Abstract
Low-grade epilepsy-associated tumors (LEATs) are common in the temporal lobe and can cause drug-resistant epilepsy. Complete resection of LEATs is sufficient for seizure relief. However, hippocampal resection might result in postoperative cognitive impairment. This study aimed to clarify the necessity of hippocampal resection for seizure and cognitive outcomes in patients with temporal lobe LEATs and a normal hippocampus. The study included 32 patients with temporal lobe LEATs and without hippocampal abnormalities. All patients underwent gross total resection as treatment for drug-resistant epilepsy at our tertiary epilepsy center from 2005 to 2020, followed by at least a 12-month follow-up period. Seizure and cognitive outcomes were compared between patients who underwent additional hippocampal resection (Resected group) and those who did not (Preserved group). Among the participants, 14 underwent additional hippocampal resection and 28 (87.5%) achieved seizure freedom irrespective of hippocampal resection. The seizure-free periods were not different between the two groups. Additional hippocampal resection resulted in a significantly negative impact on the postoperative verbal index. In conclusion, additional hippocampal resection in patients with temporal lobe LEATs without hippocampal abnormalities is unnecessary because lesionectomy alone results in good seizure control. Additional hippocampal resection may instead adversely affect the postoperative language function.
1. Introduction
Low-grade epilepsy-associated tumors (LEATs) are the primary causes of drug-resistant epilepsy, particularly in children and young adults. LEATs are known to be slow-growing and biologically benign [1], although a few cases with tumor progression or malignant transformation have been reported [2,3]. LEATs account for 2–5% of brain tumors and are the second most common etiology of surgically amenable epilepsy. The tumors arise most commonly in the temporal lobe; hence, they may cause drug-resistant limbic seizures. Complete resection of the tumor is generally sufficient for seizure relief [4,5,6]. Tumor invasion of the hippocampus or hippocampal sclerosis can be associated with temporal LEATs. In such cases, resection of the hippocampus may be considered for seizure control.
Additional hippocampal resection results in a higher likelihood of seizure resolution than lesionectomy alone in patients with World Health Organization (WHO) grade II gliomas [7]. WHO grade I tumors, often diagnosed as ganglioglioma or dysembryoplastic neuroepithelial tumors, account for more than 65% of LEATs [8]. When tumor invasion or sclerotic changes are absent in the hippocampus, additional hippocampal resection may result in postoperative cognitive impairment. The benefit of additional hippocampal resection at the expense of cognitive impairment has not been clarified, as very few studies that evaluated its effects on cognitive function included patients with a normal hippocampus. We hypothesized that tumor resection alone would provide adequate seizure control in patients with a normal hippocampus and that additional resection of the normal hippocampus could affect the postoperative cognitive status.
This study aimed to clarify the necessity of hippocampal resection in patients with temporal lobe LEATs without hippocampal abnormalities to evaluate our hypothesis and patient outcomes regarding seizure control and cognitive function.
2. Materials and Methods
Clinical information of the patients and their history of epilepsy, including the tumor location, presence of a hippocampal abnormality, extent of resection, pathological diagnosis, and postoperative seizure and cognitive outcomes, were collected retrospectively from our database based on medical records. We compared the seizure and cognitive outcomes between patients who underwent additional hippocampal resection and those who did not undergo the procedure. The study was approved by the ethics committee of the National Center of Neurology and Psychiatry, Tokyo, Japan (No. A2018-049), and the requirement for written informed consent was waived because of the study’s retrospective design.
2.1. Patients
The inclusion criteria were as follows: (1) consecutive patients with temporal lobe LEATs who underwent epilepsy surgery at our tertiary epilepsy center from 2005 to 2020; (2) absence of hippocampal abnormality, including hippocampal sclerosis and hippocampal invasion of the tumor, on magnetic resonance imaging (MRI); (3) underwent gross total resection (GTR) of the tumor; and (4) followed up for at least 12 months after resection. A total of 56 patients underwent epilepsy surgery for temporal lobe LEATs during the study period. Among them, 1 patient underwent two-stage hippocampal resection after tumor resection, 21 patients had hippocampal lesions detected by MRI, and 2 patients who failed to achieve GTR were excluded. Finally, 32 patients (13 females) were included in the analysis. Our board-certified pathologists pathologically confirmed the diagnosis of LEATs.
2.2. Presurgical Evaluation
All patients underwent comprehensive epilepsy evaluations, including medical interviews, neurological and neuropsychological examinations, long-term video-electroencephalography (EEG), and MRI. Epilepsy histories were obtained from all patients and, when necessary, from their relatives, and the semiology of their habitual seizures was confirmed. Scalp EEG was recorded with the standard 10–20 electrode placement system, including bilateral anterior temporal electrodes. Three-Tesla brain MRI was performed, including three-dimensional fluid-attenuated inversion recovery, double inversion recovery, and T1- and T2-weighted imaging, with contrast-enhanced MRI performed when necessary. We evaluated the location of LEATs and the presence of abnormalities in the hippocampus. Based on the comprehensive epilepsy evaluations, the LEATs were suspected as epileptogenic in all patients, and the institutional patient-management conference determined an indication for surgical resection.
2.3. Surgical Resection
GTR of the MRI lesion (tumor) was attempted in principle. The decision to perform hippocampal resection was usually made in advance; occasionally, it was made intraoperatively based on the electrocorticography (ECoG) findings. The hippocampus was defined as preserved when hippocampal resection was not performed, and postoperative MRI confirmed that the hippocampal head was preserved (Preserved group); otherwise, the hippocampus was considered resected (Resected group). The reasons for preserving the hippocampus were then investigated.
2.4. Postoperative Seizure Outcome
The postoperative seizure outcome was assessed during the final follow-up based on the International League Against Epilepsy (ILAE) classification [9]. The seizure-free rate was statistically compared between the Resected and Preserved groups using Fisher’s exact test (JMP version 11, SAS Institute Inc., Cary, North Carolina). The seizure-free period (SFP), defined as the seizure-free duration after surgery until the first seizure recurrence, was analyzed using the Kaplan–Meier method, and the differences in the median SFP between the two groups were assessed using the log-rank and Wilcoxon tests (R version 3.5.2, The R Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered statistically significant.
