Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Data Collection and Assessment
2.3. Statistical Analysis
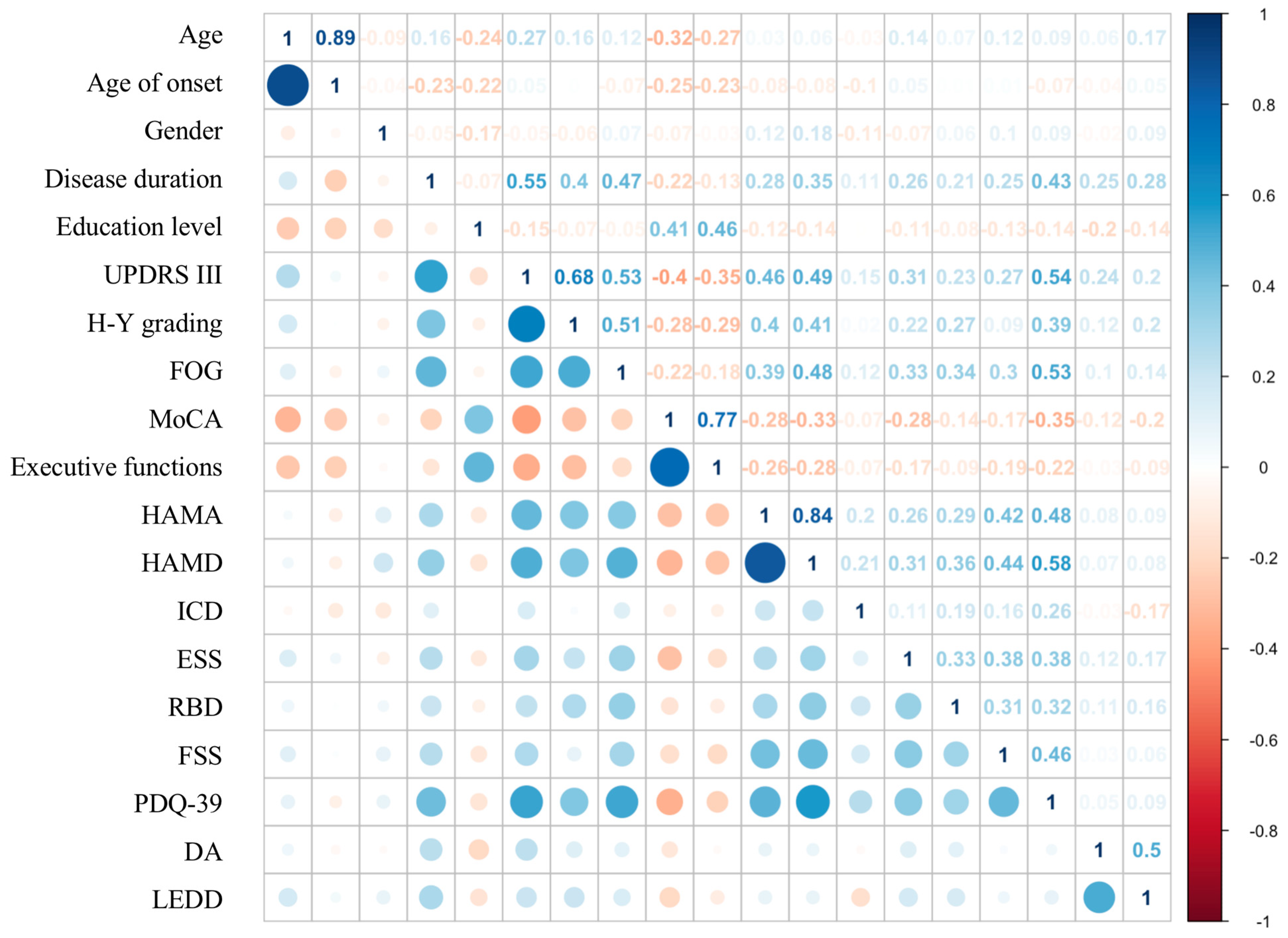
3. Results
3.1. Univariate Analysis of Factors Associated with Apathy in PD
3.2. Multifactorial Analysis of Parkinson’s Disease Apathy
4. Discussions
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lill, C.M.; Klein, C. Epidemiology and causes of Parkinson’s disease. Nervenarzt 2017, 88, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Hommel, A.; Meinders, M.J.; Lorenzl, S.; Dodel, R.; Coelho, M.; Ferreira, J.J.; Laurens, B.; Spampinato, U.; Meissner, W.; Rosqvist, K.; et al. Care of Late-Stage Parkinsonism C. The Prevalence and Determinants of Neuropsychiatric Symptoms in Late-Stage Parkinsonism. Mov. Disord. Clin. Pract. 2020, 7, 531–542. [Google Scholar] [CrossRef]
- D’Iorio, A.; Vitale, C.; Piscopo, F.; Baiano, C.; Falanga, A.P.; Longo, K.; Amboni, M.; Barone, P.; Santangelo, G. Impact of anxiety, apathy and reduced functional autonomy on perceived quality of life in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 43, 114–117. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Kulisevsky, J.; Strafella, A.P.; Krack, P. Apathy in Parkinson’s disease: Clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015, 14, 518–531. [Google Scholar] [CrossRef]
- Cubo, E.; Benito-Leon, J.; Coronell, C.; Armesto, D.; Group, A.S. Clinical correlates of apathy in patients recently diagnosed with Parkinson’s disease: The ANIMO study. Neuroepidemiology 2012, 38, 48–55. [Google Scholar] [CrossRef]
- Gorzkowska, A.; Cholewa, J.; Cholewa, J.; Wilk, A.; Klimkowicz-Mrowiec, A. Risk Factors for Apathy in Polish Patients with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 10196. [Google Scholar] [CrossRef]
- Brown, D.S.; Barrett, M.J.; Flanigan, J.L.; Sperling, S.A. Clinical and demographic correlates of apathy in Parkinson’s disease. J. Neurol. 2019, 266, 507–514. [Google Scholar] [CrossRef]
- Meyer, A.; Zimmermann, R.; Gschwandtner, U.; Hatz, F.; Bousleiman, H.; Schwarz, N.; Fuhr, P. Apathy in Parkinson’s disease is related to executive function, gender and age but not to depression. Front. Aging Neurosci. 2014, 6, 350. [Google Scholar] [CrossRef]
- Skorvanek, M.; Rosenberger, J.; Gdovinova, Z.; Nagyova, I.; Saeedian, R.G.; Groothoff, J.W.; Dijk, J.P. Apathy in elderly nondemented patients with Parkinson’s disease: Clinical determinants and relationship to quality of life. J. Geriatr. Psychiatry Neurol. 2013, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.V.; Eglit, G.M.L.; Schiehser, D.M.; Pirogovsky-Turk, E.; Litvan, I.; Lessig, S.; Filoteo, J.V. Factor Analysis of the Apathy Scale in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2019, 6, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Aho, K.; Derryberry, D.; Peterson, T. Model selection for ecologists: The worldviews of AIC and BIC. Ecology 2014, 95, 631–636. [Google Scholar] [CrossRef]
