Abstract
Background: It has been hypothesized that, whenever estrogen levels decline, psychosis symptoms in women increase. At menopause, this can happen in two main ways: (a) the loss of estrogen (mainly estradiol) can directly affect central neurotransmission, leading to increase in schizophrenia-related symptoms, and (b) the loss of estrogen can decrease the synthesis of enzymes that metabolize antipsychotic drugs, thus weakening their efficacy. Aims and Methods: The aim of this narrative review was to investigate the second possibility by searching PubMed and ClinicalTrials.gov for studies over the last two decades that investigated the metabolism of antipsychotics and their efficacy before and after menopause in women or that studied systemic and local estrogen level effects on the pharmacokinetics and pharmacodynamics of individual antipsychotic drugs. Results: The evidence suggests that symptom level in women with schizophrenia rises after menopause for many reasons beyond hormones but, importantly, there is an estrogen-dependent loss of efficacy related to antipsychotic treatment. Conclusion: Effective clinical intervention is challenging; nevertheless, several promising routes forward are suggested.
1. Introduction
1.1. Differences in Short and Long Outcomes in Women with Schizophrenia
The literature on short term treatment outcomes (under 10 years) that compare men and women with schizophrenia consistently point to female advantage. Compared to men, women are shown to have fewer and shorter hospitalizations plus a lower rate of suicide, homelessness, substance abuse, and forensic involvement [].
Women’s family and social relationships are reported as superior to men’s [,], and women are generally found to exhibit a more robust response to treatment []. Importantly, however, long term outcomes (over 10 years) are similar in men and women []. Equally important are data that show women with schizophrenia, but not men, experiencing increases in symptom severity at midlife (over age 40) []. Increases in cognitive symptoms and functional decline over time are often attributed to age, but positive, negative, and affective symptoms also worsen. Two main potential explanations for increased symptoms in women post menopause are (a) estrogen loss directly affects neurotransmitters that mediate those symptoms and (b) estrogen loss moderates the efficiency of enzymes that metabolize antipsychotic drugs such that less of the drug arrives at target sites in the brain [].
1.2. Why Estrogen Is Important
Three estrogens (estrone, 17β-estradiol (E2), and estriol) regulate many central nervous system functions [] and operate through three estrogen receptor subtypes, Erα, Erβ and G-protein-coupled estrogen receptor 1 (GPER). The three ER subtypes are genetically distinct and mediate different estrogenic actions. ERα and Erβ, initiate both genomic and non-genomic traditional sexual and reproductive functions while GPER mediates neuroprotection and cognition [].
Estrogens are synthesized from the parent molecule, cholesterol, with estradiol (E2) being the most potent of the three [,]. E2, mainly produced in ovarian oocytes, gradually decreases over the course of perimenopause []. Estrone (E1) is mainly synthesized in the adrenal glands and adipose tissue and continues to be available after menopause. Estriol (E3) is mainly produced during pregnancy. Estrogens originate in many body sites, including the brain.
At menopause (the cessation, in women, of menses due to the death of oocytes), many women experience cognitive problems because the decline in E2 is associated with a reduction in the volumes of both the hippocampus and the parietal cortex []. There are other effects of estrogen decline that can lead to a variety of symptoms at this time because E2, the estrogen that is lost at menopause, serves many functions. It insulates against the effects of injury, inflammation, ischemia and apoptosis; it activates signal transduction pathways and modulates intracellular homeostasis. It also promotes neuronal growth and activates other neuroreparative processes such as decreasing blood–brain barrier permeability, reducing oxidative stress, neuroinflammation and excitotoxicity, and promoting synaptic plasticity, axonal growth, neurogenesis, and remyelination [].
1.3. The Perimenopause
There are two stages to the years leading up to menopause—the early menopausal transition when menstrual cycles remain mostly regular, and the late transition, where amenorrhea becomes more prolonged and lasts for at least 60 days until it stops altogether []. Perimenopause usually lasts from 7 to 14 years during which, in the early phase, estrogen levels may paradoxically increase []. This is because, in order to stimulate estrogen production, follicle-stimulating hormone (FSH) secretion rises in response to decreasing levels of oocyte estrogen secretion []. Most women are able to accommodate to estrogen level fluctuations during this period because of prior exposure to menstrual period fluxes, the 9-fold gradual rise of estrogen over the course of pregnancy [] and the precipitous drop postpartum. This sharp, abrupt fall precipitates postpartum psychosis in approximately one woman in a thousand []. The drop at menopause is more gradual but no less significant and, in a substantial number of women with schizophrenia, is associated with an increase in the severity of psychotic symptoms [,,].
1.4. Estrogen, Dopamine, and Schizophrenia
The menopausal risk of increased psychosis severity may be tied to the link between estradiol and brain dopamine [,], the neurotransmitter most closely implicated in the pathogenesis of schizophrenia []. Relatively little is known about sex differences in dopamine function but D2/D3 dopamine receptors, the neuronal membrane receptors to which most antipsychotic medications preferentially bind [] do show some differences between men and women. The results of human PET studies show that women have more D2 receptors than men in the frontal and temporal cortex and in the thalamus. In the striatum, D2 receptor density declines with age faster in men than in women. Animal studies also point to similar sex differences [].
One research group studied sex differences in D2 receptor occupancy after administration of the antipsychotic drug, olanzapine, and demonstrated that women are able to achieve the same D2 receptor occupancy as men while taking a lesser daily oral dose []. Current thinking is that the dopamine transporter that returns dopamine to its presynaptic cell after secretion into the synaptic gap is key to the hyperdopaminergic state responsible for psychotic symptoms, and estradiol activity has been found at the transporter site []. It is thought that the lower the estrogen level in relevant parts of the brain, the more severe the psychosis []. The amount of antipsychotic medication that reaches the brain is determined by its level in the plasma, and that, in turn, is chiefly influenced by liver and gut enzymes that break down antipsychotic drugs. Relevant to our review is the fact that estradiol levels can speed or slow some of the enzymes that metabolize specific antipsychotic drugs [,]. This effect is most evident when the drugs are administered orally but still affects intramuscularly or intravenously administered antipsychotics.