2.5. Antiseizure Medication (ASM) Reduction
The numbers of ASMs at the time of surgery and the final follow-up were compared. The association between hippocampal resection and successful ASM withdrawal after surgery was statistically evaluated.
2.6. Postoperative Cognitive Outcome
Neuropsychological evaluations were performed pre- and postoperatively. The intelligence quotient (IQ) was measured using the Wechsler Adult Intelligence Scale (WAIS), Wechsler Intelligence Scale for Children (WISC), or Suzuki–Binet test [10]. The developmental quotient (DQ) was measured in young children by the Kinder Infant Developmental Scale (KIDS) [11] or Enjoji test [12]. Both pre- and postoperative assessments used the same tests.
Cognitive function was categorized into three components: global index (GI), verbal index (VI), and working memory index (WMI). The GI was represented by the full-scale IQ in the WAIS or WISC or by the standardized IQ/DQ when evaluated with other scales. The standardization of IQ/DQ was performed using the following formula:
GI = 10 × (x − μ)/σ + 100 (x: measured score; μ: average score; σ: standard deviation)
The average score (μ) was referenced from previous reports: 111 in the Suzuki–Binet test and 105 in the KIDS and Enjoji test [10,11,12,13].
The VI was represented by the verbal comprehension score in the WAIS or WISC or by the standardized value of the averaged sub-scores on language comprehension and language expression in KIDS [14]. The WMI was represented by the working memory score in the WAIS and WISC.
The pre- and postoperative indices between the Resected and Preserved groups were statistically compared using Student’s t-tests. The differences between the pre- and postoperative indices were statistically analyzed using the paired t-test. The preoperative cognitive indices and effects of hippocampal resection on the postoperative cognitive indices were analyzed using analysis of covariance (ANCOVA). All statistical analyses were performed using JMP software (version 11, SAS Institute Inc., Cary, North Carolina). A p-value < 0.05 was considered significant.
3. Results
3.1. Patient and Tumor Characteristics
The clinical characteristics of the 32 patients (32 tumors) are summarized in Table 1.

Table 1.
Patient characteristics.
The mean ages at initial epileptic seizure and surgery were 8.1 (range, 0–18) and 18.6 (range, 0.5–55) years, respectively. The mean duration of postsurgical follow-up was 67.7 (range, 12–170) months. Among the patients, 75.0% were right-handed; handedness was not determined in 15.6% of the patients, mainly because of their young age. The most common seizure type was focal onset impaired awareness seizures (96.9%). Seizure frequency was daily in 3 cases, weekly in 15 cases, monthly in 10 cases, and rare in 4 cases.
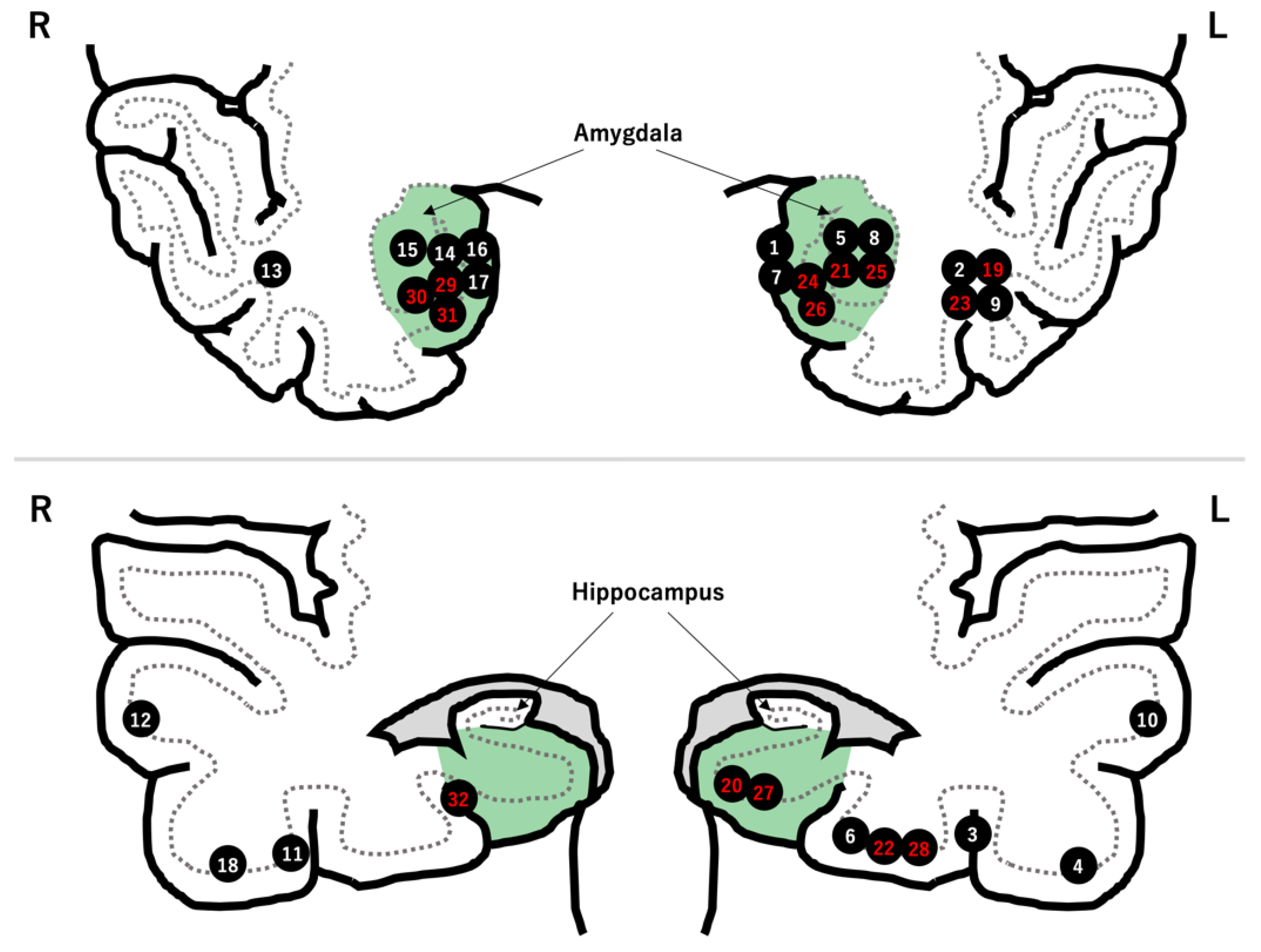
The tumors were in the left temporal lobe and medial to the collateral sulcus in 20 (62.5%) and 18 (56.3%) patients, respectively (Figure 1). Cystic formation and calcification were seen in 22 (68.8%) and 13 (40.6%) tumors, respectively. The pathological diagnosis was ganglioglioma in 15 patients (45.6%), low-grade glioma or astrocytoma in 15 patients, glioneuronal tumor in 1 patient, and dysembryoplastic neuroepithelial tumor in 1 patient (Table 2).
