patients, mainly in reactive astrocytes, and therefore influences immune proteins and cytokines. Moreover, several mouse models of HD display significantly higher levels of IL-1β. IL-1β itself is able to directly induce neurotoxicity via activation of tyrosine kinases and phosphorylation of NMDA receptors involving the NF-kappa(κ) B pathway. Inflammatory responses are initiated by different receptors, among others, including the Toll-like receptors (TLRs). TLR activation evokes NF-κB activation resulting in increased transcription of proinflammatory cytokines [132].
6.4. Axonal Transport Defect
6.4.1. Defective Synaptic Transmission
Axonal transport is necessary for appropriate transfer to neuronal membranes in order to facilitate synaptic communication. Synaptic excitability in HD is inhibited by a failure of receptor delivery, either GABAA (γ-aminobutyric acid type A) or AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors [41]. HAP1 connects these receptors to the kinesin motor factor KIF5, and mutant htt disrupts this interface [133,134]. Mutant htt also prevents the release of BDNF and cortical transport and the regressive transport of its receptor TrkB in the striatum, which is essential to trigger survival signals in the cell body [86,135].
6.4.2. Excitotoxicity and Medium Spiny Neurons (MSNs) Degeneration
In HD, MSNs in the striatum show the most noticeable deterioration [136]. MSNs were also found to be selectively influenced by glutamatergic signals, which facilitate abnormal firing and neurotransmission, NMDA receptor-mediated glutamate activation, and can cause striatal neuronal death via a process known as excitotoxicity [135,137]. Moreover, mutations in HD modify not only NMDAR trafficking in striatal neurons but also the balance of synaptic (pro-survival) and extrasynaptic (detrimental) NMDAR activity [138,139,140,141].
Consequently, several cellular pathways are involved in the evolution of HD (Figure 5).
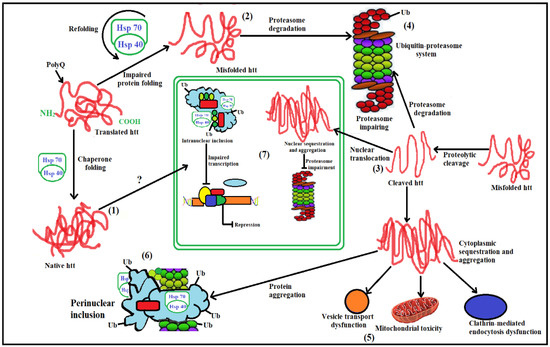
Figure 5.
Steps in cellular pathogenesis of HD. (1). Huntingtin (htt), a freshly generated protein, is encouraged to fold into a native shape by the molecular chaperones Hsp70 and Hsp40. Wild-type htt is primarily cytoplasmic and likely participates in postsynaptic signaling, clathrin-mediated endocytosis, vesicle transport, cytoskeletal anchoring, or neuronal transport. HTT might enter the nucleus and influence the control of transcription; (2). In order to promote either their refolding or their ubiquitination (Ub) and subsequent demise by the 26S proteasome, chaperones can help recognize aberrant proteins. If chaperones are not present to rectify the incorrect folding of htt caused by the HD mutation, misfolded htt will accumulate in the cytoplasm. The HD mutation causes conformational alterations; (3). Alternately, mutant htt may also be cleaved by proteolysis, resulting in amino-terminal fragments that produce β-sheet structures; (4). Finally, cleaved N-terminal fragments, which may form soluble monomers, oligomers, or huge insoluble aggregates, or full-length mutant htt may cause toxicity. Mutant versions of htt may damage the ubiquitin-proteasome system (UPS) in the cytoplasm, causing a buildup of more improperly folded proteins; (5). These harmful proteins may also interfere with clathrin-mediated endocytosis and regular vesicle transport. Additionally, the presence of mutant htt may cause mitochondrial damage, which would directly or indirectly activate pro-apoptotic proteins and increase cellular toxicity as well as other negative effects; (6). The cell gathers harmful pieces into ubiquitinated cytoplasmic perinuclear aggregates as a form of self-defense; (7). Furthermore, mutant htt can go into the nucleus and create nuclear inclusions, which can interfere with transcription and the UPS.
7. Diagnosis
The clinical manifestation of a person with proven HD is utilized to make a diagnosis. To begin, it is essential to obtain an absolute history of the person, which takes precedence over full family history. With or without cognitive or psychiatric changes, motor alterations remain the key clinical criteria [142]. The three main indications, also family history, are required for diagnosis. For all diagnostic tests, the patient must give informed consent [1] (Figure 6). In a patient with chorea, a diagnosis of HD is strongly suspected. The following is an alternative way of diagnosing HD:
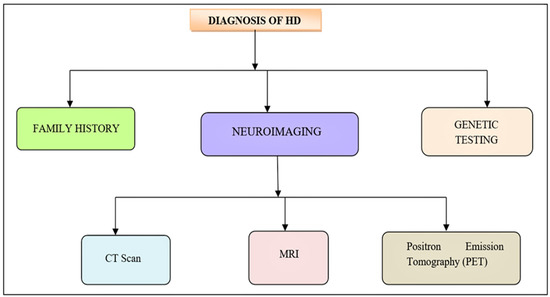
Figure 6.
Diagnosis of HD. The initial clinical manifestation of HD is similar to the neurological diseases. Neurological test, definitive genetic examination along with foremost family history is recommended for the diagnosis of HD.
- (1)
- Neuroimaging can assist confirm a diagnosis while ruling out other possibilities. A CT scan or an MRI can reveal cerebral atrophy or atrophy of the caudate nucleus. PET (Positron emission tomography) can also show diminution in striatal metabolic rate [143,144].
- (2)
- Genetic testing is a reliable technique to verify a suspected clinical diagnosis [145,146].
8. Herbal Management of HD
There have been numerous reports of herbal plants used for the management of HD. Natural constituents derived from herbal plants that have antioxidant, anti-inflammation, anti-apoptosis, immune-stimulatory, calcium antagonization, and neuroprotective properties have been shown to cure or treat HD [39,146,147,148]. Several plants and phytochemicals that have been shown to have anti-HD properties are briefly described.
8.1. Panax Ginseng
Ginseng is the dried root of the plant genus Panax [149]. Panax ginseng is derived from the Greek words “pan” meaning all and “axos” meaning cure [150]. It is native to Korea and China but is now globally popular and cultivated in countries such as Japan, US, Russia, Canada, Vietnam, Nepal, and Eastern Himalayas [151,152].
Ginseng extract improves neurological and psychological symptoms along with cognitive functions in healthy [153,154,155]. Ginseng has a beneficial effect on psychological feature performance owing to its action on the hippocampal brain [156]. Inhibition of Ca2+ entry through glutamate receptors, ginsenosides Rb1 and Rg3, safeguard cortical neurons from glutamate-induced cell damage [157,158]. The inhibition of both NMDA and glutamate-induced by saponins from ginseng increases Ca2+ entry through glutamate receptors [159]. An in vitro HD assay with MSN cultures for the investigation of the neuroprotective potential of compounds of ginseng total saponins exhibits neuroprotection by Rb1, Rg5, and Rc are interlinked with the capacity to prevent glutamate induced Ca2+ responses. These results can be credited to the active therapeutic choice to treat HD [160]. Ginsenosides Rd, Rb1, and Rb3 have also been shown to protect striatal neuronal damage caused by 3-NP [161]. Active components of ginseng demonstrate to possess beneficial potential such as antioxidant [160], anti-apoptotic [160], anti-inflammatory [161], and immune-stimulatory activities [162]. Moreover, it decreases lipid peroxidation and Ca2+ influx and suppresses neuronal excitotoxicity, stabilizes ATP levels in cells, protects neuronal structural integrity, and improves cognitive function [163,164].
8.2. Bacopa monnieri
It is a perennial, creeping herb of the family Scrophulariaceae [163,165]. It is also known as Brahmi and is found in warm wetlands [165]. They are indigenous to India and Australia [166]. It is also grown in Sri Lanka, Nepal, China, Vietnam, and Taiwan, Florida, and other southern states of the USA [167].
B. monnieri contains dammarane-type triterpenoid saponins, Bacosides A and Bacosides B, which are biomarkers for this species [165,167,168,169]. It also contains different kinds of saponin, including A–G [170,171,172] together with pseudojujubogenin or jujubogenin moieties as aglycones [173], Bacopaside I–V, X, and N1 and N2 [174,175,176]. The existence of several active constituents such as saponin, alkaloids, sterols, and flavonoids, are attributed to the pharmacological effects of B. monnieri [165,177,178]. This plant has potential activity as a memory booster, anti-inflammatory, analgesic, hepatoprotective, and antipyretic, free radical scavenging neuropharmacological disorders such as insomnia and antidepressant agent [179,180]. Bacoside A is the chief constituent for improving memory [181,182]. Due to mechanisms including metal ions complex chelation and enhanced antioxidant defense enzymes enhance the neuroprotective and memory-boosting effects of B. monneiri extracts [179,183,184]. An ethanolic extract of B. monneiri inactivates 3-nitropropionic acid (NP)-induces dysfunction of mitochondria by altering antioxidant mechanism [185]. 3-NP inactivates the succinate dehydrogenase cell enzyme (SDH) and the electron transport chain complex II–III [186]. It also reduces ROS, malondialdehyde (MDA), and free fatty acid levels [187]. The oral intake of BM’s leaf powder is reported to reduce basal concentrations of several oxidative markers and improve thiol-related antioxidant molecules, and antioxidant enzyme activity [188]. The dietary B. monneiri supplements lead to consequential defense against oxidative impairment in the brain along with a defensive effect against neuronal dysfunction due to stress. Thus, B. monneiri can be very beneficial in HD treatment [189].
8.3. Curcuma longa
Curcuma longa is generically called turmeric, a perennial plant [190] with yellow flower [191] dried rhizome of C. longa Linn (Curcuma domestica Valeton) [150] belonging to Zingiberaceae [190]. It is also cultivated expansively in Malaysia, Bangladesh, Cambodia, China, Indonesia, Thailand, and the Philippines [192,193].
Curcumin, obtained from the rhizome of C. longa Linn [194], is a natural agent with several functions and is pharmacologically safe [195]. Curcumin is a phytochemical and a crucial bioactive ingredient that is stated to possess antioxidant, antiangiogenic, anti-inflammatory, antimutagenic, antibacterial, and antiplatelet aggregation potential due to its chemical structure [196].