Further suggestive evidence of the potential link between estrogen and dopamine transmission is the well-established fact that women first express the symptoms of schizophrenia at a later age than men [,]. Important also is the high incidence rate of schizophrenia in women with Turner’s syndrome [,]. Turner’s syndrome (one X chromosome wholly or partially missing) is a chromosomal abnormality characterized by reduced serum estrogen levels. Further evidence is that both male and female schizophrenia patients show reduced levels of plasma estrogen when compared to age and sex peers []. Another confirmatory finding is that early puberty in girls (but not in boys) is associated with relatively later onset of symptoms in young people who go on to develop schizophrenia [,,]. It is also known that symptoms fluctuate over the menstrual cycle in women with schizophrenia, increasing in severity when estrogen levels are low [,]. At postpartum, when estrogen levels precipitously drop, women with schizophrenia often relapse []. An interesting epidemiological finding is that the relative male/female incidence of schizophrenia reverses after age 40 []. There is also evidence that estrogen-reducing drugs trigger acute psychosis []. Perhaps most convincingly, adding estrogen or selective estrogen receptor modulators (SERMS) to an antipsychotic regimen improves treatment efficacy [].
1.5. Estrogen and Antipsychotic Efficacy
Duffy and Epperson concluded that estrogen levels influenced both the efficacy and the side effects of antipsychotic drugs after reviewing 12 human brain imaging studies that investigated sex differences in antipsychotic response []. None of the 12, however, were looking for a menopausal effect. It has been clinically noted that, after menopause, the dose of the antipsychotic medication often needs to be increased in women to maintain symptom stability. This is either because symptoms have become more severe or because the drugs function less well. The time duration since menopause has been shown to correlate negatively with antipsychotic response in postmenopausal women. The results of a 12-week prospective study of 64 postmenopausal women diagnosed with schizophrenia [] used the duration of reproductive years (menarche to menopause) as an indirect measure of cumulative estrogen exposure. The antipsychotic response was defined as a reduction of at least 30% on the Positive and Negative Syndrome Scale. Antipsychotic adherence was assessed by plasma level monitoring at 4 weeks. Forty-two participants (66%) were found to be antipsychotic responders. Time since menopause was significantly and negatively associated with antipsychotic response, explaining almost 42% of the variance. This finding could be a consequence of a direct effect of estrogen loss on dopamine transmission and, thus, on psychotic symptoms, or, alternatively, a direct effect of estrogen loss on drug metabolism and, hence, on plasma levels of antipsychotics entering the brain.
1.6. Estrogen after Menopause
Estradiol levels continue to fluctuate to some degree after menopause. Estrone continues to be produced in the adrenal glands (until adrenopause) and in adipose tissue, and estrogens are synthesized in bone, vascular endothelium, aortic smooth muscle cells, and in numerous sites in the brain [,]. In women after menopause, estrone levels increase from premenopausal levels. Estradiol (E2) levels are the ones that drop [,].
Estradiol is synthesized in both neurons and glial cells and is classified as a neurosteroid. Neurosteroids directly modulate plasma membrane ion channels and regulate intracellular signaling and they exert powerful effects on the brain before and after menopause []. Of relevance to the effect of estrogen in schizophrenia is the distribution of estrogen receptors in the brain. They are most abundant in the subcortical structures that are involved in schizophrenia (the hippocampus, amygdala, thalamus, and nucleus accumbens) and within neurotransmitter pathways (dopaminergic, serotonergic, and glutamatergic), all of which are implicated in the pathogenesis of schizophrenia symptoms [].
1.7. Symptom Exacerbation after Menopause Due to Stress
We have not yet mentioned a potential explanation for worsening symptoms and the need for increased antipsychotic dosing in women with schizophrenia after menopause that is independent of estrogen loss. Menopause itself is a stressful time for women. The physiological symptoms of menopause (vasomotor, sleep, sexual, and cognitive) are distressing. There are further psychological and social pressures at this time (children leaving home, parents dying, marital, employment and economic constraints). The impact of fertility loss, the need to adapt to aging, and the onset of a variety of new medical conditions further augment the stress experienced by menopausal women []. The effect of stress is not altogether independent of estrogenic influence, however, since the hypothalamic-pituitary-adrenal stress axis (HPA-axis) responds, to some degree, to estrogen. This has been well shown in animal studies, but the results in human studies are more controversial. Men consistently show greater HPA reactivity than women when evaluated for achievement. Some studies have found greater reactivity in women when the stressor is social performance. Sex difference (and, by implication, estradiol level) appears to depend on the nature of the stress []. Important to note is that both antipsychotics and stress increase prolactin levels, which, in turn, decreases estrogen levels [].
1.8. Aims
The specific aim of this review paper is to examine to what extent the loss of antipsychotic efficacy in women with schizophrenia after menopause is explained by estrogen’s effect on metabolizing enzymes. We review the literature
- (a)
- On the contrast in antipsychotic effectiveness pre- and post-menopause in women with schizophrenia;
- (b)
- On systemic and local estrogen level effects on the pharmacokinetics and pharmacodynamics of individual antipsychotic drugs.
2. Methods
We carried out a narrative review based on electronic searches on PubMed of papers investigating metabolism of antipsychotics and antipsychotic efficacy at and after menopause.
We used the following search terms: antipsychotics AND (pharmacokinetics OR pharmacodynamics) AND estrogen; (menopause OR postmenopausal OR estrogen) AND schizophrenia AND antipsychotics. We targeted studies published over the last two decades; however, some older papers were also considered if they were frequently cited and relevant to our aims. Additionally, ClinicalTrials.gov was searched for relevant papers not found in the PubMed search. Reference lists of the initially selected papers were screened for frequently cited older articles.
Inclusion criteria: (1) language restricted to English, French, Spanish and German, (2) publication in peer-reviewed journals, (3) focus on effectiveness of antipsychotics in postmenopausal women or (4) focus on sex differences in pharmacodynamics and pharmacokinetics of antipsychotics. Exclusion criteria: (1) case reports or case series, (2) patient samples receiving antipsychotics but diagnosed with affective or organic medical conditions.
The screening of several hundred abstracts (N = 552) and the selection process for full length paper screening and ultimate inclusion were performed by all authors. Disagreements about inclusion were resolved by group discussion.