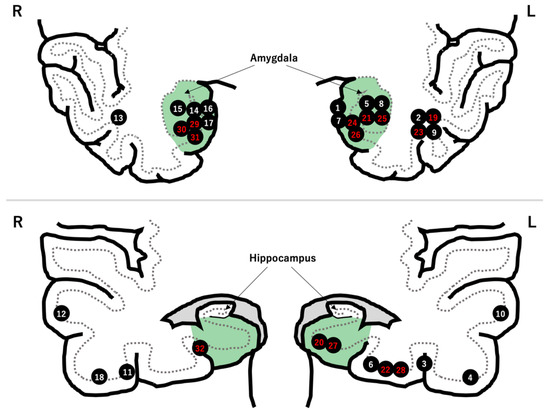
Figure 1.
Locations of the 32 LEATs. There were 18 tumors in the mesial temporal region (green zone). The patient number is highlighted in red after hippocampal resection.

Table 2.
Surgery and surgical outcome.
Focal cortical dysplasia was seen adjacent to the tumor in two patients with low-grade glioma. No patients underwent chemo/radiotherapies after surgery or showed tumor recurrence.
3.2. Hippocampal Resection
Fourteen patients underwent hippocampal resection. Of these, 10 patients had tumors located medial to the collateral sulcus (Figure 1).
Meanwhile, the hippocampus was preserved in 18 patients. The reasons for preserving the hippocampus were “distant tumor location from hippocampus” in 10 patients, “no preoperative cognitive decline” in 3 patients, “absence of abnormal epileptiform discharges on intraoperative ECoG” in 1 patient, and “unidentified” in 4 patients (Table 2 and Figure 2).
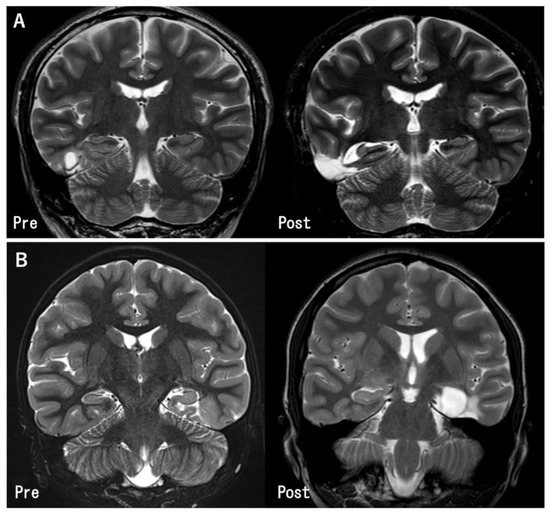
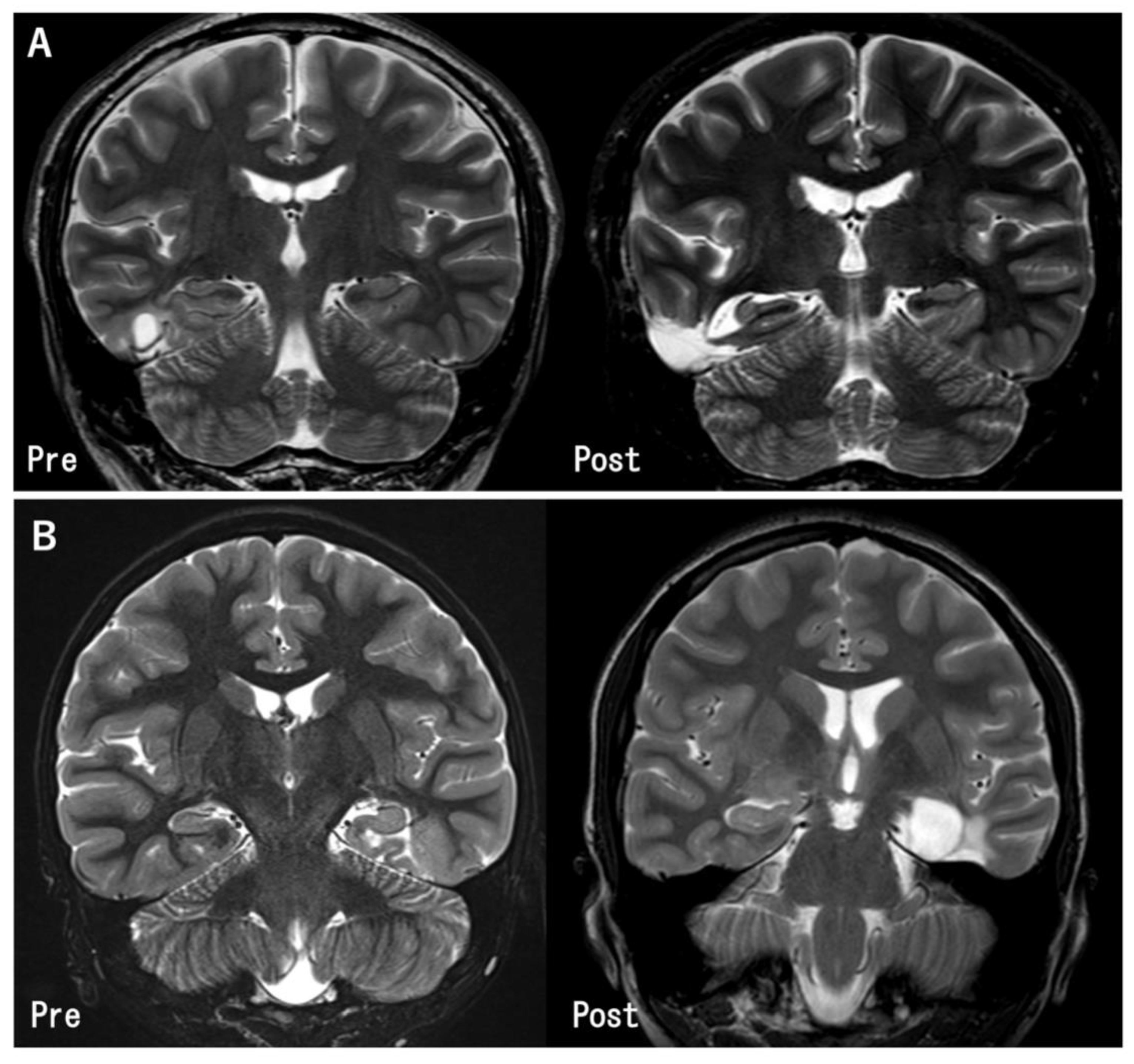
Figure 2.
