The ALFF Alterations of Spontaneous Pelvic Pain in the Patients of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Evaluated by fMRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Participants
2.2. The Adapted Clinical Scales
2.3. Resting-State fMRI Data Acquisition
2.4. Data Preprocessing Analysis
2.5. Analysis of ALFF and fALFF
2.6. Statistics Analysis
3. Results
3.1. Features of Observational Cohorts
3.2. The Abnormal Activated Brain Regions by mALFF Analysis
3.3. The Abnormal Activated Brain Regions by mfALFF Analysis
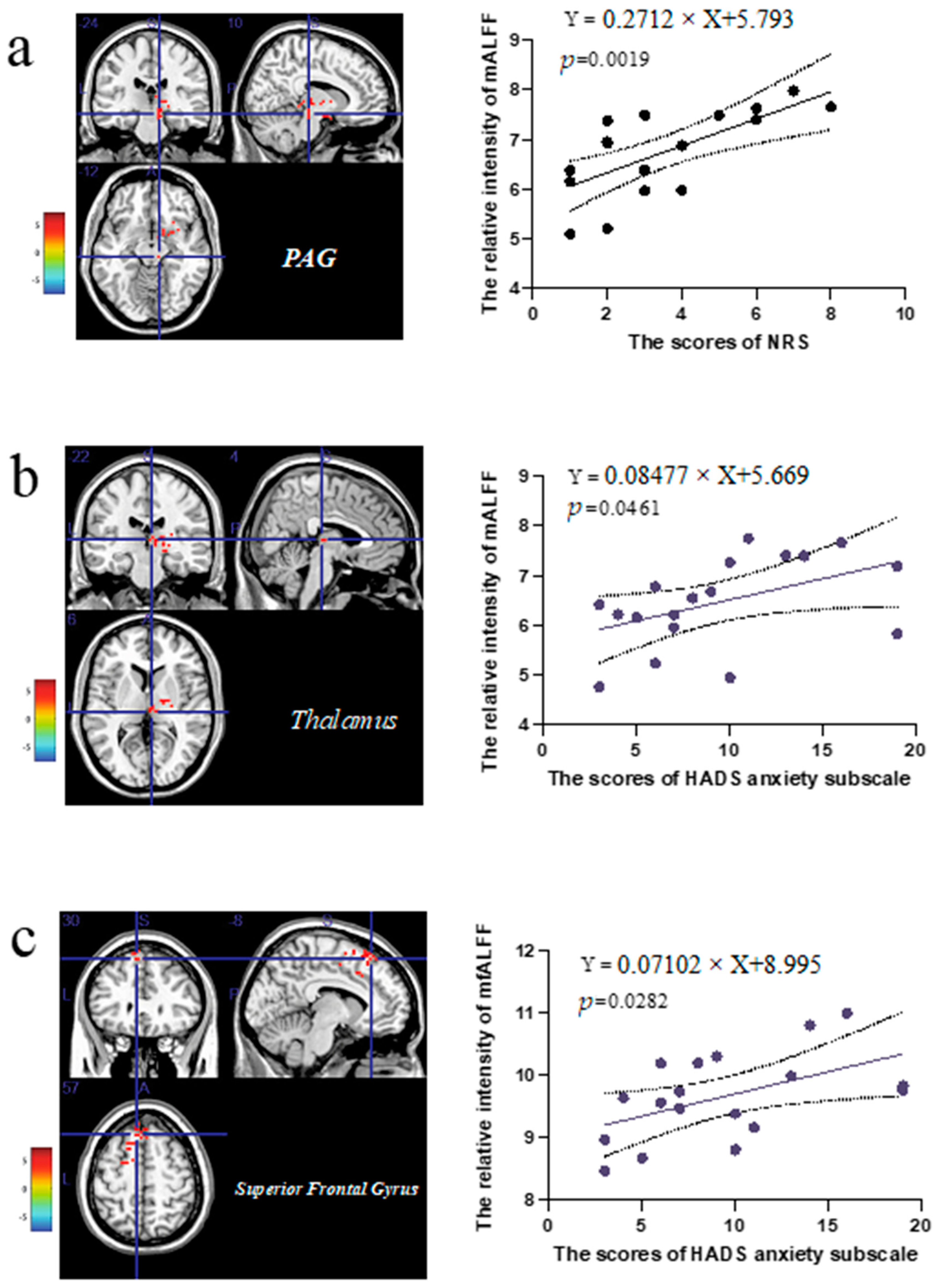
3.4. The Correlation Analysis of the Extracted Values in the Abnormal Brain Regions and Clinical Scale Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Zhang, L.; Shen, Y.; Yao, H.; Yong, S.; You, Y. Effectiveness of psychological interventions for treating chronic prostatitis/chronic pelvic pain syndrome: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e22151. [Google Scholar] [CrossRef]
- Krieger, J.N.; Nyberg, L., Jr.; Nickel, J.C. NIH consensus definition and classification of prostatitis. JAMA 1999, 282, 236–237. [Google Scholar] [CrossRef]
- Šutulović, N.; Grubač, Ž.; Šuvakov, S.; Jerotić, D.; Puškaš, N.; Macut, D.; Rašić-Marković, A.; Simić, T.; Stanojlović, O.; Hrnčić, D. Experimental Chronic Prostatitis/Chronic Pelvic Pain Syndrome Increases Anxiety-Like Behavior: The Role of Brain Oxidative Stress, Serum Corticosterone, and Hippocampal Parvalbumin-Positive Interneurons. Oxid. Med. Cell Longev. 2021, 2021, 6687493. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, K.; Zhou, M.; Xia, K.; Xu, Y.; Sun, X.; Zhu, Y.; Cui, C.; Deng, C. Influence of Experimental Autoimmune Prostatitis on Sexual Function and the Anti-inflammatory Efficacy of Celecoxib in a Rat Model. Front. Immunol. 2020, 11, 574212. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Keslar, K.S.; Gotwald, P.; Berglund, R.; Vij, S. Neuroinflammatory gene expression in chronic prostatitis/chronic pelvic pain syndrome patients: Insights into etiology and phenotype biology. Transl. Androl. Urol. 2021, 10, 3340–3347. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, C.; Kong, X.; Meng, J.; Fan, S.; Ding, Y.; Fang, Q.; Dong, T.; Zhang, H.; Ni, J.; et al. Identification of novel susceptibility factors related to CP/CPPS-like symptoms: Evidence from a multicenter case-control study. Prostate 2022, 82, 772–782. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.G.; Du, H.X.; Zhan, C.S.; Liu, Y.; Zhang, M.; Chen, X.G.; Wen, L.P.; Zhang, L.; Liang, C.Z. Melatonin attenuates prostatic inflammation and pelvic pain via Sirt1-dependent inhibition of the NLRP3 inflammasome in an EAP mouse model. Prostate 2021, 81, 1179–1190. [Google Scholar] [CrossRef]