Figure 1 shows the flow chart of papers screened and those excluded. In the end, a total of 53 studies were included in the review.
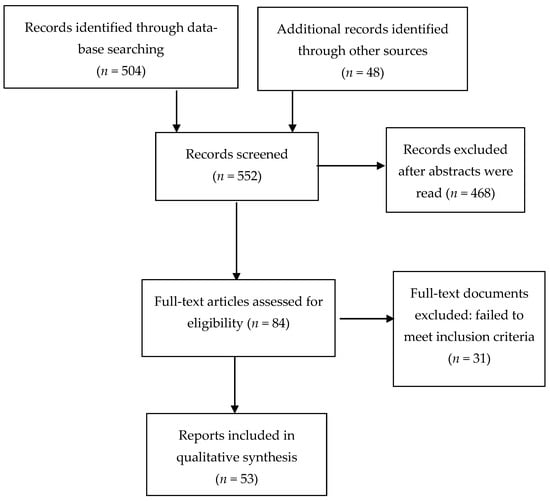
Figure 1.
Flow diagram of included studies.
3. Pharmacokinetics of Antipsychotics
3.1. Absorption of Antipsychotics
Absorption, but also distribution, metabolism and elimination are affected by women’s reproductive phases: puberty, menstrual stages, pregnancy, lactation, and menopause. The oral route for absorption is the most common one for antipsychotic drugs, although long-acting depot antipsychotics are becoming popular, and they are administered intramuscularly [].
Numerous sex differences in oral drug absorption (effect on gastric acid, gastric emptying time, intestinal contractility and transit time) have been identified and are usually attributed to hormonal differences between men and women that wane at older ages []. Recent research suggests, however, that some sex differences in drug bioavailability may persist throughout life. Castberg et al. [] tested the absorption vs. elimination of several antipsychotic drugs—clozapine olanzapine, risperidone and quetiapine. They found that host age predicted the concentrations of all four drugs. At patient age 80, concentrations of drugs adjusted by dose were almost twice as high as at age 40, suggesting increasingly impaired rates of elimination. Concentration increase by age was highest for clozapine and lowest for olanzapine, suggesting differences between the two drugs in 1st and 2nd pass metabolism. Women showed higher concentrations than men (20–30% higher) at both pre- and post-menopausal ages, implying that peripheral estrogen levels are not the only factors that account for sex differences in bioavailability.
With respect to intramuscular injections, they are reportedly more successfully administered in men than in women. This depends on the depth of fat covering the muscle at the site of the injection and the length of the needle used []. This means that women may not benefit as much from injections as men do.
3.2. Antipsychotic Drug Metabolism
While absorption, distribution, protein binding, and elimination of antipsychotics all differ by sex and age [], the key to understanding antipsychotic response in women at menopause is the effect of estrogen plasma levels on the cytochrome P450 (CYP) enzymes that metabolize antipsychotics, mainly in the liver and gut [,]. It is not always possible, however, to definitively identify which CYP enzyme is involved in the metabolism of a particular drug because, for the most part, in vivo data come only from individuals case reports [].
For most 2nd generation antipsychotics, the principal metabolizing enzymes are currently considered to be: aripiprazole CYP 2D6 and CYP 3A4; asenapine CYP 1A2 and CYP 2D6; clozapine CYP 1A2; iloperidone CYP 2D6 and CYP 3A4; lurasidone CYP 3A4; olanzapine CYP 1A2; quetiapine CYP 3A4; risperidone CYP 2D6; ziprasidone CYP 3A4 []. Specific CYP activity can be inhibited or induced by concomitant drugs or by levels of endogenous or exogenous hormones [,]. Estrogen levels change the activity of the following liver and intestinal metabolic enzymes: CYP1A2 (asenapine, clozapine, olanzapine), CYP2C9, CYP2C19, and CYP3A4 (aripiprazole, iloperidone, quetiapine, ziprasidone) [].
According to the above, doses of aripiprazole, iloperidone, quetiapine, and ziprasidone need to be lowered at and after menopause to prevent adverse effects while doses of asenapine, clozapine and olanzapine need to increase to maintain effectiveness. Cigarette smoking induces CYP 1A2, so menopausal women who smoke and are taking drugs metabolized by CYP 1A2 need significantly raised doses but, should they cease smoking, the dose needs to be adjusted downwards [,,]. Those taking drugs metabolized by CYP2C9, CYP2C19, and CYP3A4 may suffer increased side effects after menopause.
Sex hormones also influence phase II metabolism (glucuronidation, sulfation, acetylation, methylation, glutathione conjugation), although the relevant data with respect to antipsychotics are sparse []. It is to be noted, however, that female contraceptives affect phase I and phase II metabolism in opposite directions [].
A relatively neglected contributor to drug pharmacokinetics is emerging from studies of differences in the composition of male and female microbiomes [,,]. Different gut bacteria produce metabolic enzymes that are not necessarily identical. The most dominant biotransformations ascribed to bacterial enzymes in the gut involve reductive metabolism (the antipsychotic, risperidone is an example) and hydrolytic reactions and, to a lesser extent, decarboxylations, dehydroxylations, dealkylations, dehalogenations, and deaminations []. Hormones such as estrogen regulate metabolizing enzymes in gut bacteria as well as in human liver []. This has raised the possibility that menopause alters male/female composition of gut bacteria and the actions of their metabolizing enzymes []. Indeed, several observational studies have confirmed that the gut microbiome is influenced by the hormonal environment []. These investigations, however, are still at an early stage.
3.3. Antipsychotic Drug Distribution—Effect of Adipose Tissue Changes Post Menopause
Because of the action of female hormones on fat cells, women’s bodies, in general, have a greater amount of fat mass than men, the difference decreasing after menopause []. This affects the distribution of antipsychotics, which are lipophilic drugs. Changes in drug distribution affect both the effectiveness and safety of drugs [].
Once ovaries no longer produce estradiol (i.e., after menopause), estrogens continue to be produced from cholesterol in many tissues, but especially in adipose tissue. Lipid stores in women change from their principal premenopausal subcutaneous location (mainly in the thighs) to the abdomen, which then makes distribution similar to that of men [,,], a pattern that has been associated with heightened risk for adverse effects such as diabetes and cardiovascular disturbance [,]. Thus, not only the efficacy but also the safety of antipsychotic drugs changes after menopause in women, issues that need testing in clinical trials [].