Pre- and post-operative MR images in the representative cases. (A) The tumor in the inferior temporal gyrus was totally removed. The hippocampus was preserved because it was located apart from the tumor (Case 11). (B) The tumor was located at the left parahippocampal gyrus. Gross total tumor removal and hippocampectomy was performed (Case 27).
3.3. Effects of Hippocampal Resection
3.3.1. Seizure Outcome
A total of 28 patients (87.5%) remained seizure-free (ILAE class 1) at the last follow-up, including 24 (75.0%) who were seizure-free after surgery (class 1a). Two patients achieved a class 3 outcome, one achieved a class 4 outcome, and one achieved a class 5 outcome. Twelve (85.7%) and sixteen (88.9%) patients achieved seizure freedom in the Resected and Preserved groups, respectively. The seizure-free rate was not statistically associated with hippocampal resection.
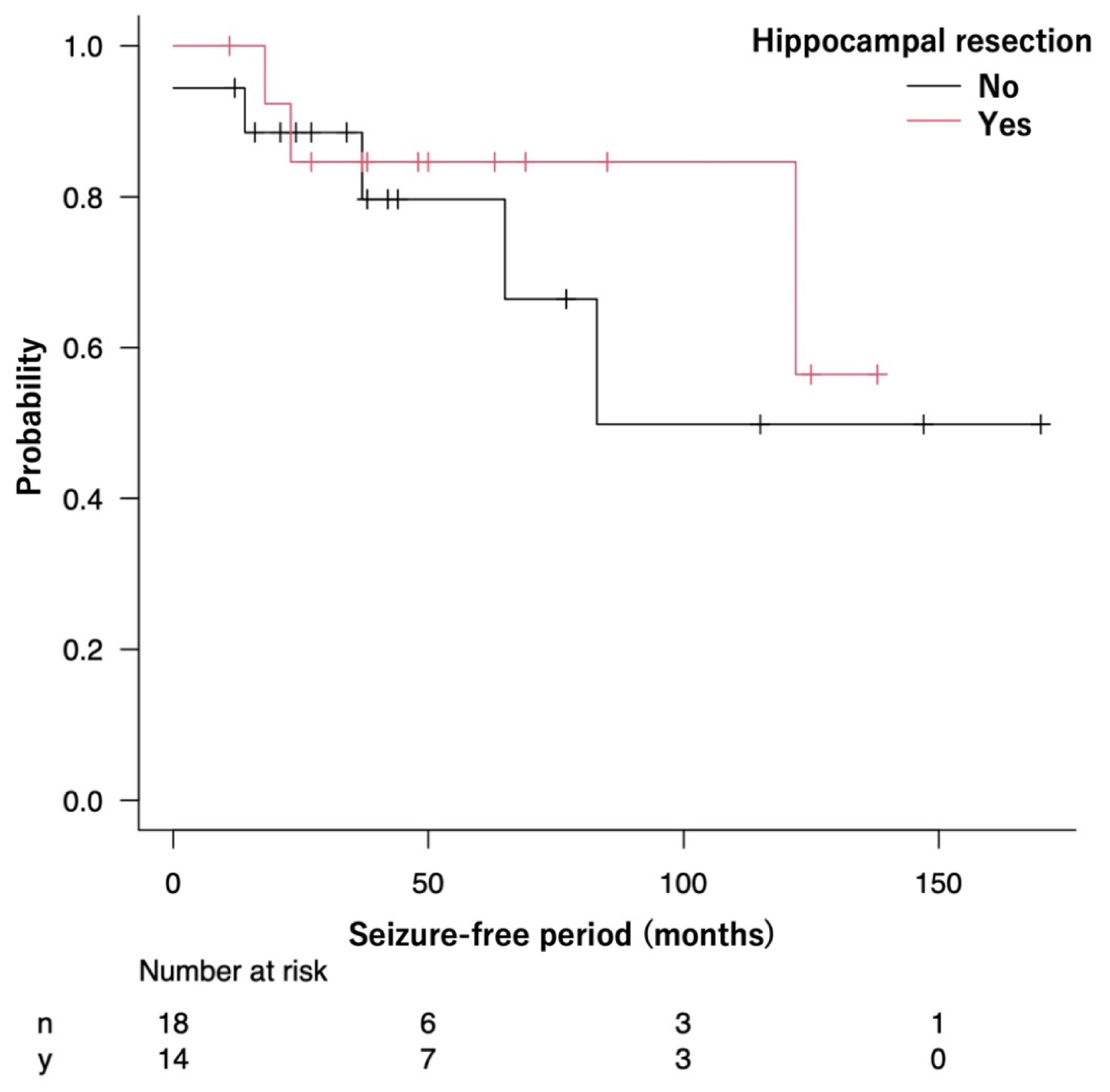
The median SFP could not be calculated in the Resected group because more than half of the patients remained seizure-free. Meanwhile, the median SFP in the Preserved group was 83 months (95% CI: 37 months—not reached). The seizure-free survival curves were not significantly different between the Resected and Preserved groups (p = 0.52) (Figure 3).