- Huang, X.; Qin, Z.; Cui, H.; Chen, J.; Liu, T.; Zhu, Y.; Yuan, S. Psychological factors and pain catastrophizing in men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): A meta-analysis. Transl. Androl. Urol. 2020, 9, 485–493. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Liang, C. Chronic Prostatitis and Pelvic Pain Syndrome: Another Autoimmune Disease? Arch. Immunol. Ther. Exp. 2021, 69, 24. [Google Scholar] [CrossRef]
- Franco, J.V.; Turk, T.; Jung, J.H.; Xiao, Y.T.; Iakhno, S.; Tirapegui, F.I.; Garrote, V.; Vietto, V. Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst. Rev. 2019, 10, CD012552. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, K.; Xu, C.; Liu, S.; Sun, Q.; Yang, Z.; Dai, X.; Li, N. Mechanism of Acupuncture and Moxibustion on Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Narrative Review of Animal Studies. Pain Res. Manag. 2021, 2021, 2678242. [Google Scholar] [CrossRef]
- Arora, H.C.; Eng, C.; Shoskes, D.A. Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Ann. Transl. Med. 2017, 5, 30. [Google Scholar] [CrossRef]
- Passavanti, M.B.; Pota, V.; Sansone, P.; Aurilio, C.; De Nardis, L.; Pace, M.C. Chronic Pelvic Pain: Assessment, Evaluation, and Objectivation. Pain Res. Treat. 2017, 2017, 9472925. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tétreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef]
- Gary, Z.Y.; Ly, M.; Karim, H.T.; Muppidi, N.; Aizenstein, H.J.; Ibinson, J.W. Accelerated brain aging in chronic low back pain. Brain Res. 2021, 1755, 147263. [Google Scholar]
- Tu, Y.; Cao, J.; Bi, Y.; Hu, L. Magnetic resonance imaging for chronic pain: Diagnosis, manipulation, and biomarkers. Sci. China Life Sci. 2021, 64, 879–896. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, Y.; Liu, P.; Yang, X.; Qin, W.; Gu, J.; Ding, D.; Tian, J.; Wang, M. Alterations in regional homogeneity of resting-state cerebral activity in patients with chronic prostatitis/chronic pelvic pain syndrome. PLoS ONE 2017, 12, e0184896. [Google Scholar] [CrossRef]
- Korkmaz, S.; Karadag, M.A.; Hamamcioglu, K.; Sofikerim, M.; Aksu, M. Electrophysiological Identification of Central Sensitization in Patients with Chronic Prostatitis. Urol. J. 2015, 12, 2280–2284. [Google Scholar]
- Yang, H.; Long, X.Y.; Yang, Y.; Yan, H.; Zhu, C.Z.; Zhou, X.P.; Zang, Y.F.; Gong, Q.Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 2007, 36, 144–152. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Harrison, T.M.; Maass, A.; Adams, J.N.; Du, R.; Baker, S.L.; Jagust, W.J. Tau deposition is associated with functional isolation of the hippocampus in aging. Nat. Commun. 2019, 10, 4900. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, L.; Wang, Q.; Hu, L.; Ding, Q.; Jia, X.; Yang, X. Altered Amplitude of Low-Frequency Fluctuations in Inactive Patients with Nonneuropsychiatric Systemic Lupus Erythematosus. Neural Plast. 2019, 2019, 9408612. [Google Scholar] [CrossRef]