Curcumin may be effective in the treatment of some ailments characterized by the accumulation of fibrillar protein deposits [197]. Especially under a neurodegenerative condition such as HD, the accumulation of abnormal forms of particular proteins, such as htt, may have a role in disease development [198]. A study showed improvement in HD-like neurodegeneration when treated with solid lipid nanoparticles of curcumin (C-SLNs) [199]. There is also a considerable increase in mitochondrial complex activity and cytochrome levels. C-SLNs significantly reduced protein carbonyl production, lipid peroxidation, ROS levels, and mitochondrial swelling by restoring levels of glutathione and superoxide dismutase (SOD) activity [200]. Moreover, treatment with curcumin is reported to enhance cognitive and motor performances, restore succinate dehydrogenase action, and reduce oxidative stress, which inhibits the 3-NP in HD [201]. Curcumin also rescues down-regulated molecular chaperones in HD, including Hsp40 and Hsp70 [202]. Curcumin therapy also restored down-regulated BDNF in HD patients [203,204].
8.4. Ginkgo biloba
Ginkgo biloba, also called ginkgo, is the deciduous gymnosperm tree in the division Ginkgophyta which belongs to the Ginkgoaceae family [38,205]. Ginkgo is indigenous to Japan, China, Korea, North America, and Europe [150]. G. biloba, alike most plant medicines, contain many bioactive constituents viz. flavanol, diterpene lactones, sesquiterpenes, ginkgolides, ascorbic acid, catechin, iron-based SOD, and p-hydroxybenzoic acid, are expected to have synergistic effects [205,206].
As inflammation and free radicals are suspected to possess a part in HD development, G. biloba is reported to possess antioxidant and anti-inflammatory characteristics [207,208,209]. Mahdy and colleagues found that Ginkgo biloba might repair some of the neurological problems caused by a toxin, 3-Nitropropionic acid (3-NP) [208]. When injected into the brains of mice, 3-NP mimics the effects of HD: it causes many of the biological and behavioral changes that are seen in people with HD. However, mice that were treated with both 3-NP and ginkgo biloba showed milder neurodegenerative problems than those treated with 3-NP alone. Several biochemical changes that occur upon exposure to 3-NP were mitigated in animals that were treated with Ginkgo biloba. Authors suggest that Ginkgo biloba’s antioxidant properties, antiapoptotic effects, and improvement of energy metabolism were responsible for the neuroprotective effects. The G. biloba extract improves the 3-NP induced neurobehavioral impairments [210] while also lowering striatal MDA levels. Glyceraldehyde-3-phosphate dehydrogenase and Bcl xl expression levels in the striatum are also down-regulated and up-regulated by standardized G. biloba extract (EGb 761). These biochemical findings, together with histological findings, suggested that EGb 761 can be utilized in HD [211].
8.5. Centella asiatica
It is popularly called Indian Pennywort, Gotu kola, and Jal Brahmi, and is a small, herbaceous, frost-tender perennial plant from the family Umbelliferae [212]. It is classified as a Rasayana in Ayurveda because of its quality to heal memory and age-related brain problems [213,214]. It is a controlling brain tonic that has long been utilized in Ayurvedic medicine to revitalize the body, boost intelligence, and treat cognitive problems such as Alzheimer’s disease [215,216].
Triterpenoid saponins, such as madecassoside, madecassic acid, asiaticoside, and asiatic acid (AA), are the key components of C. asiatica [217,218]. Research revealed the activity of AA on the neurodegenerative potential of C. asiatica in CNS directing on brain cells enhances the elongation of neuritis in an in vitro experimental model [219,220]. An in vivo study on C. asiatica leaf extract also demonstrated healing in the dendritic arborization of hippocampal CA3 neurons [221,222]. The mechanisms playing a putative role are MEK/ERK and PI3/Atk signalling pathways [223,224]. Moreover, the neurodegenerative effect of C. asiatica takes place via the MAP kinase pathway [219,223]. The most significant use of C. asiatica is regarded as a brain tonic to enhance memory function [225]. The ability to preserve mental function is attributed to its antioxidant characteristics. Various ROS scavenging experiments in vitro and in vivo have been established to discover this effect. C. asiatica improves faster functional recovery and enhanced axonal regeneration, according to a study [226]. An alternative study found a considerable elevation in dendritic length and branching sites in amygdaloid and hippocampal CA3 neurons [227]. As a result, the study’s findings suggest that C. asiatica has a possible protective action against any assault (caused by oxidative stress and mitochondrial damage), and memory-enhancing properties can help control HD and its consequences.
8.6. Xylaria Species
Xyloketal B is an extract obtained from marine mangrove fungus of Xylaria species. In the early phases of HD, the damage to brain cell connection arises in the areas that allow movement called the basal ganglia and the cortex [228]. Thus, figuring out how to prevent neuron mortality and increase the excitability of specific nerve cell connections could lead to new treatments. The identification of many unique, natural, and active chemicals often takes place in the marine environment [229]. Xyloketal B has established robust neuroprotection in contrasting models related to neuronal impairments [230,231]. Six xyloketal B derivatives were evaluated in a Caenorhabditis elegans HD model to find potent neuroprotective for HD; all six compounds demonstrated a preventive role [232]. The aromatic core structure of Xyloketal B features unique bicyclic acetal moieties that can be easily changed to ameliorate and broaden its activity [233,234]. Moreover, some xyloketal derivatives can form a hydrogen bond. Xyloketal adheres to mutant htt proteins and inhibits the htt aggregation process, hence slowing the progression of HD [235]. Molecular docking experiments demonstrate that it can bind to the mutant htt protein’s GLN369 and GLN393 residues, generating a stable trimeric complex that prevents mutant htt aggregation formation. Thus, xyloketal derivatives serve as novel drug candidates for treating HD [232].
9. Pain and HD
The increased CAG repeat in HD gene carriers leads to a gradual long polyglutamine repeat, which results in neuronal loss in the brain, most significant in the basal ganglia. The integration of motor, emotional, autonomic, and cognitive responses to pain are one of the fundamental functions of the basal ganglia in the processing and analgesia of pain. [236,237]. Compared to other patients’ symptoms, the pain might not seem like a major issue. Even still, it is misunderstood and poorly understood in HD, despite the fact that it might significantly improve the quality of life for those who are impacted. Numerous research has examined the root causes of chronic pain in HD patients. Muscle and endocrine dysfunction, which are putative sources of nociceptive and neuropathic pain in HD, may be exacerbated by mutant Huntingtin [238], perhaps through inflammatory and immune mechanisms [239]. A meta-analysis indicated that while the pain burden in HD was lower than that of the general population, the total mean prevalence of pain in HD was around 41% [240]. A cross-sectional analysis of the Enroll-HD study in carriers of the pre-manifest and manifest HD gene mutations and in carriers of the non-HD gene mutation was performed, which verified that the prevalence of pain interference was considerably higher in the middle stage of HD compared to not HD gene carriers and that the late and middle stages of HD had lower prevalence’s of painful situations [241]. According to reports, persistent pain is less common in HD patients who are evident, and it tends to get less common as the disease progresses. Patients with HD also experienced less severe pain and associated dysfunction. Additionally, with the complete phenotypical presentation of the disease in the middle and late stages, this characteristic, which was lacking in the pre-manifest phase, became relevant. Patients with more CAG expansion and decreased functional capacity appeared to have diminished pain perception [242].
10. Discussion
Huntington’s disease (HD) is a rare, neurodegenerative disorder characterized by chorea, behavioral manifestations, and dementia [243]. Although HD is rare, it does receive a great deal of research attention. One reason is that HD has some features that make it more likely to be a tractable problem than other neurodegenerative conditions. First, the autosomal dominant nature of the condition means that the diagnosis is it is possible to accurately model and study the disease in vitro and in vivo. It is estimated that the mean HD prevalence is 5 in 100,000 people. Moreover, in another study, it is estimated that one in every 10,000 persons-nearly, 30,000 in the United States, have Huntington’s disease. Juvenile Huntington’s occurs in approximately 16% of all cases [244,245]. Somatic instability of the CAG repeat occurs in the tissues that are most vulnerable to HD pathology, particularly the striatum, and the degree of instability negatively correlates with age at disease onset. Genetic association studies have shown that DNA repair components, particularly those involved in mismatch repair, modify somatic instability and disease course [117]. Mutation of Htt characterized with repeat expansion of CAG trinucleotides is the key factor in HD. Abnormal aggregation of mutant Htt protein may cause toxic effects in neurons, leading to a series of pathogenic mechanisms associated the alteration in proteostasis and protein degradation following mitochondrial dysfunction, oxidative stress, transcription and synaptic dysfunction, axonal transport impairment, and a series of metabolic impairments subsequent to neurodegeneration. Despite the fact that the pathogenesis of HD has still not been resolved and a cure is not available, many therapeutic options are available for treating symptoms and signs with a view to improving quality of life. Although many signs and symptoms can be treated, it is not always necessary to do so. To date, there are no promising treatments for the long-term unwanted effects of HD, which are being combated by symptomatic prevention and treatments for mitigating the psychiatric, cognitive, and motor deformities of HD. The patient’s limitations in daily life determine whether or not drugs are required. Very little evidence is available about the drug or the dosage to prescribe for any signs and symptoms. To overcome the above-mentioned concerns, investigation has been devoted to the isolation of novel compounds from a variety of natural products in modulating relevant neuro-degenerative disorders. Up until then, a plethora of traditional treatments based on natural products have been shown to possess a wide range of therapeutic benefits for HD under in vitro and in vivo models [246,247]. The neuroprotective effect of natural products in HD experimental models has been extensively studied. Indeed, based on relevant studies, natural products offer neuroprotection in experimental models predominantly through the antioxidant defense system, scavenging free radicals, neutralization of reactive oxygen species (ROS), reduction of oxidative stress, preservation of mitochondrial function, anti-inflammatory protection, inhibition of apoptosis, and induction of autophagy.
In future studies, it will be important to determine more precisely which components of the altered circuit contribute to deficits in learning, memory, and mood in the early stages because the development of more specific therapies for these symptoms would significantly improve the quality of life in affected individuals. Moreover, it will be critical to identify the earliest molecular mechanisms that lead to neuronal dysfunction and death in order to develop therapies that can delay the onset of overt HD.
11. Conclusions
HD is an inherited neurological illness in which htt is a protein that regulates transcription, transports intracellularly, and participates in the endosome-lysosome pathway. When this htt undergoes mutation, it causes several cellular complications viz., transcriptional dysregulation, protein aggregation and altered UPS, neuroglia dysfunction, mitochondrial dysfunction, altered synaptic plasticity, and axonal transport defect. This fatal disease is characterized by abnormal involuntary movements, impaired voluntary movements, and cognitive and psychiatric disturbances associated with neuronal death. Alternative and complementary therapies based on scientifically validated herbal ingredients may be an effective supplement to conventional medicine, which has potential drawbacks include the development of drug resistance and unpleasant side effects.