3.4. Elimination of Antipsychotics in Men and Women
The sexually dimorphic GI microbiome may affect drug elimination differently in women and men []. Renal elimination depends on tubular secretion, reabsorption, and glomerular filtration rate, which are reportedly lower in women than in men []. It also depends on body weight []. In one study of human kidney, 23 genes coding for drug transporters showed differential male/female mRNA expression. Twenty-one of these genes were expressed at higher levels in men, whereas two were expressed at higher levels in women [].
3.5. Genes and Antipsychotic Response
Importantly, individual gene polymorphisms determine antipsychotic response because they bear on all aspects of pharmacokinetics, especially metabolism [,].
Alkelai et al. used whole-genome sequencing to analyze variants of CYP2D6, responsible to a large degree for the metabolism of aripiprazole, asenapine, iloperidone, and risperidone []. They identified 57 different genotypes that predicted five metabolic phenotypes (poor metabolizers to ultra-rapid metabolizers). Genetic variants have also been reported in enzymes responsible for the metabolism of clozapine []. A current summary of drug-gene variations is available []. We know from the results of Yang et al., who recorded gene expression of 374 drug-metabolizing enzymes and transporters in male and female liver samples, that at least 77 such genes differ in the two sexes []. Sex differences in pharmacokinetics that persist post menopause [,] could result from a combination of genetic factors, e.g., genetic sex difference in drug metabolism independent of estrogen, genetic disparity between men and women in the volume of drug distribution, and genetic sex differences in glomerular filtration rates []. Furthermore, there are many variants of the dopamine receptor D2 (DRD2) gene [] and the 5-HTR2A gene [] that may theoretically affect antipsychotic response. In all patients receiving antipsychotic medications, genetic variations in both metabolic genes and neurotransmitter genes play a role in response.
Individual sex differences that are not attributable to hormones or genes can also result from disease states, diet and environmental exposures, all of which can change after menopause.
3.6. Pharmacodynamics of Antipsychotics
Pharmacodynamics refers to how a drug works once it reaches the site of its intended effect (membrane receptors, ion channels, relevant enzymes, and signaling pathway), but also how it impacts other, non-targeted body sites where it can sometimes exert unwanted effects []. For instance, a significant pharmacodynamic sex difference is the increased prevalence of QT interval prolongation in women and men (but worse in women) that is induced by many antipsychotics. This leads to an increased incidence of tachycardias, arrythmias and syncope in women. An early study investigated 32 cases of antipsychotic-induced Torsades de Pointes and found that women constituted 70% of all cases [].
A drug’s efficacy with respect to receptor binding depends on its molecular configuration; in the case of antipsychotics, this would refer mainly to D2/D3 receptors []. Psychodynamic effectiveness is also affected by host age, sex, frailty status and associated hormone levels, immune/inflammatory characteristics, microbiome composition, and genetic factors.
Estrogen levels in the brain determine, in part, the integrity of dopamine pathways and influence functional connectivity, neurotransmission, and brain structure [,,,,]. The threshold percentage of D2/D3 receptors that antipsychotics need to occupy for effectiveness, and the threshold that results in extrapyramidal adverse effects, is modulated by sex. Kaasinen et al. [], using positron emission tomography (PET) and a high-affinity radioligand to measure extrastriatal D2-like receptors, evaluated D2 binding potential in the frontal cortex, temporal cortex, and thalamus of 12 healthy men and 12 healthy women. Women showed higher D2 binding in all three regions, but the difference relative to men was only statistically significant for the frontal cortex. A more recent register-based study [] investigated how age and sex influenced striatal D2 receptor availability using [11C] raclopride. The pooled data from 5 different PET scanners yielded information on 120 healthy males and 36 healthy females, aged 19 to 71. Binding consistently declined with increasing age in both sexes, most convincingly in men and women between the ages of 20 and 60, the range for which there were most data. The decline occurred in both sexes but, on average, female binding stayed between 6–8% higher than male binding irrespective of age. Lateralization was more evident in males than in females. The investigators conducted a replication study of 135 participants that showed the same age and sex effects as the first study. The confirmation is important because their findings were not altogether in harmony with earlier results reporting sex-dependent decline in dopamine function, with males showing steeper reduction in receptors [,]. Using radioactive olanzapine as their PET ligand, Eugene and Masiak [] had found, like Malén et al. [], that, over various age ranges, women needed a smaller oral dose than men to achieve an occupancy of 70% D2 binding, 60% being the usual minimum required for antipsychotic efficacy. The amount of DA transporter (DAT) has been found to be higher in postmenopausal women compared to similarly aged men (age range 50–86, mean 70 years) []. Others had previously also found sex differences in DATs [,]. In addition, it has been shown that women show significantly higher presynaptic dopamine synthesis than men in the striatum, more in the caudate than in the putamen [].
Importantly, D2 receptor availability has been reported to vary with the level of plasma sex steroids [,]. Sex-specific hormones as well as genes evidently play a role in dopaminergic function [] and this implies that menopause, when estrogen levels sharply decline, changes women’s response to antipsychotics. Menopause may also affect the severity of adverse effects. In a cross-sectional study, Iversen et al. [] found that over 75% of individuals taking antipsychotics reported adverse effects and that twice as many women as men described these as severe. Ages and hormonal status were not specified in this study. The adverse effects that are most likely to be impacted by sex hormones and, therefore, by menopause are: extrapyramidal effects (including tardive dyskinesia), agranulocytosis, cardiac arrythmia, metabolic effects, and hyperprolactinemia. According to hospital statistics, these adverse effects of antipsychotics are, in general, more prevalent in women than in men [], but how much of this is due to menopause has never been investigated.
Finally, epigenetic modifications can play a role in male/female pharmacodynamic differences because DNA methylation and histone acetylation differ between the two sexes [,].