- Mele, B.; Van, S.; Holroyd-Leduc, J.; Ismail, Z.; Pringsheim, T.; Goodarzi, Z. Diagnosis, treatment and management of apathy in Parkinson’s disease: A scoping review. BMJ Open 2020, 10, e037632. [Google Scholar] [CrossRef]
- Mele, B.; Goodarzi, Z.; Hanson, H.M.; Holroyd-Leduc, J. Barriers and facilitators to diagnosing and managing apathy in Parkinson’s disease: A qualitative study. BMC Neurol. 2019, 19, 101. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Cubo, E.; Coronell, C.; Group, A.S. Impact of apathy on health-related quality of life in recently diagnosed Parkinson’s disease: The ANIMO study. Mov. Disord. 2012, 27, 211–218. [Google Scholar] [CrossRef]
- Toyama, M.; Okuma, Y.; Yamamoto, M.; Kashihara, K.; Yoshida, K.; Saiki, H.; Maeda, T.; Tsuboi, Y.; Takahashi, Y.; Nakayama, T. Non-motor symptoms depending on motor severity in Japanese patients with Parkinson’s disease: A multicenter cross-sectional study. J. Neurol. Sci. 2020, 412, 116641. [Google Scholar] [CrossRef]
- Terashi, H.; Ueta, Y.; Kato, H.; Mitoma, H.; Aizawa, H. Characteristics of apathy in treatment-naïve patients with Parkinson’s disease. Int. J. Neurosci. 2019, 129, 16–21. [Google Scholar] [CrossRef]
- Chao, J.Y.; Xiong, K.P.; Zhuang, S.; Zhang, J.R.; Huang, J.Y.; Li, J.; Mao, C.J.; Wu, H.H.; Wang, J.Y.; Liu, C.F. Relationship between emotional apathy and motor symptoms, sleep and cognitive function in patients with early Parkinson’s disease. Chin. J. Stomatol. 2021, 101, 2792–2797. [Google Scholar] [CrossRef]
- Costa, A.; Peppe, A.; Zabberoni, S.; Scalici, F.; Caltagirone, C.; Carlesimo, G.A. Apathy in individuals with Parkinson’s disease associated with mild cognitive impairment. A neuropsychological investigation. Neuropsychologia 2018, 118, 4–11. [Google Scholar] [CrossRef]
- Martin, G.P.; McDonald, K.R.; Allsop, D.; Diggle, P.J.; Leroi, I. Apathy as a behavioural marker of cognitive impairment in Parkinson’s disease: A longitudinal analysis. J. Neurol. 2020, 267, 214–227. [Google Scholar] [CrossRef] [PubMed]
- D’Iorio, A.; Maggi, G.; Vitale, C.; Trojano, L.; Santangelo, G. "Pure apathy" and cognitive dysfunctions in Parkinson’s disease: A meta-analytic study. Neurosci. Biobehav. Rev. 2018, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Trojano, L.; Barone, P.; Errico, D.; Grossi, D.; Vitale, C. Apathy in Parkinson’s disease: Diagnosis, neuropsychological correlates, pathophysiology and treatment. Behav. Neurol. 2013, 27, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Zgaljardic, D.J.; Borod, J.C.; Foldi, N.S.; Rocco, M.; Mattis, P.J.; Gordon, M.F.; Feigin, A.S.; Eidelberg, D. Relationship between self-reported apathy and executive dysfunction in nondemented patients with Parkinson disease. Cogn. Behav. Neurol. 2007, 20, 184–192. [Google Scholar] [CrossRef]
- Gratwicke, J.; Jahanshahi, M.; Foltynie, T. Parkinson’s disease dementia: A neural networks perspective. Brain 2015, 138, 1454–1476. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Kulisevsky, J. Apathy in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 657–678. [Google Scholar] [CrossRef]