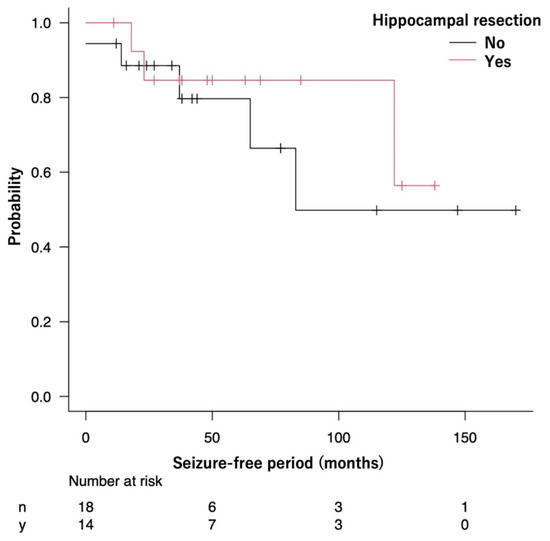
Figure 3.
The Kaplan–Meier curve shows the seizure-free survival of patients in the Resected and Preserved groups.
The same analysis was performed in the limited patients with the tumor located medial to the collateral sulcus. The seizure-free survival curves were not significantly different between the two groups (p = 0.77) (Supplementary Figure S1).
3.3.2. ASM Reduction
The number of postoperative ASMs was reduced in six (42.9%) and eight (44.4%) patients in the Resected and Preserved groups, respectively, but the difference was not significant. Meanwhile, ASMs were completely withdrawn in six patients (18.8%) (Table 2).
3.3.3. Cognitive Outcome
The IQ was evaluated with the WAIS in 15 patients, WISC in 11 patients, and Suzuki–Binet test in 1 patient. The DQ was evaluated with KIDS and Enjoji in four patients and one patient, respectively. The cognitive outcomes are summarized in Table 3 and Figure 4.

Table 3.
Pre- and postoperative cognitive outcomes.
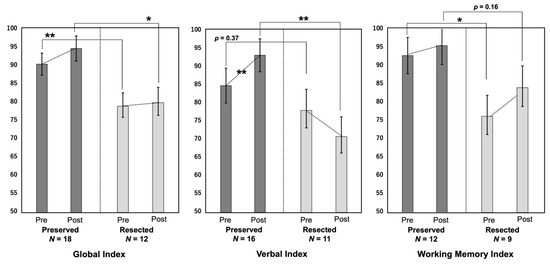
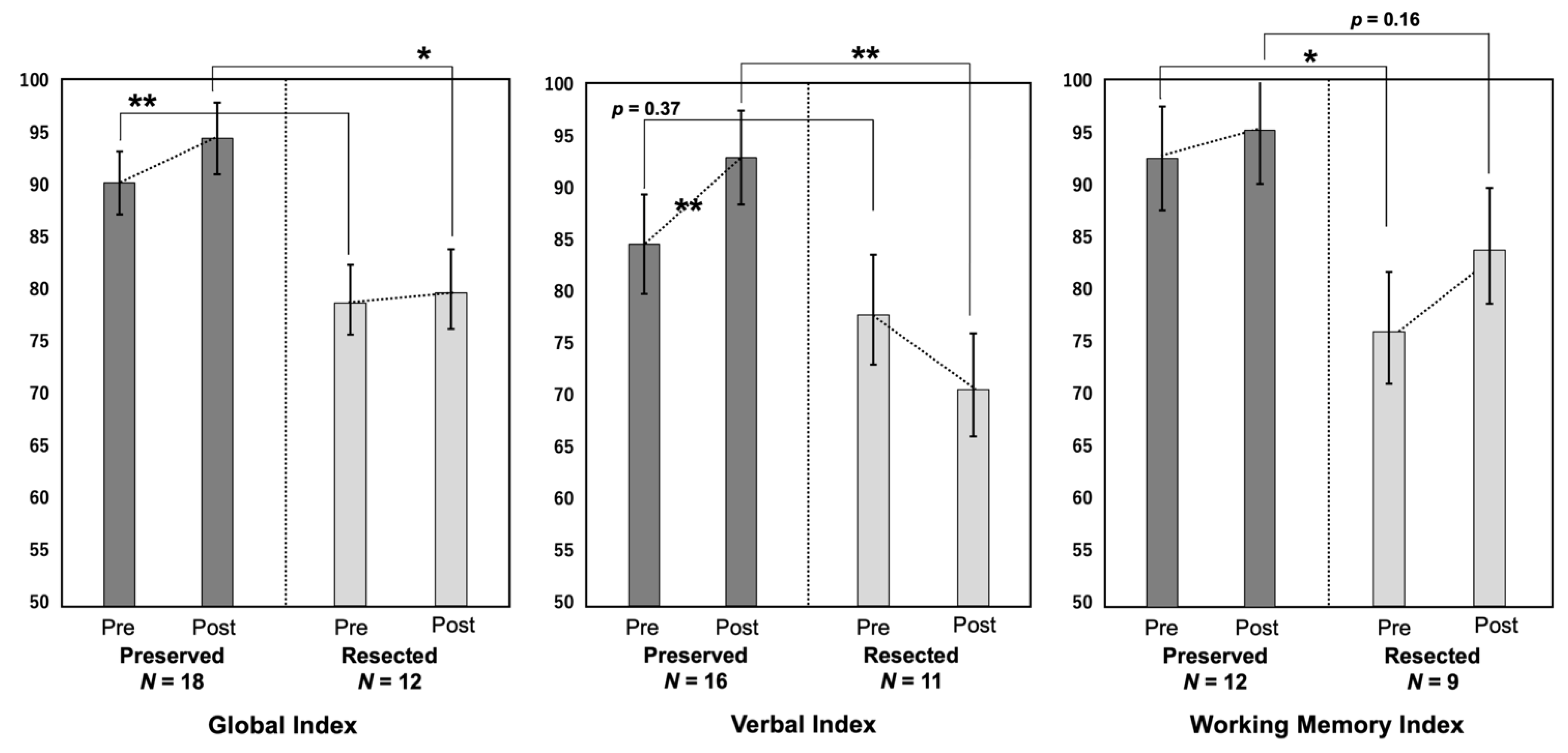
Figure 4.