3.7. Changes in Postmenopausal Clinical Response to Antipsychotics
Because estrogen, via its effect on dopamine pathways, is thought to enhance the efficacy of antipsychotics, psychotic symptoms increase in severity after menopause and, correspondingly, women become less responsive to antipsychotics than they were during reproductive life. This means that, frequently, doses need to be raised and side effects, therefore, become more likely.
A small 3-year survey from the 1980s explored the interaction between sex, age and dose in patients receiving antipsychotics []. Men at younger ages required higher doses than women matched for age. By the time of menopause, however, women needed higher doses of antipsychotics than men of comparable age, and higher doses than younger women. Many years later, González-Rodríguez and collaborators [] carried out the prospective observational study referred to earlier in this review. Participants were 64 women with schizophrenia or closely related disorders. All were postmenopausal and all required a change of antipsychotic because of failure to respond. For the assessment of psychotic symptoms and of function, the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impression Scale (CGI), and the Personal Social Performance Scale (PSP) were used. Cumulative estrogen exposure, calculated as the difference between age at menarche and age at menopause, was not predictive of overall antipsychotic response. Time since menopause, however, was negatively correlated with antipsychotic response, which suggested that the longer the time since menopause, the poorer the response to antipsychotics. This implies that, besides estrogen loss, age and increasing medical challenges may play important roles in antipsychotic response in this population.
The same research group investigated the association among gonadal hormones, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and FSH/LH ratio and clinical improvement in postmenopausal women with schizophrenia []. Because increasing levels of FSH are a marker of poor ovarian reserve in women, the FSH/LH ratio has been used in reproductive medicine to predict response to controlled ovarian stimulation and outcomes of in vitro fertilization procedures. The study authors hypothesized that FSH/LR ratio would correlate positively with antipsychotic response in postmenopausal women. Thirty-seven acutely ill postmenopausal women with schizophrenia received a newly initiated antipsychotic as part of a 12-week prospective observational study. PANSS, CGI and PSP were administered at baseline and again 12 weeks later. After correcting for potential confounders and multiple testing, there was no significant association in serum levels of estradiol, progesterone, testosterone, FSH, LH or FSH/LH ratio with clinical improvement. Prior to Bonferroni correction, however, the FSH/LH ratio did correlate positively with improvement, so that this remains a promising area of investigation.
Table 1 summarizes changes in clinical outcomes in women with schizophrenia after menopause.

Table 1.
Effects of menopause on clinical outcomes in women with schizophrenia.
In summary, the literature suggests that clinical status worsens and antipsychotic response decreases after menopause in women. Many postmenopausal women with schizophrenia require higher doses of antipsychotics than their premenopausal peers [,,]. The progressive decline of response to antipsychotics is partly explained by estrogen decline, but the evidence suggests that other factors are implicated as well.
Table 2 summarizes the main factors associated with variations of clinical response in postmenopausal women with schizophrenia.

Table 2.
Changes in clinical response in postmenopausal women with schizophrenia.
4. Discussion and Future Directions
The estrogen protection hypothesis postulates a neuroprotective role for estrogens in the brain that shields it, to some extent, from the development of psychotic symptoms. The decline of estradiol at the time of menopause correlates with an increase (relative to men) in the incidence of schizophrenia in women after menopause. Symptoms in those with a pre-existing schizophrenia diagnosis intensify at this time. Although several ways in which estradiol loss at menopause can increase symptom severity have been identified, this review has focused on the theoretical effects of estrogen loss on the pharmacokinetics and pharmacodynamics of antipsychotics. This is a complex area which involves estrogen effects on 1st and 2nd pass liver enzymes, and on neurotransmitter receptors in the brain. There is reason to believe that some antipsychotics are more affected by estrogen loss than others. Furthermore, from a pharmacodynamic point of view, brain estradiol levels influence the integrity of dopamine pathways, neurotransmission, brain structure and functions.
Raloxifene, a selective estrogen receptor modulator (SERM), has been found to exert neuroprotective effects in the central nervous system and has proven to be a good adjunctive drug for psychotic symptoms in the menopausal period. Most recently, a study carried out by Huerta-Ramos and collaborators (2020) investigated the efficacy of raloxifene as a treatment of cognitive symptoms when added to antipsychotics in postmenopausal schizophrenia []. The addition of raloxifene 60 mg/daily did not show any effect on cognitive function but improved negative and general psychopathological symptoms []. Recent meta-analyses have reported that raloxifene appears to be efficacious and safe for women at the time of menopause, particularly for those whose symptoms are not severe [,].
Clinical recommendations are:
- When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice [,] because it increases the risk of weight gain, cardiovascular and cerebrovascular events.
- Changing to an antipsychotic that is less affected by estrogen loss may work better, [,,] because estrogen levels change the activity of CYP1A2 and CYP3A4, which principally affects clozapine and olanzapine. The work of Hoekstra et al. [], suggests that amisulpride and aripiprazole work well post menopause.
- Changing to a depot or skin patch antipsychotic that obviates 1st pass metabolism will improve levels of drugs affected by of CYP1A2 and CYP3A4 enzymes.
- Adding hormone replacement or a selective estrogen receptor modulator such as raloxifene or newer SERMS or including phytoestrogens (bioidenticals) in the diet may prove effective [,,,].
- Weight maintenance may be important because extra adipose tissue yields more estrogen [,] although this will be estrone rather than estradiol.
- High prolactin levels, whether induced by antipsychotics, other drugs, or by stress, reduce estrogen levels. Prolactin-sparing antipsychotics are recommended [].
Our findings also suggest several promising strategies for future drug trials:
- (1)
- Comparing the effectiveness of different antipsychotics in postmenopausal women with schizophrenia
- (2)
- Recruiting pre- and post- menopausal women in trials of antipsychotic drugs.
- (3)
- Stratifying by hormonal status when analyzing results of antipsychotic trials []
- (4)
- Comparing D2/D3 occupancy in pre- and post-menopausal women and matched age men in patients being treated for schizophrenia
- (5)
- Comparing antipsychotic side effects in pre- and post- menopausal women and men with schizophrenia [,].