- Wang, B.; Niu, Y.; Miao, L.; Cao, R.; Yan, P.; Guo, H.; Li, D.; Guo, Y.; Yan, T.; Wu, J.; et al. Decreased Complexity in Alzheimer’s Disease: Resting-State fMRI Evidence of Brain Entropy Mapping. Front. Aging Neurosci. 2017, 9, 378. [Google Scholar] [CrossRef]
- Ge, S.; Hu, Q.; Guo, Y.; Xu, K.; Xia, G.; Sun, C. Potential Alterations of Functional Connectivity Analysis in the Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Neural Plast. 2021, 2021, 6690414. [Google Scholar] [CrossRef]
- Hayasaka, S.; Peiffer, A.M.; Hugenschmidt, C.E.; Laurienti, P.J. Power and sample size calculation for neuroimaging studies by non-central random field theory. Neuroimage 2007, 37, 721–730. [Google Scholar] [CrossRef]
- Litwin, M.S.; McNaughton-Collins, M.; Fowler, F.J., Jr.; Nickel, J.C.; Calhoun, E.A.; Pontari, M.A.; Alexander, R.B.; Farrar, J.T.; O’Leary, M.P. The National Institutes of Health chronic prostatitis symptom index: Development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J. Urol. 1999, 162, 369–375. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; van Till, J.O.; Magri, V.; Perletti, G.; Houbiers, J.G.; Weidner, W.; Nickel, J.C. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur. Urol. 2013, 63, 953–959. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), S240–S252. [Google Scholar]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef]
- Doiron, R.C.; Shoskes, D.A.; Nickel, J.C. Male CP/CPPS: Where do we stand? World J. Urol. 2019, 37, 1015–1022. [Google Scholar] [CrossRef]
- Šutulović, N.; Grubač, Ž.; Šuvakov, S.; Jovanović, Đ.; Puškaš, N.; Macut, Đ.; Marković, A.R.; Simić, T.; Stanojlović, O.; Hrnčić, D. Chronic prostatitis/chronic pelvic pain syndrome increases susceptibility to seizures in rats and alters brain levels of IL-1beta and IL-6. Epilepsy Res. 2019, 153, 19–27. [Google Scholar] [CrossRef]
- Domínguez Vivero, C.; Leira, Y.; Saavedra Piñeiro, M.; Rodríguez-Osorio, X.; Ramos-Cabrer, P.; Villalba Martín, C.; Sobrino, T.; Campos, F.; Castillo, J.; Leira, R. Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine. Toxins 2020, 12, 479. [Google Scholar] [CrossRef]
- Mokhtar, M.; Singh, P. Neuroanatomy, Periaqueductal Gray; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Yu, R.; Gollub, R.L.; Spaeth, R.; Napadow, V.; Wasan, A.; Kong, J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014, 6, 100–108. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Mao, C.; Zhang, P.; Zhang, Q.; Jiang, L.; Yang, Y.; Ma, J.; Ye, L.; Lee, K.O.; et al. Increased thalamo-cortical functional connectivity in patients with diabetic painful neuropathy: A resting-state functional MRI study. Exp. Ther. Med. 2021, 21, 509. [Google Scholar] [CrossRef]