Naturally derived products having entrenched cell reinforcement and neuron safeguarding potential have indicated useful impacts against the manifestations of HD in both in vivo and in vitro studies. In this review, the roles of a number of plants are investigated in various neurotoxic animal models and transgenics are discussed, highlighting their ability to influence signalling pathways, leading to neuromodulation and probable neuroprotection. Moreover, the brief knowledge that the review provides on the pathologic mechanisms involved in HD are crucial points to consider while investigating therapeutic solutions and target specific relief. However, more investigation is needed to abundantly understand the potential therapeutic activity of phytochemicals in the prevention of HD. Hence, clinical outcomes of various studies are also needed to be evaluated in order to accept the efficacy of herbal medication in mainstream medicine.
Author Contributions
Conceptualization: S.M.F. and A.K.; writing—Original draft preparation: Z.I., S.K. and V.K.; writing—Review and editing: B.S.I.A.E.K., I.K. and M.U.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| HD | Huntington’s Disease |
| Htt | huntingtin |
| VEGF | vascular endothelial growth factor |
| TRP | tryptophan |
| KYN | Kynurenine |
| ANS | autonomic nervous system |
| CBP | CREB—binding protein |
| CREB | cAMP response element-binding |
| NRSE | Neuron restrictive silencer elements |
| CTD | carboxy-terminal domain |
| TBP | TATA-binding protein |
| HDACs | histone deacetylase complexes |
| UPS | ubiquitin-proteasome system |
| Hsp70 | Heat-shock protein 70 |
| HAP1 | Huntingtin-associated protein1 |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| MDA | malondialdehyde |
| NO | nitric oxide |
| TGF-β | transforming growth factor beta |
| TLRs | Toll-like receptors |
| GABAA | γ-aminobutyric acid type A |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| MSNs | Medium Spiny Neurons |
References
- Roos, R. Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G. Handbook of Clinical Neurology; I0 Leucodystrophies Lipidoses; Elsevier: Amsterdam, The Netherlands, 1968; Volume 4. [Google Scholar]
- Walker, F.O. Huntington’s Disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Novak, M.J.; Tabrizi, S.J. Huntington’s Disease. BMJ 2010, 340, c3109. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.; Ghisi, N. Evaluating the Current State of the Art of Huntington Disease Research: A Scientometric Analysis. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Mahalingam, S.; Levy, L.M. Genetics of Huntington Disease. Am. J. Neuroradiol. 2014, 35, 1070–1072. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s Disease: From Molecular Pathogenesis to Clinical Treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Gusella, J.F.; Wexler, N.S.; Conneally, P.M.; Naylor, S.L.; Anderson, M.A.; Tanzi, R.E.; Watkins, P.C.; Ottina, K.; Wallace, M.R.; Sakaguchi, A.Y. A Polymorphic DNA Marker Genetically Linked to Huntington’s Disease. Nature 1983, 306, 234–238. [Google Scholar] [CrossRef]
- Choudhary, S.; Kumar, P.; Malik, J. Plants and Phytochemicals for Huntington’s Disease. Pharmacogn. Rev. 2013, 7, 81. [Google Scholar]
- Vuono, R.; Winder-Rhodes, S.; de Silva, R.; Cisbani, G.; Drouin-Ouellet, J.; Spillantini, M.G.; Cicchetti, F.; Barker, R.A. The Role of the European Huntington’s Disease Network. Brain 2015, 138, 1907–1918. [Google Scholar] [CrossRef]
- Landles, C.; Bates, G.P. Huntingtin and the Molecular Pathogenesis of Huntington’s Disease: Fourth in Molecular Medicine Review Series. EMBO Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef]
- Penney, J.B., Jr.; Vonsattel, J.; Macdonald, M.E.; Gusella, J.F.; Myers, R.H. CAG Repeat Number Governs the Development Rate of Pathology in Huntington’s Disease. J. Am. Neurol. Assoc. Child Neurol. Soc. 1997, 41, 689–692. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Leggo, J.; Coles, R.; Almqvist, E.; Biancalana, V.; Cassiman, J.-J.; Chotai, K.; Connarty, M.; Craufurd, D.; Curtis, A. Phenotypic Characterization of Individuals with 30–40 CAG Repeats in the Huntington Disease (HD) Gene Reveals HD Cases with 36 Repeats and Apparently Normal Elderly Individuals with 36–39 Repeats. Am. J. Hum. Genet. 1996, 59, 16. [Google Scholar]
- Imarisio, S.; Carmichael, J.; Korolchuk, V.; Chen, C.-W.; Saiki, S.; Rose, C.; Krishna, G.; Davies, J.E.; Ttofi, E.; Underwood, B.R. Huntington’s Disease: From Pathology and Genetics to Potential Therapies. Biochem. J. 2008, 412, 191–209. [Google Scholar] [CrossRef]
- Conneally, P.M. Huntington Disease: Genetics and Epidemiology. Am. J. Hum. Genet. 1984, 36, 506. [Google Scholar]
- Andrew, S.E.; Paul Goldberg, Y.; Kremer, B.; Telenius, H.; Theilmann, J.; Adam, S.; Starr, E.; Squitieri, F.; Lin, B.; Kalchman, M.A. The Relationship between Trinucleotide (CAG) Repeat Length and Clinical Features of Huntington’s Disease. Nat. Genet. 1993, 4, 398–403. [Google Scholar] [CrossRef]
- Gusella, J.F.; MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P. Molecular Genetics of Huntington’s Disease. Arch. Neurol. 1993, 50, 1157–1163. [Google Scholar] [CrossRef]
- Swami, M.; Hendricks, A.E.; Gillis, T.; Massood, T.; Mysore, J.; Myers, R.H.; Wheeler, V.C. Somatic Expansion of the Huntington’s Disease CAG Repeat in the Brain Is Associated with an Earlier Age of Disease Onset. Hum. Mol. Genet. 2009, 18, 3039–3047. [Google Scholar] [CrossRef]
- Harjes, P.; Wanker, E.E. The Hunt for Huntingtin Function: Interaction Partners Tell Many Different Stories. Trends Biochem. Sci. 2003, 28, 425–433. [Google Scholar] [CrossRef]
- Harper, P.S.; Jones, L. Huntington’s Disease: Genetic and Molecular Studies. Oxf. Monogr. Med. Genet. 2002, 45, 113–158. [Google Scholar]
- Politis, M.; Piccini, P. Positron Emission Tomography Imaging in Neurological Disorders. J. Neurol. 2012, 259, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. Positron Emission Tomography Provides Molecular Imaging of Biological Processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Dragatsis, I.; Dietrich, P. Genetics and Neuropathology of Huntington’s Disease. Int. Rev. Neurobiol. 2011, 98, 325–372. [Google Scholar] [PubMed]
- Tai, Y.F.; Pavese, N.; Gerhard, A.; Tabrizi, S.J.; Barker, R.A.; Brooks, D.J.; Piccini, P. Imaging Microglial Activation in Huntington’s Disease. Brain Res. Bull. 2007, 72, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Wu, Y.-R.; Chen, Y.-C.; Chen, C.-M. Plasma Inflammatory Biomarkers for Huntington’s Disease Patients and Mouse Model. Brain. Behav. Immun. 2015, 44, 121–127. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef]
- Kersten, H.M.; Danesh-Meyer, H.V.; Kilfoyle, D.H.; Roxburgh, R.H. Optical Coherence Tomography Findings in Huntington’s Disease: A Potential Biomarker of Disease Progression. J. Neurol. 2015, 262, 2457–2465. [Google Scholar] [CrossRef]
- Andrade, C.; Beato, J.; Monteiro, A.; Costa, A.; Penas, S.; Guimarães, J.; Reis, F.F.; Garrett, C. Spectral-Domain Optical Coherence Tomography as a Potential Biomarker in Huntington’s Disease. Mov. Disord. 2016, 31, 377–383. [Google Scholar] [CrossRef]
- Dhalla, A.; Pallikadavath, S.; Hutchinson, C.V. Visual Dysfunction in Huntington’s Disease: A Systematic Review. J. Huntingt. Dis. 2019, 8, 233–242. [Google Scholar] [CrossRef]
- Paulus, W.; Schwarz, G.; Werner, A.; Lange, H.; Bayer, A.; Hofschuster, M.; Müller, N.; Zrenner, E. Impairment of Retinal Increment Thresholds in Huntington’s Disease. J. Am. Neurol. Assoc. Child Neurol. Soc. 1993, 34, 574–578. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Béré, E.; Cantereau-Becq, A.; Meunier-Balandre, A.-C.; Merceron, B.; Burel, A.; Merienne, K.; Ravel, C.; Becq, F.; Bourmeyster, N. Myelinosome Organelles in the Retina of R6/1 Huntington Disease (Hd) Mice: Ubiquitous Distribution and Possible Role in Disease Spreading. Int. J. Mol. Sci. 2021, 22, 12771. [Google Scholar] [CrossRef]
- Widner, B.; Leblhuber, F.; Walli, J.; Tilz, G.; Demel, U.; Fuchs, D. Degradation of Tryptophan in Neurodegenerative Disorders. In Tryptophan, Serotonin, and Melatonin; Springer: Singapore, 1999; pp. 133–138. [Google Scholar]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Spiden, S.L.; Taylor, R.; Stone, T.W.; Darlington, L.G. Blood Levels of Kynurenines, Interleukin-23 and Soluble Human Leucocyte Antigen-G at Different Stages of Huntington’s Disease. J. Neurochem. 2010, 112, 112–122. [Google Scholar] [CrossRef]
- Adam, O.R.; Jankovic, J. Symptomatic Treatment of Huntington Disease. Neurotherapeutics 2008, 5, 181–197. [Google Scholar] [CrossRef]