- (6)
- Studying the effect on antipsychotic requirements of adrenopause in postmenopausal women and men with schizophrenia
- (7)
- Studying the effect of sex hormones on the blood–brain barrier for antipsychotic medications []
- (8)
- Studying the effect of phytoestrogens [] in the diet and xenoestrogens [] in the environment on symptoms and antipsychotic requirements in postmenopausal women and men with schizophrenia.
Lastly, the findings suggest a need for different antipsychotic dosing guidelines for men and women (pre- and post-menopausal).
Research recommendations: Information on pharmacogenetics and the use of ‘big data’ will inform future research. New genetic editing techniques applied to new animal models can aid in resolving questions about best treatments for postmenopausal women. Neuroimaging techniques that explore new ways of visualizing brain circuitry will help to pave the way toward precision psychiatry [].
5. Conclusions
Menopause is a critical period of a woman’s life characterized by a significant decline in estrogen plasma levels. It is associated with a worsening of psychotic symptoms in women with schizophrenia. This is at least in part due to a direct effect on the brain of a decline in estrogen neuroprotection and an indirect effect of estrogen loss on antipsychotic pharmacokinetics and pharmacodynamics. Other factors, such as aging, co-morbidity, drug interactions, and the psychosocial stresses that are associated with menopause all play additional roles in the severity of women’s psychotic symptoms after menopause.
We have considered several reasons why psychotic symptoms worsen in women with schizophrenia at menopause. Two important ones are: (a) the low level of plasma estradiol that directly affects central neurotransmission, and (b) the low level of plasma estradiol that affects the synthesis of metabolizing enzymes, affecting the levels of some antipsychotics more than others.
It is important in clinical trials of women participants to investigate and aggregate data according not only to sex, but also to hormonal status. Guidelines should provide a concise and precise description of up-to-date experimental evidence, including sex-and-age-specific prescribing information that takes hormone levels into account.
Author Contributions
A.G.-R. and M.V.S. wrote the first draft of the manuscript. M.V.S. collaborated with A.G.-R. on subsequent revisions. J.A.M. collaborated in writing the paper and producing Tables, and M.V.S. supervised and critically reviewed the content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this review are available on request from the corresponding author.
Conflicts of Interest
A.G.-R. has received free registration or travel funds for congresses from Janssen, Lundbeck-Otsuka, and Angelini. J.A.-M. has received consultancy and/or lecture honoraria from Sanofi, Pfizer, Servier, Janssen, and Lundbeck-Otsuka.
References
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Bucci, P.; Mucci, A.; Pezzella, P.; Galderisi, S. Gender differences in clinical and psychosocial features among persons with schizophrenia: A mini review. Front. Psychiatry 2021, 12, 789179. [Google Scholar] [CrossRef] [PubMed]
- Shafie, S.; Samari, E.; Jeyagurunathan, A.; Abdon, E.; Chang, A.; Chong, S.A.; Subramaniam, M. Gender difference in quality of life (QoL) among outpatients with schizophrenia in a tertiary care setting. BMC Psychiatry 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Does gender influence outcome in schizophrenia? Psychiatr. Q. 2019, 90, 173–184. [Google Scholar] [CrossRef]
- Riecher-Rössler, A.; Butler, S.; Kulkarni, J. Sex and gender differences in schizophrenic psychoses—A critical review. Arch. Women Ment. Health 2018, 21, 627–648. [Google Scholar] [CrossRef]
- Culbert, K.M.; Thakkar, K.N.; Klump, K.L. Risk for midlife psychosis in women: Critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk. Psychol. Med. 2022, 52, 1612–1620. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Catalán, R.; Penadés, R.; Ruiz Cortés, V.; Torra, M.; Seeman, M.V.; Bernardo, M. Antipsychotic response worsens with postmenopausal duration in women with schizophrenia. J. Clin. Psychopharmacol. 2016, 36, 580–587. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; van Waarde, A.; Doorduin, J.; de Vries, E.F.J. Sex steroid hormones and brain function: PET imaging as a tool for research. J. Neuroendocrinol. 2018, 30, e12565. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Eyster, K.M. The estrogen receptors: An overview from different perspectives. In Estrogen Receptors; Springer: Berlin, Germany, 2016; Volume 1366, pp. 1–10. [Google Scholar]
- Rehbein, E.; Hornung, J.; Sundström Poromaa, B.; Derntl, B. Shaping of the female human brain by sex hormones: A review. Neuroendocrinology 2021, 111, 183–206. [Google Scholar] [CrossRef]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019, 55, 100787. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.C. Ovarian aging and the perimenopausal transition: The paradox of endogenous ovarian overstimulation. Endocrine 2005, 26, 297–300. [Google Scholar] [CrossRef]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The menopause transition: Signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sundström Poromaa, I.; Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 2014, 8, 380. [Google Scholar] [PubMed]
- Jones, I. Postpartum psychosis: An important clue to the etiology of mental illness. World Psychiatry 2020, 19, 334–336. [Google Scholar] [CrossRef]
- Brzezinski, A.; Brzezinski-Sinai, N.A.; Seeman, M.V. Treating schizophrenia during menopause. Menopause 2017, 24, 582–588. [Google Scholar] [CrossRef]
- Marin, R.; Diaz, M. Estrogen interactions with lipid rafts related to neuroprotection. Impact of brain ageing and menopause. Front. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef]
- Szeliga, A.; Stefanowski, B.; Meczekalski, B.; Snopek, M.; Kostrzak, A.; Smolarczyk, R.; Bala, G.; Duszewska, A.; Smolarczyk, K.; Maciejewska-Jeske, M. Menopause in women with schizophrenia, schizoaffective disorder and bipolar disorder. Maturitas 2021, 152, 57–62. [Google Scholar] [CrossRef]