- Alshelh, Z.; Di Pietro, F.; Youssef, A.M.; Reeves, J.M.; Macey, P.M.; Vickers, E.R.; Peck, C.C.; Murray, G.M.; Henderson, L.A. Chronic Neuropathic Pain: It’s about the Rhythm. J. Neurosci. 2016, 36, 1008–1018. [Google Scholar] [CrossRef]
- Linley, S.B.; Athanason, A.C.; Rojas, A.K.P.; Vertes, R.P. Role of the reuniens and rhomboid thalamic nuclei in anxiety-like avoidance behavior in the rat. Hippocampus 2021, 31, 756–769. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, H.; Wu, Z.; He, Q.; Liu, J.; Xu, Y.; Yao, S.; He, X.; Chen, Y.; Liang, Y. Electroacupuncture Alleviates Chronic Pain-Induced Anxiety Disorders by Regulating the rACC-Thalamus Circuitry. Front. Neurosci. 2020, 14, 615395. [Google Scholar] [CrossRef]
- Ong, W.Y.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Li, B.; Su, S. Effects of Acupuncture at Neiguan in Neural Activity of Related Brain Regions: A Resting-State fMRI Study in Anxiety. Neuropsychiatr. Dis. Treat. 2022, 18, 1375–1384. [Google Scholar] [CrossRef]
- Azqueta-Gavaldon, M.; Youssef, A.M.; Storz, C.; Lemme, J.; Schulte-Göcking, H.; Becerra, L.; Azad, S.C.; Reiners, A.; Ertl-Wagner, B.; Borsook, D.; et al. Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain 2020, 161, 595–608. [Google Scholar] [CrossRef]
- Chen, C.; Yan, M.; Yu, Y.; Ke, J.; Xu, C.; Guo, X.; Lu, H.; Wang, X.; Hu, L.; Wang, J.; et al. Alterations in Regional Homogeneity Assessed by fMRI in Patients with Migraine Without Aura. J. Med. Syst. 2019, 43, 298. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, J.; Xia, S.H.; Gutstein, H.B.; Huang, Y.H.; Schlüter, O.M.; Cao, J.L.; Dong, Y. Neuropathic pain generates silent synapses in thalamic projection to anterior cingulate cortex. Pain 2021, 162, 1322–1333. [Google Scholar] [CrossRef]
- Neshatian, L.; Karmonik, C.; Khavari, R.; Shi, Z.; Elias, S.; Boone, T.; Quigley, E.M. Alterations in brain activation patterns in women with functional defecatory disorder: A novel fMRI rectal balloon expulsion study. Neurogastroenterol. Motil. 2022, e14389. [Google Scholar] [CrossRef]
- Constantinidis, C.; Klingberg, T. The neuroscience of working memory capacity and training. Nat. Rev. Neurosci. 2016, 17, 438–449. [Google Scholar] [CrossRef]
- Sun, N.; Li, Y.; Zhang, A.; Yang, C.; Liu, P.; Liu, Z.; Wang, Y.; Jin, R.; Zhang, K. Fractional amplitude of low-frequency fluctuations and gray matter volume alterations in patients with bipolar depression. Neurosci. Lett. 2020, 730, 135030. [Google Scholar] [CrossRef]