- Phillips, W.; Shannon, K.M.; Barker, R.A. The Current Clinical Management of Huntington’s Disease. J. Mov. Disord. Soc. 2008, 23, 1491–1504. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Neuroprotective Therapeutics from Botanicals and Phytochemicals against Huntington’s Disease and Related Neurodegenerative Disorders. J. Herb. Med. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Wang, F.; Chen, J. Natural Compounds from Traditional Medicinal Herbs in the Treatment of Cerebral Ischemia/Reperfusion Injury. Acta Pharmacol. Sin. 2010, 31, 1523–1531. [Google Scholar] [CrossRef]
- Singh, Y.; Paswan, S.K.; Kumar, R.; Otia, M.K.; Acharya, S.; Kumar, D.; Keshamma, E. Plant & Its Derivative Shows Therapeutic Activity on Neuroprotective Effect. J. Res. Appl. Sci. Biotechnol. 2022, 1, 10–24. [Google Scholar]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef]
- Byun, S.; Lee, M.; Kim, M. Gene Therapy for Huntington’s Disease: The Final Strategy for a Cure? J. Mov. Disord. 2022, 15, 15. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Wexler, N.S.; Wexler, A.R.; Tabrizi, S.J.; Douglas, I.; Evans, S.J.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef]
- Harper, P.S. The Epidemiology of Huntington’s Disease. Hum. Genet. 1992, 89, 365–376. [Google Scholar] [CrossRef]
- Bates, G.; Harper, P.S.; Jones, L. Huntington’s Disease, 3rd ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Kay, C.; Collins, J.A.; Wright, G.E.; Baine, F.; Miedzybrodzka, Z.; Aminkeng, F.; Semaka, A.J.; McDonald, C.; Davidson, M.; Madore, S.J. The Molecular Epidemiology of Huntington Disease Is Related to Intermediate Allele Frequency and Haplotype in the General Population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 346–357. [Google Scholar] [CrossRef]
- Kumar, P.; Naidu, P.; Padi, S.; Kumar, A. Huntington’s Disease: A Review. Indian J. Pharm. Educ. Res. 2007, 41, 287–294. [Google Scholar] [CrossRef]
- Jha, S.; Patel, R. Some Observations on the Spectrum of Dementia. Neurol. India 2004, 52, 213. [Google Scholar]
- Hamilton, J.; Salmon, D.; Corey-Bloom, J.; Gamst, A.; Paulsen, J.; Jerkins, S.; Jacobson, M.; Peavy, G. Behavioural Abnormalities Contribute to Functional Decline in Huntington’s Disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 120–122. [Google Scholar] [CrossRef]
- Eddy, C.M.; Parkinson, E.G.; Rickards, H.E. Changes in Mental State and Behaviour in Huntington’s Disease. Lancet Psychiatry 2016, 3, 1079–1086. [Google Scholar] [CrossRef]
- Brinkman, R.; Mezei, M.; Theilmann, J.; Almqvist, E.; Hayden, M. The Likelihood of Being Affected with Huntington Disease by a Particular Age, for a Specific CAG Size. Am. J. Hum. Genet. 1997, 60, 1202. [Google Scholar]
- Franklin, G.L.; Camargo, C.H.F.; Meira, A.T.; Pavanelli, G.M.; Milano, S.S.; Germiniani, F.B.; Lima, N.S.; Raskin, S.; Barsottini, O.G.P.; Pedroso, J.L. Is Ataxia an Underestimated Symptom of Huntington’s Disease? Front. Neurol. 2020, 11, 571843. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Pearson, J.; Slow, E.J.; Hossain, S.M.; Leavitt, B.R.; Hayden, M.R. Cognitive Dysfunction Precedes Neuropathology and Motor Abnormalities in the YAC128 Mouse Model of Huntington’s Disease. J. Neurosci. 2005, 25, 4169–4180. [Google Scholar] [CrossRef]
- Wheelock, V. The Motor Disorder. In A Physician’s Guide to the Management of Huntington’s Disease, 3rd ed.; Nance, M., Paulsen, J.S., Rosenblatt, A., Wheelock, V., Eds.; Huntington’s Disease Society of America: New York, NY, USA, 2011; p. 39. [Google Scholar]
- Berardelli, A.; Noth, J.; Thompson, P.D.; Bollen, E.L.; Currà, A.; Deuschl, G.; Gert van Dijk, J.; Töpper, R.; Schwarz, M.; Roos, R.A. Pathophysiology of Chorea and Bradykinesia in Huntington’s Disease. J. Mov. Disord. Soc. 1999, 14, 398–403. [Google Scholar] [CrossRef]
- Rudzińska, M.; Krawczyk, M.; Wójcik-Pędziwiatr, M.; Szczudlik, A.; Tomaszewski, T. Tremor in Neurodegenerative Ataxias, Huntington Disease and Tic Disorder. Neurol. Neurochir. Pol. 2013, 47, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Verge, L. Cognitive Deterioration in Huntington Disease. Rev. Neurol. 2001, 32, 82–85. [Google Scholar] [PubMed]
- Paulsen, J. The Cognitive Disorder. In A Physician’s Guide to the Management of Huntington’s Disease, 3rd ed.; Lovecky, D., Tarapata, K., Eds.; Huntington’s Disease Society of America: New York, NY, USA, 2011; pp. 51–62. [Google Scholar]
- Rosenblatt, A. The Psychiatric Disorder. In A Physician’s Guide to the Management of Huntington’s Disease, 3rd ed.; Lovecky, D., Tarapata, K., Eds.; Huntington’s Disease Society of America: New York, NY, USA, 2011; pp. 63–81. [Google Scholar]
- Dale, M.; van Duijn, E. Anxiety in Huntington’s Disease. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 262–271. [Google Scholar] [CrossRef]
- Beglinger, L.J.; Paulsen, J.S.; Watson, D.B.; Wang, C.; Duff, K.; Langbehn, D.R.; Moser, D.J.; Paulson, H.L.; Aylward, E.H.; Carlozzi, N.E. Obsessive and Compulsive Symptoms in Prediagnosed Huntington’s Disease. J. Clin. Psychiatry 2008, 69, 1758–1765. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 503–506. [Google Scholar] [CrossRef]
- Kirkwood, S.C.; Su, J.L.; Conneally, P.M.; Foroud, T. Progression of Symptoms in the Early and Middle Stages of Huntington Disease. Arch. Neurol. 2001, 58, 273–278. [Google Scholar] [CrossRef]
- Saldert, C.; Fors, A.; Ströberg, S.; Hartelius, L. Comprehension of Complex Discourse in Different Stages of Huntington’s Disease. Int. J. Lang. Commun. Disord. 2010, 45, 656–669. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Nehl, C.; Hoth, K.F.; Kanz, J.E.; Benjamin, M.; Conybeare, R.; McDowell, B.; Turner, B. Depression and Stages of Huntington’s Disease. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 496–502. [Google Scholar] [CrossRef]
- Witjes-Ané, M.-N.W.; Vegter-van der Vlis, M.; van Vugt, J.P.; Lanser, J.B.; Hermans, J.; Zwinderman, A.H.; van Ommen, G.-J.B.; Roos, R.A. Cognitive and Motor Functioning in Gene Carriers for Huntington’s Disease: A Baseline Study. J. Neuropsychiatry Clin. Neurosci. 2003, 15, 7–16. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Clinical Features of Huntington’s Disease. Polyglutamine Disord. 2018, 1049, 1–28. [Google Scholar]
- Bennett, E.J.; Bence, N.F.; Jayakumar, R.; Kopito, R.R. Global Impairment of the Ubiquitin-Proteasome System by Nuclear or Cytoplasmic Protein Aggregates Precedes Inclusion Body Formation. Mol. Cell 2005, 17, 351–365. [Google Scholar] [CrossRef]
- Pogoda, A.; Chmielewska, N.; Maciejak, P.; Szyndler, J. Transcriptional Dysregulation in Huntington’s Disease: The Role in Pathogenesis and Potency for Pharmacological Targeting. Curr. Med. Chem. 2021, 28, 2783–2806. [Google Scholar] [CrossRef]
- Luthi-Carter, R.; Strand, A.; Peters, N.L.; Solano, S.M.; Hollingsworth, Z.R.; Menon, A.S.; Frey, A.S.; Spektor, B.S.; Penney, E.B.; Schilling, G. Decreased Expression of Striatal Signaling Genes in a Mouse Model of Huntington’s Disease. Hum. Mol. Genet. 2000, 9, 1259–1271. [Google Scholar] [CrossRef]
- Jin, Y.N.; Johnson, G.V. The Interrelationship between Mitochondrial Dysfunction and Transcriptional Dysregulation in Huntington Disease. J. Bioenerg. Biomembr. 2010, 42, 199–205. [Google Scholar] [CrossRef]
- Steffan, J.S.; Kazantsev, A.; Spasic-Boskovic, O.; Greenwald, M.; Zhu, Y.-Z.; Gohler, H.; Wanker, E.E.; Bates, G.P.; Housman, D.E.; Thompson, L.M. The Huntington’s Disease Protein Interacts with P53 and CREB-Binding Protein and Represses Transcription. Proc. Natl. Acad. Sci. USA 2000, 97, 6763–6768. [Google Scholar] [CrossRef]
- Nucifora, F.C., Jr.; Sasaki, M.; Peters, M.F.; Huang, H.; Cooper, J.K.; Yamada, M.; Takahashi, H.; Tsuji, S.; Troncoso, J.; Dawson, V.L. Interference by Huntingtin and Atrophin-1 with Cbp-Mediated Transcription Leading to Cellular Toxicity. Science 2001, 291, 2423–2428. [Google Scholar] [CrossRef]
- Sugars, K.L.; Brown, R.; Cook, L.J.; Swartz, J.; Rubinsztein, D.C. Decreased CAMP Response Element-Mediated Transcription: An Early Event in Exon 1 and Full-Length Cell Models of Huntington’S Disease that Contributes to Polyglutamine Pathogenesis. J. Biol. Chem. 2004, 279, 4988–4999. [Google Scholar] [CrossRef]
- Gines, S.; Seong, I.S.; Fossale, E.; Ivanova, E.; Trettel, F.; Gusella, J.F.; Wheeler, V.C.; Persichetti, F.; MacDonald, M.E. Specific Progressive CAMP Reduction Implicates Energy Deficit in Presymptomatic Huntington’s Disease Knock-in Mice. Hum. Mol. Genet. 2003, 12, 497–508. [Google Scholar] [CrossRef]
- Bae, B.-I.; Xu, H.; Igarashi, S.; Fujimuro, M.; Agrawal, N.; Taya, Y.; Hayward, S.D.; Moran, T.H.; Montell, C.; Ross, C.A. P53 Mediates Cellular Dysfunction and Behavioral Abnormalities in Huntington’s Disease. Neuron 2005, 47, 29–41. [Google Scholar] [CrossRef]
- Dawson, T.M.; Ginty, D.D. CREB Family Transcription Factors Inhibit Neuronal Suicide. Nat. Med. 2002, 8, 450–451. [Google Scholar] [CrossRef]