- le Heron, C.; Apps, M.A.J.; Husain, M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia 2018, 118, 54–67. [Google Scholar] [CrossRef]
- Thobois, S.; Prange, S.; Sgambato-Faure, V.; Tremblay, L.; Broussolle, E. Imaging the Etiology of Apathy, Anxiety, and Depression in Parkinson’s Disease: Implication for Treatment. Curr. Neurol. Neurosci. Rep. 2017, 17, 76. [Google Scholar] [CrossRef]
- le Heron, C.; Plant, O.; Manohar, S.; Ang, Y.S.; Jackson, M.; Lennox, G.; Hu, M.T.; Husain, M. Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain 2018, 141, 1455–1469. [Google Scholar] [CrossRef]
- den Brok, M.G.; van Dalen, J.W.; van Gool, W.A.; van Charante, E.P.M.; de Bie, R.M.; Richard, E. Apathy in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2015, 30, 759–769. [Google Scholar] [CrossRef]
- Kirsch-Darrow, L.; Fernandez, H.H.; Marsiske, M.; Okun, M.S.; Bowers, D. Dissociating apathy and depression in Parkinson disease. Neurology 2006, 67, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Darrow, L.; Marsiske, M.; Okun, M.S.; Bauer, R.; Bowers, D. Apathy and depression: Separate factors in Parkinson’s disease. J. Int. Neuropsychol. Soc. 2011, 17, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.C.; Chan, L.L.; Tan, L.C.; Tan, E.K. Depression, anxiety, and apathy in Parkinson’s disease: Insights from neuroimaging studies. Eur. J. Neurol. 2016, 23, 1001–1019. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; le Bars, D.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 2016, 139, 2486–2502. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H.; Inoue, Y.; Sakurai, H.; Kanetaka, H.; Nakamura, M.; Miyamoto, T.; Sasai, T.; Iwamoto, T. Regional cerebral blood flow changes in patients with idiopathic REM sleep behavior disorder. Eur. J. Neurol. 2011, 18, 784–788. [Google Scholar] [CrossRef]
- Bargiotas, P.; Ntafouli, M.; Lachenmayer, M.L.; Krack, P.; Schupbach, W.M.M.; Bassetti, C.L.A. Apathy in Parkinson’s disease with REM sleep behavior disorder. J. Neurol. Sci. 2019, 399, 194–198. [Google Scholar] [CrossRef]
- Mahmood, Z.; van Patten, R.; Nakhla, M.Z.; Twamley, E.W.; Filoteo, J.V.; Schiehser, D.M. REM Sleep Behavior Disorder in Parkinson’s Disease: Effects on Cognitive, Psychiatric, and Functional outcomes. J. Int. Neuropsychol. Soc. 2020, 26, 894–905. [Google Scholar] [CrossRef]
- Schulte, E.C.; Winkelmann, J. When Parkinson’s disease patients go to sleep: Specific sleep disturbances related to Parkinson’s disease. J. Neurol. 2011, 258, S328–S335. [Google Scholar] [CrossRef]