- Kusters, C.D.J.; Paul, K.C.; Duarte Folle, A.; Keener, A.M.; Bronstein, J.M.; Bertram, L.; Hansen, J.; Horvath, S.; Sinsheimer, J.S.; Lill, C.M.; et al. Increased menopausal age reduces the risk of Parkinson’s disease: A Mendelian randomization approach. Mov. Disord. 2021, 36, 2264–2272. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Chen, G.C.; Li, G.; Rui, Y.; Qin, L.; Wan, Z. Reproductive factors and risk of Parkinson’s disease in women: A meta-analysis of observational studies. Behav. Brain Res. 2017, 335, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C. Assessing striatal dopamine in schizophrenia. Biol. Psychiatry 2022, 91, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Cumming, P.; Abi-Dargham, A.; Gründer, G. Molecular imaging of schizophrenia: Neurochemical findings in a heterogeneous and evolving disorder. Behav. Brain Res. 2021, 398, 113004. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.O.F.; Coppolino, M.; George, S.R.; Perreault, M.L. Sex differences in dopamine receptors and relevance to neuropsychiatric disorders. Brain Sci. 2021, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Eugene, A.R.; Masiak, J. A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord. J. Psychiatry 2017, 71, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Nam, H.Y.; Lee, M.J.; Pak, K.; Kim, K.; Kim, I. Effect of sex on aging-related decline of dopamine transporter in healthy subjects. Ann. Nucl. Med. 2021, 35, 76–82. [Google Scholar] [CrossRef]
- Brand, B.A.; Haveman, Y.R.A.; de Beer, F.; de Boer, J.N.; Dazzan, P.; Sommer, I.E.C. Antipsychotic medication for women with schizophrenia spectrum disorders. Psychol. Med. 2022, 52, 649–663. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Sommer, E.C. Estrogens in schizophrenia: Progress, current challenges and opportunities. Curr. Opin. 2021, 34, 228–237. [Google Scholar] [CrossRef]
- Romanescu, M.; Buda, V.; Lombrea, A.; Andor, M.; Ledeti, I.; Dehelean, L. Sex-related differences in pharmacological response to CNS drugs: A narrative review. J. Pers. Med. 2022, 12, 907. [Google Scholar] [CrossRef]
- Seeman, M.V. Gender and the onset of schizophrenia: Neurohumoral influences. Psychiatr. J. Univ. Ott. 1981, 6, 136–138. [Google Scholar]
- Häfner, H.; Riecher, A.; Maurer, K.; Löffler, W.; Munk-Jørgensen, P.; Strömgren, E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol. Med. 1989, 19, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Björlin Avdic, H.; Butwicka, A.; Nordenström, A.; Almqvist, C.; Nordenskjöld, A.; Hedvig Engberg, H.; Frisén, L. Neurodevelopmental and psychiatric disorders in females with Turner syndrome: A population-based study. J. Neurodev. Disord. 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.I.; Chue, P.S.; Tibbo, P. Investigation of Turner syndrome in schizophrenia. Am. J. Med. Genet. 2002, 96, 373–378. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry 2017, 4, 63–72. [Google Scholar] [CrossRef]
- Cohen, R.Z.; Seeman, M.V.; Gotowiec, A.; Kopala, L. Earlier puberty as a predictor of later onset of schizophrenia in women. Am. J. Psychiatry 1999, 156, 1059–1064. [Google Scholar] [CrossRef]
- Kiliçaslan, E.E.; Erol, A.; Zengin, B.; Çetinay Aydin, P.; Mete, L. Association between age at onset of schizophrenia and age at menarche. Nöro Psikiyatri Arşivi. 2014, 51, 211–215. [Google Scholar] [CrossRef]
- Ruiz, A.; Blanco, R.; Santander, J.; Miranda, E. Relationship between sex differences in onset of schizophrenia and puberty. J. Psychiatr. Res. 2000, 34, 349–353. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Dazza, P.; Sommer, I.E. Towards better care for women with schizophrenia-spectrum disorders. Lancet Psychiatry 2022, 9, 330–336. [Google Scholar] [CrossRef]
- Taylor, C.L.; Stewart, R.J.; Howard, L.M. Relapse in the first three months postpartum in women with history of serious mental illness. Schizophr. Res. 2019, 204, 46–54. [Google Scholar] [CrossRef]
- van der Werf, M.; Hanssen, M.; Köhler, S.; Verkaaik, M.; Verhey, F.R.; van Winkel, R.; van Os, J.; Allardyce, J. Rise investigators. Systematic review and collaborative recalculation of 133 693 incident cases of schizophrenia. Psychol. Med. 2012, 44, 9–16. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Cobo, J.; Soria, V.; Usall, J.; Garcia-Rizo, C.; Bioque, M.; Monreal, J.A.; Labad, J. Women undergoing hormonal treatments for infertility: A systematic review on psychopathology and newly diagnosed mood and psychotic disorders. Front. Psychiatry 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; Butler, S.; Riecher-Rössler, A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front. Neuroendocrinol. 2019, 53, 100743. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Epperson, C.N. Evaluating the evidence for sex differences: A scoping review of human neuroimaging in psychopharmacology research. Neuropsychopharmacology 2022, 47, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Androvičovà, R.; Pfaus, J.G.; Ovsepian, S.V. Estrogen pendulum in schizophrenia and Alzheimer’s disease: Review of therapeutic benefits and outstanding questions. Neurosci. Lett. 2021, 759, 136038. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid. Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Lloyd-Evans, E.; Lloyd-Evans, H. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays Biochem. 2020, 64, 591–606. [Google Scholar]
- Seeman, M.V.; González-Rodríguez, A. Stratification by sex and hormone level when contrasting men and women in schizophrenia trials will improve personalized treatment. J. Pers. Med. 2021, 11, 929. [Google Scholar] [CrossRef]
- Handa, R.J.; Sheng, J.A.; Castellanos, E.A.; Templeton, H.N.; McGivern, R.F. Sex differences in acute neuroendocrine responses to stressors in rodents and humans. Cold Spring Harb. Perspect. Biol. 2022, 14, a039081. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and gender-based pharmacological response to drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- Castberg, I.; Westin, A.A.; Skogvoll, E.; Spigset, O. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr. Scand. 2017, 136, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Ranjan, S.; Xu, T.; Gee, C.; Harker, A.; Barrera, A.; Geddes, J. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Bio-Des. Manuf. 2018, 1, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.I.; Baker, G.B. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J. Psychiatry Neurosci. 2003, 28, 99–112. [Google Scholar] [PubMed]