- McCampbell, A.; Taylor, J.P.; Taye, A.A.; Robitschek, J.; Li, M.; Walcott, J.; Merry, D.; Chai, Y.; Paulson, H.; Sobue, G. CREB-Binding Protein Sequestration by Expanded Polyglutamine. Hum. Mol. Genet. 2000, 9, 2197–2202. [Google Scholar] [CrossRef]
- Shimohata, T.; Nakajima, T.; Yamada, M.; Uchida, C.; Onodera, O.; Naruse, S.; Kimura, T.; Koide, R.; Nozaki, K.; Sano, Y. Expanded Polyglutamine Stretches Interact with TAFII130, Interfering with CREB-Dependent Transcription. Nat. Genet. 2000, 26, 29–36. [Google Scholar] [CrossRef]
- Lonze, B.E.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Dunah, A.W.; Jeong, H.; Griffin, A.; Kim, Y.-M.; Standaert, D.G.; Hersch, S.M.; Mouradian, M.M.; Young, A.B.; Tanese, N.; Krainc, D. Sp1 and TAFII130 Transcriptional Activity Disrupted in Early Huntington’s Disease. Science 2002, 296, 2238–2243. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Lee, B.; Cho, H.-Y.; Reyes, I.B.; Pu, X.-A.; Saido, T.C.; Hoyt, K.R.; Obrietan, K. CREB Is a Key Regulator of Striatal Vulnerability in Chemical and Genetic Models of Huntington’s Disease. Neurobiol. Dis. 2009, 36, 259–268. [Google Scholar] [CrossRef]
- Li, S.-H.; Li, X.-J. Huntingtin–Protein Interactions and the Pathogenesis of Huntington’s Disease. TRENDS Genet. 2004, 20, 146–154. [Google Scholar] [CrossRef]
- Zuccato, C.; Tartari, M.; Crotti, A.; Goffredo, D.; Valenza, M.; Conti, L.; Cataudella, T.; Leavitt, B.R.; Hayden, M.R.; Timmusk, T. Huntingtin Interacts with REST/NRSF to Modulate the Transcription of NRSE-Controlled Neuronal Genes. Nat. Genet. 2003, 35, 76–83. [Google Scholar] [CrossRef]
- McFarland, K.N.; Huizenga, M.N.; Darnell, S.B.; Sangrey, G.R.; Berezovska, O.; Cha, J.-H.J.; Outeiro, T.F.; Sadri-Vakili, G. MeCP2: A Novel Huntingtin Interactor. Hum. Mol. Genet. 2014, 23, 1036–1044. [Google Scholar] [CrossRef]
- Gauthier, L.R.; Charrin, B.C.; Borrell-Pagès, M.; Dompierre, J.P.; Rangone, H.; Cordelières, F.P.; De Mey, J.; MacDonald, M.E.; Leßmann, V.; Humbert, S. Huntingtin Controls Neurotrophic Support and Survival of Neurons by Enhancing BDNF Vesicular Transport along Microtubules. Cell 2004, 118, 127–138. [Google Scholar] [CrossRef]
- Plotkin, J.L.; Day, M.; Peterson, J.D.; Xie, Z.; Kress, G.J.; Rafalovich, I.; Kondapalli, J.; Gertler, T.S.; Flajolet, M.; Greengard, P. Impaired TrkB Receptor Signaling Underlies Corticostriatal Dysfunction in Huntington’s Disease. Neuron 2014, 83, 178–188. [Google Scholar] [CrossRef] [PubMed]
- McCampbell, A.; Taye, A.A.; Whitty, L.; Penney, E.; Steffan, J.S.; Fischbeck, K.H. Histone Deacetylase Inhibitors Reduce Polyglutamine Toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 15179–15184. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Kubilus, J.K.; Lee, J.; Ryu, H.; Beesen, A.; Zucker, B.; Smith, K.; Kowall, N.W.; Ratan, R.R.; Luthi-Carter, R. Histone Deacetylase Inhibition by Sodium Butyrate Chemotherapy Ameliorates the Neurodegenerative Phenotype in Huntington’s Disease Mice. J. Neurosci. 2003, 23, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Hockly, E.; Richon, V.M.; Woodman, B.; Smith, D.L.; Zhou, X.; Rosa, E.; Sathasivam, K.; Ghazi-Noori, S.; Mahal, A.; Lowden, P.A. Suberoylanilide Hydroxamic Acid, a Histone Deacetylase Inhibitor, Ameliorates Motor Deficits in a Mouse Model of Huntington’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2041–2046. [Google Scholar] [CrossRef]
- Fink, A.L. Chaperone-Mediated Protein Folding. Physiol. Rev. 1999, 79, 425–449. [Google Scholar] [CrossRef]
- Turturici, G.; Sconzo, G.; Geraci, F. Hsp70 and Its Molecular Role in Nervous System Diseases. Biochem. Res. Int. 2011, 2011, 618127. [Google Scholar] [CrossRef]
- Baldo, B.; Weiss, A.; Parker, C.N.; Bibel, M.; Paganetti, P.; Kaupmann, K. A Screen for Enhancers of Clearance Identifies Huntingtin as a Heat Shock Protein 90 (Hsp90) Client Protein. J. Biol. Chem. 2012, 287, 1406–1414. [Google Scholar] [CrossRef]
- Voges, D.; Zwickl, P.; Baumeister, W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999, 68, 1015–1068. [Google Scholar] [CrossRef]
- Hartl, F.; Hayar-Hartl, M. Complex Environment of Nascent Chain to Folded Protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef]
- Sakahira, H.; Breuer, P.; Hayer-Hartl, M.K.; Hartl, F.U. Molecular Chaperones as Modulators of Polyglutamine Protein Aggregation and Toxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 16412–16418. [Google Scholar] [CrossRef]
- Wacker, J.L.; Zareie, M.H.; Fong, H.; Sarikaya, M.; Muchowski, P.J. Hsp70 and Hsp40 Attenuate Formation of Spherical and Annular Polyglutamine Oligomers by Partitioning Monomer. Nat. Struct. Mol. Biol. 2004, 11, 1215–1222. [Google Scholar] [CrossRef]
- Ciechanover, A.; Brundin, P. The Ubiquitin Proteasome System in Neurodegenerative Diseases: Sometimes the Chicken, Sometimes the Egg. Neuron 2003, 40, 427–446. [Google Scholar] [CrossRef]
- Stanley, C.B.; Perevozchikova, T.; Berthelier, V. Structural Formation of Huntingtin Exon 1 Aggregates Probed by Small-Angle Neutron Scattering. Biophys. J. 2011, 100, 2504–2512. [Google Scholar] [CrossRef][Green Version]
- Bence, N.F.; Sampat, R.M.; Kopito, R.R. Impairment of the Ubiquitin-Proteasome System by Protein Aggregation. Science 2001, 292, 1552–1555. [Google Scholar] [CrossRef]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Scaravilli, F.; Easton, D.F.; Duden, R.; O’Kane, C.J. Inhibition of MTOR Induces Autophagy and Reduces Toxicity of Polyglutamine Expansions in Fly and Mouse Models of Huntington Disease. Nat. Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Bendotti, C.; Marino, M.; Cheroni, C.; Fontana, E.; Crippa, V.; Poletti, A.; De Biasi, S. Dysfunction of Constitutive and Inducible Ubiquitin-Proteasome System in Amyotrophic Lateral Sclerosis: Implication for Protein Aggregation and Immune Response. Prog. Neurobiol. 2012, 97, 101–126. [Google Scholar] [CrossRef]
- Nollen, E.A.; Garcia, S.M.; Van Haaften, G.; Kim, S.; Chavez, A.; Morimoto, R.I.; Plasterk, R.H. Genome-Wide RNA Interference Screen Identifies Previously Undescribed Regulators of Polyglutamine Aggregation. Proc. Natl. Acad. Sci. USA 2004, 101, 6403–6408. [Google Scholar] [CrossRef]
- Hay, D.G.; Sathasivam, K.; Tobaben, S.; Stahl, B.; Marber, M.; Mestril, R.; Mahal, A.; Smith, D.L.; Woodman, B.; Bates, G.P. Progressive Decrease in Chaperone Protein Levels in a Mouse Model of Huntington’s Disease and Induction of Stress Proteins as a Therapeutic Approach. Hum. Mol. Genet. 2004, 13, 1389–1405. [Google Scholar] [CrossRef]
- Usdin, M.T.; Shelbourne, P.F.; Myers, R.M.; Madison, D.V. Impaired Synaptic Plasticity in Mice Carrying the Huntington’s Disease Mutation. Hum. Mol. Genet. 1999, 8, 839–846. [Google Scholar] [CrossRef]
- Li, H.; Li, S.-H.; Yu, Z.-X.; Shelbourne, P.; Li, X.-J. Huntingtin Aggregate-Associated Axonal Degeneration Is an Early Pathological Event in Huntington’s Disease Mice. J. Neurosci. 2001, 21, 8473–8481. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.M.; Yoshihara, M.; Littleton, J.T. Cytoplasmic Aggregates Trap Polyglutamine-Containing Proteins and Block Axonal Transport in a Drosophila Model of Huntington’s Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Caviston, J.P.; Ross, J.L.; Antony, S.M.; Tokito, M.; Holzbaur, E.L. Huntingtin Facilitates Dynein/Dynactin-Mediated Vesicle Transport. Proc. Natl. Acad. Sci. USA 2007, 104, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Colin, E.; Zala, D.; Liot, G.; Rangone, H.; Borrell-Pagès, M.; Li, X.; Saudou, F.; Humbert, S. Huntingtin Phosphorylation Acts as a Molecular Switch for Anterograde/Retrograde Transport in Neurons. EMBO J. 2008, 27, 2124–2134. [Google Scholar] [CrossRef]
- McGuire, J.R.; Rong, J.; Li, S.-H.; Li, X.-J. Interaction of Huntingtin-Associated Protein-1 with Kinesin Light Chain: Implications in Intracellular Trafficking in Neurons. J. Biol. Chem. 2006, 281, 3552–3559. [Google Scholar] [CrossRef]
- Leenders, K.; Frackowiak, R.; Quinn, N.; Marsden, C. Brain Energy Metabolism and Dopaminergic Function in Huntington’s Disease Measured in Vivo Using Positron Emission Tomography. J. Mov. Disord. Soc. 1986, 1, 69–77. [Google Scholar] [CrossRef]
- Stahl, W.L.; Swanson, P.D. Biochemical Abnormalities in Huntington’s Chorea Brains. Neurology 1974, 24, 813. [Google Scholar] [CrossRef]
- Cramer, H.; Warter, J.-M.; Renaud, B. Analysis of Neurotransmitter Metabolites and Adenosine 3′, 5′-Monophosphate in the CSF of Patients with Extrapyramidal Motor Disorders. Adv. Neurol. 1984, 40, 431–435. [Google Scholar]