- Tessitore, A.; Giordano, A.; de Micco, R.; Caiazzo, G.; Russo, A.; Cirillo, M.; Esposito, F.; Tedeschi, G. Functional connectivity underpinnings of fatigue in "Drug-Naive" patients with Parkinson’s disease. Mov. Disord. 2016, 31, 1497–1505. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; de Micco, R.; Giordano, A.; Russo, A.; Tedeschi, G.; Chiorri, C.; Tessitore, A. Predictors of fatigue severity in early, de novo Parkinson disease patients: A 1-year longitudinal study. Parkinsonism Relat. Disord. 2020, 79, 3–8. [Google Scholar] [CrossRef]
- Saez-Francas, N.; Hernandez-Vara, J.; Roso, M.C.; Martin, J.A.; Brugue, M.C. The association of apathy with central fatigue perception in patients with Parkinson’s disease. Behav. Neurosci. 2013, 127, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Carnicella, S.; Strafella, A.P.; Bichon, A.; Lhommee, E.; Castrioto, A.; Chabardes, S.; Thobois, S.; Krack, P. Apathy and Impulse Control Disorders: Yin & Yang of Dopamine Dependent Behaviors. J. Parkinsons Dis. 2015, 5, 625–636. [Google Scholar] [CrossRef]
- Scott, B.M.; Eisinger, R.S.; Burns, M.R.; Lopes, J.; Okun, M.S.; Gunduz, A.; Bowers, D. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology 2020, 95, e2769–e2780. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Francàs, N.; Ramirez, N.; Alegre-Martin, J.; Fabregues, O.D.; Alvarez-Sabin, J.; Hernandez-Vara, J. Apathy and impulse control disorders association: A study in a sample of Parkinson’s disease patients. Eur. Psychiatry 2016, 33, S396. [Google Scholar] [CrossRef]
- Eisinger, R.S.; Ramirez-Zamora, A.; Carbunaru, S.; Ptak, B.; Peng-Chen, Z.; Okun, M.S.; Gunduz, A. Medications, Deep Brain Stimulation, and Other Factors Influencing Impulse Control Disorders in Parkinson’s Disease. Front. Neurol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Petitet, P.; Scholl, J.; Attaallah, B.; Drew, D.; Manohar, S.; Husain, M. The relationship between apathy and impulsivity in large population samples. Sci. Rep. 2021, 11, 4830. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Brockman, S. Apathy and Parkinson’s disease. Curr Treat Options Neurol 2011, 13, 267–273. [Google Scholar] [CrossRef]
- Sokoloff, P.; Diaz, J.; le Foll, B.; Guillin, O.; Leriche, L.; Bezard, E.; Gross, C. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol. Disord. Drug Targets 2006, 5, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, K.; Odin, P.; Hagell, P.; Iwarsson, S.; Nilsson, M.H.; Storch, A. Dopaminergic Effect on Non-Motor Symptoms in Late Stage Parkinson’s Disease. J. Parkinsons Dis. 2018, 8, 409–420. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, J.J.; Ham, J.H.; Lee, P.H.; Sohn, Y.H. Apathy and striatal dopamine defects in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 23, 62–65. [Google Scholar] [CrossRef]

| Apathetic Group (n = 138) | Nonapathetic Group (n = 62) | ||
|---|---|---|---|
| Demographics Characteristics | |||
| Gender | 0.6770 | ||
| Male | 63 (45.65%) | 31 (50%) | |
| Female | 75 (54.35%) | 31 (50%) | |
| Age (years) | 65.00 (58.00, 69.00) | 62.50 (53.50, 69.75) | 0.0857 |
| Age of onset (years) | 58.00 (53.00, 65.00) | 58.00 (49.25, 65.75) | 0.2586 |
| Education level (years) | 9.00 (7.25, 12.00) | 12.00 (9.00, 15.00) | <0.001 |