The pre- and postoperative scores in the three cognitive indices (global index, verbal index, and working memory index) are compared. *: p < 0.05, **: p < 0.01.
The preoperative GI and WMI and postoperative GI and VI were lower in the Resected group than in the Preserved group. The other indices also tended to be lower in the Resected group. The VI significantly improved postoperatively in the Preserved group (p < 0.01), whereas it tended to worsen in the Resected group, although the difference was not statistically significant (p = 0.31). The GI and WMI tended to improve after surgery in both groups, although the difference was not significant.
Controlling for the preoperative VI, ANCOVA revealed a statistically significant difference between the Resected and Preserved groups on the postoperative VI. The differences in the GI and WMI between both groups were not significant (Table 4).

Table 4.
Analysis of covariance for the effect of hippocampal resection on postoperative cognitive index.
4. Discussion
We investigated the influence of additional hippocampal resection on seizure and cognitive outcomes in a series of patients with temporal lobe LEATs and a radiologically normal hippocampus. The novelty of this study is that it only included patients with temporal LEATs and a normal hippocampus on MRI. Based on our results, additional hippocampal resection did not necessarily provide positive effects on seizure outcomes for patients with a normal hippocampus and instead had the potential of worsening verbal function.
Our results suggest that additional hippocampal resection has limited significance on seizure outcomes, further strengthening the evidence from previous reports. Research has shown that additional hippocampal resection does not increase the probability of seizure freedom [6,15,16]. Vogt et al. reported that hippocampal resection had no significant effect on postoperative seizure outcomes in a large cohort with temporal lobe LEATs [6]. Fried et al. reported that 87% of their 41 patients achieved seizure freedom from lesionectomy alone, and they did not find any association between the extent of hippocampal resection and seizure freedom [15]. In a study of 27 patients with temporal lobe LEAT performed by Morris et al., 81% achieved seizure freedom, but removing the mesial structures did not contribute to attaining seizure freedom [16]. These results suggest that the hippocampus should be preserved unless it has structural abnormalities. However, these retrospective studies included relatively small numbers of patients, and the number of patients with a normal hippocampus was not specified. An important issue that needs to be addressed is whether a structurally and functionally intact hippocampus should be resected; hence, our study strictly focused on patients without structural abnormalities in the hippocampus.
It may be reasonable to perform hippocampal resection to achieve better seizure outcomes when a hippocampal abnormality is present. An extensive review by Englot et al. reported that additional hippocampal resection significantly increased the seizure-free rate in patients with temporal lobe LEATs [4]. Cataltepe et al. and Mintzer et al. also supported the suitability of additional hippocampal resection [17,18]. However, these studies did not provide an answer for the issue we addressed because they did not exclusively include patients with a normal hippocampus. A few studies have proposed the efficacy of additional hippocampal resection in patients with a normal hippocampus. In a study of 15 paralimbic glioma patients without hippocampal invasion, Ghareeb et al. reported that additional hippocampal resection resulted in seizure freedom in all patients [7]. Morioka et al. performed hippocampal resection in patients without hippocampal invasion if the intraoperative electrocorticogram detected epileptic discharges and found pathological degeneration, such as neuronal loss or dysplastic neurons, in the resected hippocampus. The authors concluded that pathological abnormalities in the hippocampus supported the necessity of hippocampal resection [19]. In some cases, reoperation to remove the mesial temporal structures was necessary to control seizures after lesionectomy. In such cases, the hippocampus can be pathologically normal [16]. However, the studies mentioned above were based on small case series.
The indication for hippocampal resection may be based on the tumor’s location. In our study, most patients with a tumor lateral to the collateral sulcus underwent lesionectomy alone. In contrast, half of patients with a tumor located medial to the collateral sulcus underwent hippocampal resection. Yu et al. investigated temporal lobe epilepsy (TLE) patients with a normal hippocampus and concluded that the distance between the tumor and hippocampus was not a factor responsible for achieving seizure freedom [20]. The hippocampus is not necessarily epileptogenic, even if the tumor is located close to it. However, 96.9% of our patients showed impaired awareness during seizures, which is a typical sign of mesial TLE [21]. Hence, the hippocampus may be involved in seizures.
Intraoperative ECoG is commonly used to decide whether hippocampal resection should be performed. Sugano et al. proposed additional hippocampal resection based on the ECoG results because frequent spikes were observed in the hippocampus after resecting the lateral temporal lesions [22]. However, Yu et al. found that the intraoperative spikes and high-frequency oscillations of the hippocampus were not associated with postoperative seizure outcomes [20]. These data imply that intraoperative ECoG is not always reliable when determining whether the hippocampus is truly epileptogenic or not. A decision based on the intraoperative ECoG results should be carefully considered as it may lead to unnecessary surgical intervention.