- Farooqui, T.; Farooqui, A.A. Aging: An Important Factor for the Pathogenesis of Neurodegenerative Diseases. Mech. Ageing Dev. 2009, 130, 203–215. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Qin, Z. Molecular Mechanisms of Excitotoxicity and Their Relevance to Pathogenesis of Neurodegenerative Diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Bogdanov, M.B.; Andreassen, O.A.; Dedeoglu, A.; Ferrante, R.J.; Beal, M.F. Increased Oxidative Damage to DNA in a Transgenic Mouse Model of Huntington’s Disease. J. Neurochem. 2001, 79, 1246–1249. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington Disease: New Insights into Molecular Pathogenesis and Therapeutic Opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mecocci, P.; Browne, S.E.; Senin, U.; Beal, M.F. Oxidative Damage to Mitochondrial DNA in Huntington’s Disease Parietal Cortex. Neurosci. Lett. 1999, 272, 53–56. [Google Scholar] [CrossRef]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative Stress in Huntington’s Disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A.; Tomoda, T.; Bae, B.-I. Mechanisms of Neuronal Cell Death in Huntington’s Disease. Cytogenet. Genome Res. 2003, 100, 287–295. [Google Scholar] [CrossRef]
- Túnez, I.; Tasset, I.; Pérez-De La Cruz, V.; Santamaría, A. 3-Nitropropionic Acid as a Tool to Study the Mechanisms Involved in Huntington’s Disease: Past, Present and Future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef]
- Shin, J.; Fang, Z.; Yu, Z.; Wang, C.; Li, S.; Li, X. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 2005, 171, 1001–1012. [Google Scholar] [CrossRef]
- Tydlacka, S.; Wang, C.-E.; Wang, X.; Li, S.; Li, X.-J. Differential Activities of the Ubiquitin–Proteasome System in Neurons versus Glia May Account for the Preferential Accumulation of Misfolded Proteins in Neurons. J. Neurosci. 2008, 28, 13285–13295. [Google Scholar] [CrossRef]
- Bradford, J.; Shin, J.-Y.; Roberts, M.; Wang, C.-E.; Li, X.-J.; Li, S. Expression of Mutant Huntingtin in Mouse Brain Astrocytes Causes Age-Dependent Neurological Symptoms. Proc. Natl. Acad. Sci. USA 2009, 106, 22480–22485. [Google Scholar] [CrossRef]
- Chou, S.-Y.; Weng, J.-Y.; Lai, H.-L.; Liao, F.; Sun, S.H.; Tu, P.-H.; Dickson, D.W.; Chern, Y. Expanded-Polyglutamine Huntingtin Protein Suppresses the Secretion and Production of a Chemokine (CCL5/RANTES) by Astrocytes. J. Neurosci. 2008, 28, 3277–3290. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Role of Microglial M1/M2 Polarization in Relapse and Remission of Psychiatric Disorders and Diseases. Pharmaceuticals 2014, 7, 1028–1048. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M. Reactive Microgliosis: Extracellular Micro-Calpain and Microglia-Mediated Dopaminergic Neurotoxicity. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, F.; Wang, J.; Wu, J.; Han, R.; Zhu, L.; DiFiglia, M.; Qin, Z. Expression of Mutant N-Terminal Huntingtin Fragment (Htt552-100Q) in Astrocytes Suppresses the Secretion of BDNF. Brain Res. 2012, 1449, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Träger, U.; Andre, R.; Lahiri, N.; Magnusson-Lind, A.; Weiss, A.; Grueninger, S.; McKinnon, C.; Sirinathsinghji, E.; Kahlon, S.; Pfister, E.L. HTT-Lowering Reverses Huntington’s Disease Immune Dysfunction Caused by NFκB Pathway Dysregulation. Brain 2014, 137, 819–833. [Google Scholar] [CrossRef]
- Björkqvist, M.; Wild, E.J.; Thiele, J.; Silvestroni, A.; Andre, R.; Lahiri, N.; Raibon, E.; Lee, R.V.; Benn, C.L.; Soulet, D. A Novel Pathogenic Pathway of Immune Activation Detectable before Clinical Onset in Huntington’s Disease. J. Exp. Med. 2008, 205, 1869–1877. [Google Scholar] [CrossRef]
- Ellrichmann, G.; Reick, C.; Saft, C.; Linker, R.A. The Role of the Immune System in Huntington’s Disease. Clin. Dev. Immunol. 2013, 2013, 541259. [Google Scholar] [CrossRef]
- Twelvetrees, A.E.; Yuen, E.Y.; Arancibia-Carcamo, I.L.; MacAskill, A.F.; Rostaing, P.; Lumb, M.J.; Humbert, S.; Triller, A.; Saudou, F.; Yan, Z. Delivery of GABAARs to Synapses Is Mediated by HAP1-KIF5 and Disrupted by Mutant Huntingtin. Neuron 2010, 65, 53–65. [Google Scholar] [CrossRef]
- Mandal, M.; Wei, J.; Zhong, P.; Cheng, J.; Duffney, L.J.; Liu, W.; Yuen, E.Y.; Twelvetrees, A.E.; Li, S.; Li, X.-J. Impaired α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptor Trafficking and Function by Mutant Huntingtin. J. Biol. Chem. 2011, 286, 33719–33728. [Google Scholar] [CrossRef]
- Liot, G.; Zala, D.; Pla, P.; Mottet, G.; Piel, M.; Saudou, F. Mutant Huntingtin Alters Retrograde Transport of TrkB Receptors in Striatal Dendrites. J. Neurosci. 2013, 33, 6298–6309. [Google Scholar] [CrossRef]
- Anitha, M.; Nandhu, M.; Anju, T.; Jes, P.; Paulose, C. Targeting Glutamate Mediated Excitotoxicity in Huntington’s Disease: Neural Progenitors and Partial Glutamate Antagonist–Memantine. Med. Hypotheses 2011, 76, 138–140. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Preventing Ca2+-Mediated Nitrosative Stress in Neurodegenerative Diseases: Possible Pharmacological Strategies. Cell Calcium 2010, 47, 190–197. [Google Scholar] [CrossRef]
- Coyle, J.; Schwarcz, R. Model for Huntington’s Chorea: Lesion of Striatal Neurons with Kainic Acid. Nature 1976, 263, 3. [Google Scholar] [CrossRef]
- Fan, M.M.; Fernandes, H.B.; Zhang, L.Y.; Hayden, M.R.; Raymond, L.A. Altered NMDA Receptor Trafficking in a Yeast Artificial Chromosome Transgenic Mouse Model of Huntington’s Disease. J. Neurosci. 2007, 27, 3768–3779. [Google Scholar] [CrossRef]
- Okamoto, S.; Pouladi, M.A.; Talantova, M.; Yao, D.; Xia, P.; Ehrnhoefer, D.E.; Zaidi, R.; Clemente, A.; Kaul, M.; Graham, R.K. Balance between Synaptic versus Extrasynaptic NMDA Receptor Activity Influences Inclusions and Neurotoxicity of Mutant Huntingtin. Nat. Med. 2009, 15, 1407–1413. [Google Scholar] [CrossRef]
- Milnerwood, A.J.; Gladding, C.M.; Pouladi, M.A.; Kaufman, A.M.; Hines, R.M.; Boyd, J.D.; Ko, R.W.; Vasuta, O.C.; Graham, R.K.; Hayden, M.R. Early Increase in Extrasynaptic NMDA Receptor Signaling and Expression Contributes to Phenotype Onset in Huntington’s Disease Mice. Neuron 2010, 65, 178–190. [Google Scholar] [CrossRef]
- Liu, D.; Long, J.D.; Zhang, Y.; Raymond, L.A.; Marder, K.; Rosser, A.; McCusker, E.A.; Mills, J.A.; Paulsen, J.S. Motor Onset and Diagnosis in Huntington Disease Using the Diagnostic Confidence Level. J. Neurol. 2015, 262, 2691–2698. [Google Scholar] [CrossRef]
- Kloeppel, S.; Henley, S.; Hobbs, N.Z.; Wolf, R.C.; Kassubek, J.; Tabrizi, S.J.; Frackowiak, R. Magnetic Resonance Imaging of Huntington’s Disease: Preparing for Clinical Trials. Neuroscience 2009, 164, 205–219. [Google Scholar] [CrossRef][Green Version]
- Versluis, M.; van der Grond, J.; van Buchem, M.; van Zijl, P.; Webb, A. High-Field Imaging of Neurodegenerative Diseases. Neuroimaging Clin. 2012, 22, 159–171. [Google Scholar] [CrossRef]
- Nold, C.S. Huntington Disease. JAAPA 2017, 30, 46–47. [Google Scholar] [CrossRef]
- Shin, H.; Kim, M.H.; Lee, S.J.; Lee, K.-H.; Kim, M.-J.; Kim, J.S.; Cho, J.W. Decreased Metabolism in the Cerebral Cortex in Early-Stage Huntington’s Disease: A Possible Biomarker of Disease Progression? J. Clin. Neurol. 2013, 9, 21–25. [Google Scholar] [CrossRef]
- Sandhya, S.; Vinod, K.R.; Kumar, S. Herbs used for brain disorders. Hygeia J. Drugs Med. 2010, 2, 38–45. [Google Scholar]
- Khan, A.; Jahan, S.; Alshahrani, S.; Alshehri, B.M.; Sameer, A.S.; Arafah, A.; Ahmad, A.; Rehman, M.U. Phytotherapeutic Agents for Neurodegenerative Disorders: A Neuropharmacological Review. In Phytomedicine; Academic Press: Cambridge, MA, USA, 2021; pp. 581–620. [Google Scholar]
- Hu, S.Y. The GenusPanax (Ginseng) in Chinese Medicine. Econ. Bot. 1976, 30, 11–28. [Google Scholar] [CrossRef]
- Kar, A. Pharmacognosy and Pharmacobiotechnolgy, 2nd ed.; New Age International (P) Limited: New Delhi, India, 2007. [Google Scholar]
- Yun, T.K. Brief Introduction of Panax Ginseng CA Meyer. J. Korean Med. Sci. 2001, 16, S3–S5. [Google Scholar] [CrossRef]
- Baker, J.T.; Borris, R.P.; Carté, B.; Cordell, G.A.; Soejarto, D.D.; Cragg, G.M.; Gupta, M.P.; Iwu, M.M.; Madulid, D.R.; Tyler, V.E. Natural Product Drug Discovery and Development: New Perspectives on International Collaboration. J. Nat. Prod. 1995, 58, 1325–1357. [Google Scholar] [CrossRef]
- Terasawa, K.; Shimada, Y.; Kita, T.; Yamamoto, T.; Tosa, H.; Tanaka, N.; Saito, Y.; Kanaki, E.; Goto, S.; Mizushima, N. Choto-San in the Treatment of Vascular Dementia: A Double-Blind, Placebo-Controlled Study. Phytomedicine 1997, 4, 15–22. [Google Scholar] [CrossRef]
- D’angelo, L.; Grimaldi, R.; Caravaggi, M.; Marcoli, M.; Perucca, E.; Lecchini, S.; Frigo, G.; Crema, A. A Double-Blind, Placebo-Controlled Clinical Study on the Effect of a Standardized Ginseng Extract on Psychomotor Performance in Healthy Volunteers. J. Ethnopharmacol. 1986, 16, 15–22. [Google Scholar] [CrossRef]
- Cho, I.-H. Effects of Panax Ginseng in Neurodegenerative Diseases. J. Ginseng Res. 2012, 36, 342. [Google Scholar] [CrossRef]