| Disease duration (years) | 5.00 (3.00, 7.00) | 4.00 (2.00, 7.00) | 0.0143 |
| Motor Symptoms | |||
| UPDRS III | 47.00 (33.25, 64.00) | 32.00 (22.25, 54.00) | <0.001 |
| H-Y grading | 2.00 (1.50, 3.00) | 2.00 (1.00, 2.00) | 0.0038 |
| Freezing of gait | 7.00 (2.00, 13.00) | 3.00 (1.00, 8.50) | 0.0063 |
| Non-motor Symptoms | |||
| MoCA | 22.00 (17.25, 24.00) | 24.00 (22.00, 26.00) | <0.001 |
| HAMD | 15.00 (9.00, 23.00) | 9.00 (5.00, 14.25) | <0.001 |
| HAMA | 11.00 (6.00, 17.00) | 8.00 (4.75, 12.25) | 0.0037 |
| ESS | 5.50 (1.00, 9.00) | 2.00 (1.00, 7.00) | 0.0042 |
| RBD | 3.00 (1.00, 7.00) | 1.00 (0.00, 4.00) | 0.0114 |
| FSS | 40.00 (20.50, 54.00) | 22.00 (11.25, 40.75) | < 0.001 |
| Executive functions | 5.00 (3.25, 6.00) | 6.00 (4.25, 7.00) | 0.0007 |
| ICD | 0.0638 | ||
| Yes | 20 (14.49%) | 4 (6.45%) | |
| No | 118 (85.51%) | 58 (93.55%) | |
| PDQ-39 | 44.50 (26.25, 61.00) | 20.00 (8.00, 33.75) | <0.001 |
| Medication Taking | |||
| LEDD | 400.00 (281.20, 593.80) | 300.00 (0.00, 534.4) | 0.3420 |
| Dopamine receptor agonists | 0.00 (0.00, 75.00) | 0.00 (0.00, 75.00) | 0.0098 |
| Variables | IV |
|---|---|
| Disease duration | 0.7475 |
| MoCA | 0.7375 |
| Age | 0.6570 |
| HAMD | 0.6068 |
| Age of onset | 0.5604 |
| Freezing of gait | 0.4932 |
| PDQ-39 | 0.4926 |
| UPDRSIII | 0.4116 |
| FSS | 0.4049 |
| HAMA | 0.3611 |
| Education level | 0.3501 |
| LEDD | 0.3443 |
| ESS | 0.2844 |
| RBD | 0.2542 |
| H-Y grading | 0.2001 |
| ICD | 0.1309 |
| Dopamine receptor agonists | 0.1016 |
| Gender | 0.0076 |
| Executive functions | 0.0056 |
| Coefficients | SD | Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| (Intercept) | 0.7475 | 0.1631 | <0.001 | 2.1118 | (1.5341, 2.9069) |
| Education level | −0.0153 | 0.0056 | 0.0065 | 0.9848 | (0.9741, 0.9956) |
| MoCA | −0.0091 | 0.0061 | 0.1397 | 0.9909 | (0.9791, 1.0029) |
| PDQ-39 | 0.0057 | 0.0012 | <0.0001 | 1.0057 | (1.0033, 1.0080) |
| Dopamine receptor agonists | −0.0012 | 0.0006 | 0.0276 | 0.9987 | (0.9976, 0.9999) |
| LEDD | 0.0003 | 0.0001 | 0.0058 | 1.0003 | (1.0001, 1.0005) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, R.; Qi, Y.; He, J.; Zheng, X.; Ren, W.; Chang, Y. Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease. Brain Sci. 2022, 12, 1343. https://doi.org/10.3390/brainsci12101343
Luo R, Qi Y, He J, Zheng X, Ren W, Chang Y. Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease. Brain Sciences. 2022; 12(10):1343. https://doi.org/10.3390/brainsci12101343
Chicago/Turabian StyleLuo, Ruirui, Yumeng Qi, Jiuqin He, Xiaoqi Zheng, Wenhua Ren, and Ying Chang. 2022. "Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease" Brain Sciences 12, no. 10: 1343. https://doi.org/10.3390/brainsci12101343
APA StyleLuo, R., Qi, Y., He, J., Zheng, X., Ren, W., & Chang, Y. (2022). Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease. Brain Sciences, 12(10), 1343. https://doi.org/10.3390/brainsci12101343