The preoperative cognitive function was lower in the Resected group than in the Preserved group. A mesial tumor can easily affect hippocampal function more than a lateral tumor; therefore, the difference in the preoperative cognitive function between both groups can be attributed to the fact that 71.4% of patients in the Resected group had a tumor in the mesial temporal area. In addition to the VI, each cognitive index improved after surgery in the Resected group, although the changes were not significant. The results may imply that resection of a normal hippocampus has a negative impact on verbal function after surgery. Yu et al. described a significant improvement in intelligence, facial recognition, and logical memory after lesionectomy alone, and other cognitive functions showed no significant decline after surgery in patients with a normal hippocampus [20]. Previous studies including a small number of cases reported deterioration of memory in patients following lesionectomy on the dominant side [22,23]. Generally, hippocampal resection can worsen language and memory functions, especially following surgery on the dominant side [24]. Vogt et al. reported that patients with LEAT who underwent additional hippocampal resection showed significantly lower verbal memory scores than those who underwent lesionectomy alone [6]. The authors also reported that complete resection of the hippocampus resulted in lower verbal memory scores than partial resection. Wagner et al. reported that resection of the left-sided parahippocampal gyrus caused the decline in verbal learning performance even if the hippocampus was preserved [24]. The parahippocampal gyrus, a structure closely connected to the hippocampus, was resected together with the hippocampus in our patients with tumors in the parahippocampal gyrus. We suggest that, to achieve better functional outcomes, additional hippocampal resection should be carefully considered in patients with a normal hippocampus. A less invasive technique, such as multiple hippocampal transections, may also be a treatment option for lesions in the hippocampus.
This study had few limitations. The small sample size limited the statistical power of the study. The verbal memory score is a more appropriate index when considering the impact of resecting a normal dominant-sided hippocampus on cognitive function, although we had to use the VI and WMI to evaluate verbal and memory functions because of the limited number of patients who underwent the Wechsler Memory Scale test. This study possibly introduced selection bias due to its retrospective nature. The study was not designed to select patients randomly for hippocampal resection indication. The tumor location and preoperative cognitive function may have preoperatively influenced the indication of hippocampal resection. However, this study is novel in that it only included patients with a normal hippocampus, and the results may provide new evidence for surgical strategies for patients with temporal LEATs with the normal hippocampus.
5. Conclusions
In patients with temporal lobe LEATs, additional hippocampal resection is not necessary because lesionectomy alone results in good seizure control and additional hippocampal resection can adversely affect the postoperative language function. The results may provide a clue for establishing an optimal surgical strategy in these patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci12101381/s1, Figure S1: The Kaplan–Meier curve shows the seizure-free survival of patients in the Resected and Preserved groups in the limited patients with the tumor located medial to the collateral sulcus.
Author Contributions
Conception and design: M.I. and Y.T. Acquisition of data: Y.T. Analysis and interpretation of data: M.I., Y.T., N.I., K.I., Y.K. (Yuiko Kimura), K.K., S.Y. and Y.K. (Yuu Kaneko). Drafting the article: Y.T. Critically revising the article: M.I., Y.T., N.I. and T.Y. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported, in part, by Grants-in-Aid for Scientific Research (KAKENHI) [grant number JP19K09494, JP22K09296] from the Japan Society for the Promotion of Science (JSPS), and Intramural Research Grant (28-4: Clinical Research for Diagnostic and Therapeutic Innovations in Developmental Disorders, 1-4: Integrative research on pathomechanism, diagnostic methodology and therapeutics for epilepsy) for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry.
Institutional Review Board Statement
The study was approved by the ethics committee of the National Center of Neurology and Psychiatry, Tokyo, Japan (No. A2018-049), and the requirement for written informed consent was waived because of the study’s retrospective design.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pelliccia, V.; Deleo, F.; Gozzo, F.; Sartori, I.; Mai, R.; Cossu, M.; Tassi, L. Early and Late Epilepsy Surgery in Focal Epilepsies Associated With Long-Term Epilepsy-Associated Tumors. J. Neurosurg. 2017, 127, 1147–1152. [Google Scholar] [CrossRef]
- Kim, N.R.; Wang, K.C.; Bang, J.S.; Choe, G.; Park, Y.; Kim, S.K.; Cho, B.K.; Chi, J.G. Glioblastomatous Transformation of Ganglioglioma: Case Report With Reference to Molecular Genetic and Flow Cytometric Analysis. Pathol. Int. 2003, 53, 874–882. [Google Scholar] [CrossRef]
- Luyken, C.; Blümcke, I.; Fimmers, R.; Urbach, H.; Elger, C.E.; Wiestler, O.D.; Schramm, J. The Spectrum of Long-Term Epilepsy-Associated Tumors: Long-Term Seizure and Tumor Outcome and Neurosurgical Aspects. Epilepsia 2003, 44, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Berger, M.S.; Barbaro, N.M.; Chang, E.F. Factors Associated With Seizure Freedom in the Surgical Resection of Glioneuronal Tumors. Epilepsia 2012, 53, 51–57. [Google Scholar] [CrossRef]