- Kurimoto, H.; Nishijo, H.; Uwano, T.; Yamaguchi, H.; Zhong, Y.-M.; Kawanishi, K.; Ono, T. Effects of Nonsaponin Fraction of Red Ginseng on Learning Deficits in Aged Rats. Physiol. Behav. 2004, 82, 345–355. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Markelonis, G.; Oh, T. Ginsenosides Rb1 and Rg3 Protect Cultured Rat Cortical Cells from Glutamate-induced Neurodegeneration. J. Neurosci. Res. 1998, 53, 426–432. [Google Scholar] [CrossRef]
- Lu, J.-M.; Yao, Q.; Chen, C. Ginseng Compounds: An Update on Their Molecular Mechanisms and Medical Applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jeong, H.K.; Bulin, S.E.; Kwon, S.W.; Park, J.H.; Bezprozvanny, I. Ginsenosides Protect Striatal Neurons in a Cellular Model of Huntington’s Disease. J. Neurosci. Res. 2009, 87, 1904–1912. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Park, K.-K.; Lee, J.-M.; Chun, K.-S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surh, Y.-J. Antioxidant and Anti-Tumor Promoting Activities of the Methanol Extract of Heat-Processed Ginseng. Cancer Lett. 2000, 150, 41–48. [Google Scholar] [CrossRef]
- Li, X.; Li, S. Effects of Total Saponins of Sanchi (Panax Pseudo-Ginseng Var. Notoginseng) on TNF, NO and Its Mechanisms. Chin. Tradit. Herb. Drugs 1999, 30, 514–517. [Google Scholar]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.-D. Use of Ginseng in Medicine with Emphasis on Neurodegenerative Disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef]
- Rai, K.; Gupta, N.; Dharamdasani, L.; Nair, P.; Bodhankar, P. Bacopa monnieri: A Wonder Drug Changing Fortune of People. Int. J. Appl. Sci. Biotechnol. 2017, 5, 127–132. [Google Scholar] [CrossRef]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting multiple pathways by naturally occurring phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef]
- Prasad, R.; Bagde, U.; Pushpangadan, P.; Varma, A. Bacopa monniera, L.: Pharmacological Aspects and Case Study Involving Piriformospora Indica. Int. J. Integr. Biol. 2008, 3, 100–110. [Google Scholar]
- Stough, C.; Singh, H.; Zangara, A. Mechanisms, Efficacy, and Safety of Bacopa monnieri (Brahmi) for Cognitive and Brain Enhancement. Evid. Based Complement. Altern. Med. 2015, 2015, 717605. [Google Scholar] [CrossRef]
- Barrett, S.C.; Strother, J.L. Taxonomy and Natural History of Bacopa (Scrophulariaceae) in California. Syst. Bot. 1978, 3, 408–419. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A. A Review on Bacopa monniera: Current Research and Future Prospects. Int. J. Green Pharm. IJGP 2010, 4. [Google Scholar]
- Bammidi, S.R.; Volluri, S.S.; Chippada, S.C.; Avanigadda, S.; Vangalapati, M. A Review on Pharmacological Studies of Bacopa monniera. J. Chem. Biol. Phys. Sci. JCBPS 2011, 1, 250. [Google Scholar]
- Hou, C.; Lin, S.; Cheng, J.; Hsu, F. Bacopaside III, Bacopasaponin G, and Bacopasides A, B., and C from Bacopa monniera. J. Nat. Prod. 2002, 65, 1759–1763. [Google Scholar] [CrossRef]
- Mahato, S.B.; Garai, S.; Chakravarty, A.K. Bacopasaponins E and F: Two Jujubogenin Bisdesmosides from Bacopa monniera. Phytochemistry 2000, 53, 711–714. [Google Scholar] [CrossRef]
- Garai, S.; Mahato, S.B.; Ohtani, K.; Yamasaki, K. Dammarane-Type Triterpenoid Saponins from Bacopa monniera. Phytochemistry 1996, 42, 815–820. [Google Scholar] [CrossRef]
- Garai, S.; Mahato, S.B.; Ohtani, K.; Yamasaki, K. Bacopasaponin DA Pseudojujubogenin Glycoside from Bacopa monniera. Phytochemistry 1996, 43, 447–449. [Google Scholar] [CrossRef]
- Murthy, P.B.S.; Raju, V.R.; Ramakrisana, T.; Chakravarthy, M.S.; Kumar, K.V.; Kannababu, S.; Subbaraju, G.V. Estimation of Twelve Bacopa Saponins in Bacopa monnieri Extracts and Formulations by High-Performance Liquid Chromatography. Chem. Pharm. Bull. 2006, 54, 907–911. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Garai, S.; Masuda, K.; Nakane, T.; Kawahara, N. Bacopasides III—V: Three New Triterpenoid Glycosides from Bacopa monniera. Chem. Pharm. Bull. 2003, 51, 215–217. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Sarkar, T.; Masuda, K.; Shiojima, K.; Nakane, T.; Kawahara, N. Bacopaside I and II: Two Pseudojujubogenin Glycosides from Bacopa monniera. Phytochemistry 2001, 58, 553–556. [Google Scholar] [CrossRef]
- Kawai, K.-I.; Shibata, S. Pseudojujubogenin, a New Sapogenin from Bacopa monniera. Phytochemistry 1978, 17, 287–289. [Google Scholar] [CrossRef]
- Kapoor, R.; Srivastava, S.; Kakkar, P. Bacopa monnieri Modulates Antioxidant Responses in Brain and Kidney of Diabetic Rats. Environ. Toxicol. Pharmacol. 2009, 27, 62–69. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhattacharya, A.; Kumar, A.; Ghosal, S. Antioxidant Activity of Bacopa monniera in Rat Frontal Cortex, Striatum and Hippocampus. Phytother. Res. 2000, 14, 174–179. [Google Scholar] [CrossRef]
- Kishore, K.; Singh, M. Effect of Bacosides, Alcoholic Extract of Bacopa monniera Linn. (Brahmi), on Experimental Amnesia in Mice. IJEB 2005, 43. [Google Scholar]
- Vohora, S.; Khanna, T.; Athar, M.; Ahmad, B. Analgesic Activity of Bacosine, a New Triterpene Isolated from Bacopa monnieri. Fitoter. Milano 1997, 68, 361–365. [Google Scholar]
- Vohora, D.; Pal, S.; Pillai, K. Protection from Phenytoin-Induced Cognitive Deficit by Bacopa monniera, a Reputed Indian Nootropic Plant. J. Ethnopharmacol. 2000, 71, 383–390. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Chaurasia, S.; Tripathi, E.; Upadhyay, A.; Dubey, G. Bacopa monniera Linn. as an Antioxidant: Mechanism of Action. Indian J. Exp. Biol. 1996, 34, 523–526. [Google Scholar]
- Russo, A.; Izzo, A.A.; Borrelli, F.; Renis, M.; Vanella, A. Free Radical Scavenging Capacity and Protective Effect of Bacopa monniera, L. on DNA Damage. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 870–875. [Google Scholar]
- Shinomol, G.K.; Bharath, M.S. Pretreatment with Bacopa monnieri Extract Offsets 3-Nitropropionic Acid Induced Mitochondrial Oxidative Stress and Dysfunctions in the Striatum of Prepubertal Mouse Brain. Can. J. Physiol. Pharmacol. 2012, 90, 595–606. [Google Scholar] [CrossRef]
- Andreassen, O.A.; Ferrante, R.J.; Hughes, D.B.; Klivenyi, P.; Dedeoglu, A.; Ona, V.O.; Friedlander, R.M.; Beal, M.F. Malonate and 3-Nitropropionic Acid Neurotoxicity Are Reduced in Transgenic Mice Expressing a Caspase-1 Dominant-Negative Mutant. J. Neurochem. 2000, 75, 847–852. [Google Scholar] [CrossRef]
- Kim, G.W.; Copin, J.-C.; Kawase, M.; Chen, S.F.; Sato, S.; Gobbel, G.T.; Chan, P.H. Excitotoxicity Is Required for Induction of Oxidative Stress and Apoptosis in Mouse Striatum by the Mitochondrial Toxin, 3-Nitropropionic Acid. J. Cereb. Blood Flow Metab. 2000, 20, 119–129. [Google Scholar] [CrossRef]
- Vishwas, S.; Gulati, M.; Kapoor, B.; Gupta, S.; Singh, S.K.; Awasthi, A.; Khan, A.; Goyal, A.; Bansal, A.; Baishnab, S. Expanding the Arsenal against Huntington’s Disease-Herbal Drugs and Their Nanoformulations. Curr. Neuropharmacol. 2021, 19, 957–989. [Google Scholar] [CrossRef]
- Shinomol, G.K. Bacopa monnieri Modulates Endogenous Cytoplasmic and Mitochondrial Oxidative Markers in Prepubertal Mice Brain. Phytomedicine 2011, 18, 317–326. [Google Scholar] [CrossRef]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and Neurodegenerative Diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Jain, S.; Shrivastava, S.; Nayak, S.; Sumbhate, S. Recent Trends in Curcuma Longa Linn. Pharmacogn. Rev. 2007, 1, 119–128. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Mullaicharam, A.; Maheswaran, A. Pharmacological Effects of Curcumin. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 92. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Kikis, E.A.; Gidalevitz, T.; Morimoto, R.I. Protein Homeostasis in Models of Aging and Age-Related Conformational Disease. Protein Metab. Homeost. Aging 2010, 694, 138–159. [Google Scholar]
- Bates, G. Huntingtin Aggregation and Toxicity in Huntington’s Disease. Lancet 2003, 361, 1642–1644. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Gharaibeh, A.; Maiti, P.; Culver, R.; Heileman, S.; Srinageshwar, B.; Story, D.; Spelde, K.; Paladugu, L.; Munro, N.; Muhn, N. Solid Lipid Curcumin Particles Protect Medium Spiny Neuronal Morphology, and Reduce Learning and Memory Deficits in the YAC128 Mouse Model of Huntington’s Disease. Int. J. Mol. Sci. 2020, 21, 9542. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Kumar, P.; Padi, S.; Naidu, P.; Kumar, A. Possible Neuroprotective Mechanisms of Curcumin in Attenuating 3-Nitropropionic Acid-Induced Neurotoxicity. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 19–26. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF Is Essential to Promote Persistence of Long-Term Memory Storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef]
- Liu, Z.; Dou, W.; Zheng, Y.; Wen, Q.; Qin, M.; Wang, X.; Tang, H.; Zhang, R.; Lv, D.; Wang, J. Curcumin Upregulates Nrf2 Nuclear Translocation and Protects Rat Hepatic Stellate Cells against Oxidative Stress. Mol. Med. Rep. 2016, 13, 1717–1724. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin Modulates Cell Death and Is Protective in Huntington’s Disease Model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef]