- Fu, X.; Li, H.; Yan, M.; Chen, J.; Liu, F.; Zhao, J.; Guo, W. Shared and Distinct Fractional Amplitude of Low-Frequency Fluctuation Patterns in Major Depressive Disorders with and Without Gastrointestinal Symptoms. Front. Psychiatry 2021, 12, 744898. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Zhang, L.; Wang, X.; Wang, X.; Cheng, B.; Luo, K.; Gong, Q. Stress and the brain: Perceived stress mediates the impact of the superior frontal gyrus spontaneous activity on depressive symptoms in late adolescence. Hum. Brain Mapp. 2019, 40, 4982–4993. [Google Scholar] [CrossRef]


| Patients of Chronic Prostatitis/Chronic Pelvic Pain (n = 18) | Healthy Control (n = 20) | p Value | |
|---|---|---|---|
| Gender | Male | Male | - |
| Age/years | 34.28± 8.61 | 39.67 ± 13.32 | 0.1497 |
| Duration of spontaneous pelvic pain /months | 19.56 ± 5.38 | 0 | <0.001 |
| Total National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) score (item1 + 2 + 3 + 4 + 5 + 6 + 7 + 8 + 9) | 27.78 ± 11.60 | 0 | <0.001 |
| Pain and discomfort (1 + 2 + 3 + 4) | 13.27 ± 8.29 | 0 | <0.001 |
| NRS (4) | 3.50 ± 2.35 | 0 | <0.001 |
| Lower urinary tract symptoms (5 + 6) | 6.61 ± 3.05 | 0 | <0.001 |
| Impact on quality of life (7 + 8 + 9) | 8.44 ± 3.58 | 0 | <0.001 |
| Severity of symptoms (1 + 2 + 3 + 4 + 5 + 6) | 20.33 ± 10.39 | 0 | <0.001 |
| Hospital Anxiety and Depression Scale (HADS) | 15.32 ± 7.99 | 0 | <0.001 |
| HADS (anxiety) | 9.44 ± 5.54 | 0 | <0.001 |
| HADS (depression) | 6.17 ± 3.60 | 0 | <0.001 |
| Region Label | Cluster/Voxels | Peak t-Value | Montreal Neurological Institute (MNI) Coordinates | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive | Thalamus | 158 | 9.730 | 6 | −21 | 9 |
| Inferior Parietal Lobule | 220 | 10.612 | −45 | −27 | 24 | |
| Cingulate Gyrus | 110 | 7.665 | −6 | −15 | 45 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Hu, Q.; Xia, G.; Tan, Y.; Guo, Y.; Sun, C. The ALFF Alterations of Spontaneous Pelvic Pain in the Patients of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Evaluated by fMRI. Brain Sci. 2022, 12, 1344. https://doi.org/10.3390/brainsci12101344
Ge S, Hu Q, Xia G, Tan Y, Guo Y, Sun C. The ALFF Alterations of Spontaneous Pelvic Pain in the Patients of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Evaluated by fMRI. Brain Sciences. 2022; 12(10):1344. https://doi.org/10.3390/brainsci12101344
Chicago/Turabian StyleGe, Shengyang, Qingfeng Hu, Guowei Xia, Yifan Tan, Yijun Guo, and Chuanyu Sun. 2022. "The ALFF Alterations of Spontaneous Pelvic Pain in the Patients of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Evaluated by fMRI" Brain Sciences 12, no. 10: 1344. https://doi.org/10.3390/brainsci12101344
APA StyleGe, S., Hu, Q., Xia, G., Tan, Y., Guo, Y., & Sun, C. (2022). The ALFF Alterations of Spontaneous Pelvic Pain in the Patients of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Evaluated by fMRI. Brain Sciences, 12(10), 1344. https://doi.org/10.3390/brainsci12101344