- Ravat, S.; Iyer, V.; Muzumdar, D.; Shah, U.; Pradhan, P.; Jain, N.; Godge, Y. Clinical Characteristics, Surgical and Neuropsychological Outcomes in Drug Resistant Tumoral Temporal Lobe Epilepsy. Int. J. Surg. 2016, 36, 436–442. [Google Scholar] [CrossRef]
- Vogt, V.L.; Witt, J.A.; Delev, D.; Grote, A.; von Lehe, M.; Becker, A.J.; Schramm, J.; Elger, C.E.; Helmstaedter, C. Cognitive Features and Surgical Outcome of Patients With Long-Term Epilepsy-Associated Tumors (LEATs) Within the Temporal Lobe. Epilepsy Behav. 2018, 88, 25–32. [Google Scholar] [CrossRef]
- Ghareeb, F.; Duffau, H. Intractable Epilepsy in Paralimbic Word Health Organization Grade II Gliomas: Should the Hippocampus Be Resected When Not Invaded by the Tumor? J. Neurosurg. 2012, 116, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Aronica, E.; Urbach, H.; Alexopoulos, A.; Gonzalez-Martinez, J.A. A Neuropathology-Based Approach to Epilepsy Surgery in Brain Tumors and Proposal for a New Terminology Use for Long-Term Epilepsy-Associated Brain Tumors. Acta Neuropathol. 2014, 128, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.G.; Blume, W.T.; Fish, D.; Goldensohn, E.; Hufnagel, A.; King, D.; Sperling, M.R.; Lüders, H.; Pedley, T.A. Commission on Neurosurgery of the International League Against Epilepsy (ILAE). ILAE Commission Report. Proposal for a New Classification of Outcome With Respect to Epileptic Seizures Following Epilepsy Surgery. Epilepsia 2001, 42, 282–286. [Google Scholar] [CrossRef]
- Suzuki, T. A Comparative Study of the Tanaka-Binet Intelligence Test and the Suzuki-Binet Intelligence Test. Jpn Assoc. Educ. Psychol. 1996, 38, 179. [Google Scholar] [CrossRef]
- Miyake, K.; Ohmura, M.; Takashima, M.; Yamanouchi, S.; Hashimoto, T.; Kobayashi, K. A New Test Developmental Screening Scale? Kinder Infant Development Scale? Hum. Dev. Res. 1990, 6, 147–163. [Google Scholar]
- Nakahara, J. Correlations of an Investigation Into the Development and the Level of Adaptability of Development With the Adaptability to Dental Treatment in Children Aged 3 Years. Jpn J. Pediatr. Dent. 2007, 45, 458–468. [Google Scholar] [CrossRef]
- Esteso Orduña, B.; de la Fournier del Castillo, M.C.; Cámara Barrio, S.; García Fernández, M.; Andrés Esteban, E.M.; Álvarez-Linera Prado, J.; Budke, M.; Maldonado Belmonte, M.J.; González Marqués, J.; Pérez Jiménez, M.Á. Cognitive and Behavioral Profiles of Pediatric Surgical Candidates With Frontal and Temporal Lobe Epilepsy. Epilepsy Behav. 2021, 117, 107808. [Google Scholar] [CrossRef]
- Ikegaya, N.; Iwasaki, M.; Kaneko, Y.; Kaido, T.; Kimura, Y.; Yamamoto, T.; Sumitomo, N.; Saito, T.; Nakagawa, E.; Sugai, K.; et al. Cognitive and Developmental Outcomes After Pediatric Insular Epilepsy Surgery for Focal Cortical Dysplasia. J. Neurosurg. Pediatr. 2020, 26, 543–551. [Google Scholar] [CrossRef]
- Fried, I.; Kim, J.H.; Spencer, D.D. Limbic and Neocortical Gliomas Associated With Intractable Seizures: A Distinct Clinicopathological Group. Neurosurgery. 1994, 34, 815–823. [Google Scholar] [CrossRef]
- Morris, H.H.; Matkovic, Z.; Estes, M.L.; Prayson, R.A.; Comair, Y.G.; Turnbull, J.; Najm, I.; Kotagal, P.; Wyllie, E. Ganglioglioma and Intractable Epilepsy: Clinical and Neurophysiologic Features and Predictors of Outcome After Surgery. Epilepsia. 1998, 39, 307–313. [Google Scholar] [CrossRef]
- Cataltepe, O.; Turanli, G.; Yalnizoglu, D.; Topçu, M.; Akalan, N. Surgical Management of Temporal Lobe Tumor-Related Epilepsy in Children. J. Neurosurg. 2005, 102 (Suppl. 3), 280–287. [Google Scholar] [CrossRef]
- Mintzer, S.; Sperling, M.R. When Should a Resection Sparing Mesial Structures Be Considered for Temporal Lobe Epilepsy? Epilepsy Behav. 2008, 13, 7–11. [Google Scholar] [CrossRef]
- Morioka, T.; Hashiguchi, K.; Nagata, S.; Miyagi, Y.; Yoshida, F.; Shono, T.; Mihara, F.; Koga, H.; Sasaki, T. Additional Hippocampectomy in the Surgical Management of Intractable Temporal Lobe Epilepsy Associated With Glioneuronal Tumor. Neurol. Res. 2007, 29, 807–815. [Google Scholar] [CrossRef]
- Yu, H.Y.; Lin, C.F.; Chou, C.C.; Lu, Y.J.; Hsu, S.P.C.; Lee, C.C.; Chen, C. Outcomes of Hippocampus-Sparing Lesionectomy for Temporal Lobe Epilepsy and the Significance of Intraoperative Hippocampography. Clin. Neurophysiol. 2021, 132, 746–755. [Google Scholar] [CrossRef]
- Varotto, G.; Burini, A.; Didato, G.; Deleo, F.; Pastori, C.; Dominese, A.; Tringali, G.; Panzica, F.; de Curtis, M.; Di Giacomo, R. Impaired Awareness in Mesial Temporal Lobe Epilepsy: Network Analysis of Foramen Ovale and Scalp EEG. Clin. Neurophysiol. 2021, 132, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Sugano, H.; Shimizu, H.; Sunaga, S. Efficacy of Intraoperative Electrocorticography for Assessing Seizure Outcomes in Intractable Epilepsy Patients With Temporal-Lobe-Mass Lesions. Seizure 2007, 16, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kashida, Y.; Usui, N.; Matsuda, K.; Terada, K.; Baba, K.; Kondo, A.; Hirozawa, D.; Tottori, T.; Mihara, T.; Hanaya, R.; et al. Is Additional Mesial Temporal Resection Necessary for Intractable Epilepsy With Cavernous Malformations in the Temporal Neocortex? Epilepsy Behav. 2019, 92, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Uherek, M.; Horstmann, S.; Kadish, N.E.; Wisniewski, I.; Mayer, H.; Buschmann, F.; Metternich, B.; Zentner, J.; Schulze-Bonhage, A. Memory Outcome After Hippocampus Sparing Resections in the Temporal Lobe. J. Neurol. Neurosurg. Psychiatry. 2013, 84, 630–636. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).