- Mullaicharam, A. A Review on Evidence Based Practice of Ginkgo Biloba in Brain Health. Int. J. Pharm. Chem. Anal. 2013, 1, 24–30. [Google Scholar]
- Jacobs, B.P.; Browner, W.S. Ginkgo Biloba: A Living Fossil. Am. J. Med. 2000, 108, 341–342. [Google Scholar] [CrossRef]
- Maltas, E.; Vural, H.C.; Yildiz, S. Antioxidant Activity and Fatty Acid Composition of Ginkgo Biloba from Turkey. J. Food Biochem. 2011, 35, 803–818. [Google Scholar] [CrossRef]
- Pietri, S.; Maurelli, E.; Drieu, K.; Culcasi, M. Cardioprotective and Anti-Oxidant Effects of the Terpenoid Constituents of Ginkgo BilobaExtract (EGb 761). J. Mol. Cell. Cardiol. 1997, 29, 733–742. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted Therapeutic Benefits of Ginkgo Biloba, L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar] [CrossRef]
- Mahdy, H.M.; Tadros, M.G.; Mohamed, M.R.; Karim, A.M.; Khalifa, A.E. The Effect of Ginkgo Biloba Extract on 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neurochem. Int. 2011, 59, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Amri, H.; Ogwuegbu, S.; Boujrad, N.; Drieu, K.; Papadopoulos, V. In Vivo Regulation of Peripheral-Type Benzodiazepine Receptor and Glucocorticoid Synthesis by Ginkgo Biloba Extract EGb 761 and Isolated Ginkgolides. Endocrinology 1996, 137, 5707–5718. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-All. Indian J. Pharm. Sci. 2010, 72, 546. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S. Rasaayana Drugs Part I. Pharm. Times 1993, 25, 915. [Google Scholar]
- Jana, U.; Sur, T.; Maity, L.; Debnath, P.; Bhattacharyya, D. A Clinical Study on the Management of Generalized Anxiety Disorder with Centella asiatica. Nepal Med. Coll. J. 2010, 12, 8–11. [Google Scholar]
- Vohra, K.; Pal, G.; Gupta, V.K.; Singh, S.; Bansal, Y. An Insight on Centella asiatica Linn.: A Review on Recent Research. Pharmacol. Online 2011, 2, 440–463. [Google Scholar]
- Singh, S.; Gautam, A.; Sharma, A.; Batra, A. Centella asiatica (L.): A Plant with Immense Medicinal Potential but Threatened. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 9–17. [Google Scholar]
- Singh, B.; Rastogi, R.P. A Reinvestigation of the Triterpenes of Centella asiatica. Phytochemistry 1969, 8, 917–921. [Google Scholar] [CrossRef]
- Randriamampionona, D.; Diallo, B.; Rakotoniriana, F.; Rabemanantsoa, C.; Cheuk, K.; Corbisier, A.-M.; Mahillon, J.; Ratsimamanga, S.; El Jaziri, M. Comparative Analysis of Active Constituents in Centella asiatica Samples from Madagascar: Application for Ex Situ Conservation and Clonal Propagation. Fitoterapia 2007, 78, 482–489. [Google Scholar] [CrossRef]
- Lokanathan, Y.; Omar, N.; Puzi, N.N.A.; Saim, A.; Idrus, R.H. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. MJMS 2016, 23, 4–14. [Google Scholar]
- Mohandas Rao, K.G.; Muddanna Rao, S.; Gurumadhva Rao, S. Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Complement. Altern. Med. 2006, 3, 349–357. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrong, P.; Tantisira, M.H. Acute and sub-chronic toxicity studies of a standardized extract of Centella asiatica ECa 233. Thai J. Pharm. Sci. 2011, 35, 55–64. [Google Scholar]
- Kumar, M.V.; Gupta, Y. Effect of Different Extracts of Centella asiatica on Cognition and Markers of Oxidative Stress in Rats. J. Ethnopharmacol. 2002, 79, 253–260. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Wanakhachornkrai, O.; Pongrakhananon, V.; Chunhacha, P.; Wanasuntronwong, A.; Vattanajun, A.; Tantisira, B.; Chanvorachote, P.; Tantisira, M.H. Neuritogenic Effect of Standardized Extract of Centella asiatica ECa233 on Human Neuroblastoma Cells. BMC Complement. Altern. Med. 2013, 13, 204. [Google Scholar] [CrossRef]
- Bhavna, D.; Jyoti, K. Centella asiatica: The elixir of life. Delhi Inst. Pharm. Sci. Res. 2011, 2, 431–438. [Google Scholar]
- Soumyanath, A.; Zhong, Y.P.; Yu, X.; Bourdette, D.; Koop, D.R.; Gold, S.A.; Bruce, G.G. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J. Pharm. Pharmacol. 2005, 57, 1221–1229. [Google Scholar] [CrossRef]
- Rao, S.B.; Chetana, M.; Devi, P.U. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol. Behav. 2005, 86, 449–457. [Google Scholar]
- Hedreen, J.C.; Folstein, S.E. Early Loss of Neostriatal Striosome Neurons in Huntington’s Disease. J. Neuropathol. Exp. Neurol. 1995, 54, 105–120. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.G.; Krohn, K.; Steingröver, K.; et al. Five unique compounds: Xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef]
- Li, S.; Shen, C.; Guo, W.; Zhang, X.; Liu, S.; Liang, F.; Xu, Z.; Pei, Z.; Song, H.; Qiu, L.; et al. Synthesis and neuroprotective action of xyloketal derivatives in Parkinson’s disease models. Mar. Drugs 2013, 11, 5159–5189. [Google Scholar] [CrossRef]
- Long, S.M.; Liang, F.Y.; Wu, Q.; Lu, X.L.; Yao, X.L.; Li, S.C.; Li, J.; Su, H.; Pang, J.Y.; Pei, Z. Identification of marine neuroactive molecules in behaviour-based screens in the larval zebrafish. Mar. Drugs 2014, 12, 3307–3322. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, W.; Xu, G.; Wang, Q.; Feng, L.; Long, S.; Liang, F.; Huang, Y.; Lu, X.; Li, S.; et al. Xyloketal-derived small molecules show protective effect by decreasing mutant Huntingtin protein aggregates in Caenorhabditis elegans model of Huntington’s disease. Drug Des. Dev. Ther. 2016, 10, 1443–1451. [Google Scholar] [CrossRef]
- Qin, Z.H.; Gu, Z.L. Huntingtin processing in pathogenesis of Huntington disease. Acta Pharmacol. Sin. 2004, 25, 1243–1249. [Google Scholar]
- Pettigrew, J.D.; Cadieux, J.A.; So, S.S.; Wilson, P.D. Phenylboronic acid mediated triple condensation reactions of phloroglucinol and unsaturated carbonyl compounds. Org. Lett. 2005, 7, 467–470. [Google Scholar] [CrossRef]
- Gong, H.; Bandura, J.; Wang, G.-L.; Feng, Z.-P.; Sun, H.-S. Xyloketal B: A Marine Compound with Medicinal Potential. Pharmacol. Ther. 2021, 230, 107963. [Google Scholar] [CrossRef]
- Borsook, D.; Upadhyay, J.; Chudler, E.H.; Becerra, L. A Key Role of the Basal Ganglia in Pain and Analgesia-Insights Gained through Human Functional Imaging. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef]
- Boecker, H.; Ceballos-Baumann, A.; Bartenstein, P.; Weindl, A.; Siebner, H.; Fassbender, T.; Munz, F.; Schwaiger, M.; Conrad, B. Sensory Processing in Parkinson’s and Huntington’s Disease: Investigations with 3D H215O-PET. Brain 1999, 122, 1651–1665. [Google Scholar] [CrossRef]
- Przybyl, L.; Wozna-Wysocka, M.; Kozlowska, E.; Fiszer, A. What, When and How to Measure—Peripheral Biomarkers in Therapy of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 1561. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, G.P.; van der Zwaan, K.F.; Roos, R.A.; Achterberg, W.P. The Prevalence and the Burden of Pain in Patients with Huntington Disease: A Systematic Review and Meta-Analysis. Pain 2019, 160, 773–783. [Google Scholar] [CrossRef]
- Sprenger, G.P.; Roos, R.A.; van Zwet, E.; Reijntjes, R.H.; Achterberg, W.P.; Susanne, T. The Prevalence of Pain in Huntington’s Disease in a Large Worldwide Cohort. Parkinsonism Relat. Disord. 2021, 89, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, A.; Morgante, F.; Edwards, M.J.; Brown, P.; Pereira, E.A. Pain in Parkinson’s Disease and the Role of the Subthalamic Nucleus. Brain 2021, 144, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Wiltshire, K.; Day, L.; Dykeman, J.; Steeves, T.; Jette, N. The Incidence and Prevalence of Huntington’s Disease: A Systematic Review and Meta-analysis. Mov. Disord. 2012, 27, 1083–1091. [Google Scholar] [CrossRef]
- Illarioshkin, S.; Klyushnikov, S.; Vigont, V.; Seliverstov, Y.A.; Kaznacheyeva, E. Molecular Pathogenesis in Huntington’s Disease. Biochem. Mosc. 2018, 83, 1030–1039. [Google Scholar] [CrossRef]
- Baig, S.S.; Strong, M.; Quarrell, O.W. The Global Prevalence of Huntington’s Disease: A Systematic Review and Discussion. Neurodegener. Dis. Manag. 2016, 6, 331–343. [Google Scholar] [CrossRef]
- Lum, P.T.; Sekar, M.; Gan, S.H.; Bonam, S.R.; Shaikh, M.F. Protective Effect of Natural Products against Huntington’s Disease: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. ACS Chem. Neurosci. 2021, 12, 391–418. [Google Scholar] [CrossRef]
- Khan, A.; Alshahrani, S.; Arafah, A.; Qamar, W.; Shoaib, A.; Wali, A.F.; Amin, I.; Alqahtani, S.S.; Rehman, M.U. Possible Therapeutic Potential of Flavonoids and Phenolic Acids from Honey in Age-Related Neurodegenerative Diseases Via Targeting NAD+ Degradation. In Therapeutic Applications of Honey and Its Phytochemicals; Springer: Singapore, 2020; pp. 19–43. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).