- Preskorn, S.H. Clinically important differences in the pharmacokinetics of the ten newer “atypical” antipsychotics: Part 1. J. Psychiatr. Pract. 2012, 18, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; De Leon, J. Metabolic drug interactions with newer antipsychotics: A comparative review. Basic Clin. Pharmacol. Toxicol. 2007, 100, 4–22. [Google Scholar] [CrossRef]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The potential effects of phytoestrogens: The role in neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Madla, C.M.; Gavins, F.K.H.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Seeman, M.V. The association between hormones and antipsychotic use: A focus on postpartum and menopausal women. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319859973. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: Interaction with biological differences between men and women. Br. J. Pharmacol. 2013, 171, 580–594. [Google Scholar] [CrossRef]
- Venter, G.; van der Berg, C.L.; van der Westhuizen, F.H.; Erasmus, E. Health status is affected, and phase I/II biotransformation activity altered in young women using oral contraceptives containing drospirenone/rthinyl estradiol. Int. J. Environ. Res. Public Health 2021, 18, 10607. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex differences in gut bacteria. World J. Mens. Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, W. How and why men and women differ in their microbiomes: Medical ecology and network analyses of the microgenderome. Adv. Sci. 2019, 6, 1902054. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.M.; Gonzales, C.; Jarmusch, A.K.; Momper, J.D.; Ma, J.D. Contribution of the gut microbiome to drug disposition, pharmacokinetic and pharmacodynamic variability. Clin. Pharm. 2021, 60, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Miftahussuouo, M.; Alshawsh, A. Pharmacomicrobiomics: Influence of gut microbiota on drug and xenobiotic metabolism. FASEB J. 2022, 36, e22350. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Connecting microbiome and menopause for healthy ageing. Nat. Microbiol. 2022, 7, 354–358. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The gut microbiome and sex hormone-related diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef]
- Farkouh, A.; Riedl, T.; Gottardi, R.; Czejka, M.; Kautzky-Willer, A. Sex-related differences in pharmacokinetics and pharmacodynamics of frequently prescribed drugs: A review of the literature. Adv. Ther. 2020, 37, 644–655. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fascian, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Arnold, A.P.; Bangasser, D.A.; Denton, K.M.; Gupta, A.; Hilliard Krause, L.M.; Mayer, E.A.; McCarthy, M.; Miller, W.L.; Raznahan, A.; et al. Considering sex as a biological variable in basic and clinical studies: An endocrine society scientific statement. Endocr. Rev. 2021, 42, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharm. 2019, 48, 143–157. [Google Scholar] [CrossRef]
- Joseph, S.; Nicolson, T.J.; Hammons, G.; Word, B.; Green-Knox, B.; Lyn-Cook, B. Expression of drug transporters in human kidney: Impact of sex, age, and ethnicity. Biol. Sex Differ. 2015, 6, 4. [Google Scholar] [CrossRef]
- Tracy, T.S.; Chaudhry, A.S.; Prasad, B.; Thummel, K.E.; Schuetz, E.G.; Zhong, X.B.; Tiene, Y.C.; Jeong, H.; Pan, X.; Shireman, L.M.; et al. Interindividual variability in Cytochrome P450-mediated drug metabolism. Drug Metab. Dispos. 2016, 44, 343–351. [Google Scholar] [CrossRef]
- Alkelai, A.; Greenbaum, L.; Docherty, A.R.; Shabalin, A.A.; Povysil, G.; Malakar, A.; Hughes, D.; Delaney, S.L.; Peabody, E.P.; McNamara, J.; et al. The benefit of diagnostic whole genome sequencing in schizophrenia and other psychotic disorders. Mol. Psychiatry 2022, 27, 1435–1447. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Semedo, A.T.; Neri, H.F.S.; Vianello, R.P.; Galaviz-Hernández, C.; Sosa-Macías, M.; de Brito, R.B.; Ghedini, P.C. The CYP2C19*2 and CYP2C19*17 polymorphisms influence responses to clozapine for the treatment of schizophrenia. Neuropsychiatr. Dis. Treat. 2020, 16, 427–432. [Google Scholar] [CrossRef]
- Hays, P. Evidence basis for pharmacogenetic testing in psychiatry. J. Med. Res. Health Sci. 2022, 5, 1838–1859. [Google Scholar]
- Yang, L.; Li, Y.; Hong, H.; Chang, C.W.; Guo, L.W.; Lyn-Cook, B.; Shi, L.; Ning, B. Sex differences in the expression of drug-metabolizing and transporter genes in human liver. J. Drug Metab. Toxicol. 2012, 3, 1000119. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 2008, 82, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Stader, F.; Marzolini, C. Sex-related pharmacokinetic differences with aging. Eur. Geriatr. Med. 2022, 13, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, E.G.; Nöthen, M.M.; Grünhage, F.; Farde, L.; Nakashima, Y.; Propping, P.; Sedvall, G.C. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 1999, 4, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Olajossy-Hilkesberger, L.; Godlewska, B.; Schosser-Haupt, A.; Olajossy, M.; Wojcierowski, J.; Landowski, J.; Marmurowska-Michałowska, H.; Kasper, S. Polymorphisms of the 5-HT2A receptor gene and clinical response to olanzapine in paranoid schizophrenia. Neuropsychobiology 2011, 64, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Sex differences in schizophrenia relevant to clinical care. Expert Rev. Neurother. 2021, 21, 443–453. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fromm, B.S.; Steinman, R.T.; Meissner, M.D.; Lehmann, M.H. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993, 270, 2590–2597. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Dluzen, D.E.; Horstink, M.W.I.M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system. Endocrine 2003, 21, 67–75. [Google Scholar] [CrossRef]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef] [PubMed]
- Kaasinen, V.; Någren, K.; Hitala, J.; Farde, L.; Rinne, J.O. Sex differences in extrastriatal dopamine D2-Like receptors in the human brain. Am. J. Psychiatry 2001, 158, 308–311. [Google Scholar] [CrossRef]
- Malén, T.; Karjalainen, T.; Isojärvi, J.; Vehtari, A.; Bürkner, P.C.; Putkinen, V.; Kaasinen, V.; Hietala, J.; Nuutila, P.; Rinne, J.; et al. Atlas of type 2 dopamine receptors in the human brain: Age and sex dependent variability in a large PET cohort. NeuroImage 2022, 255, 119149. [Google Scholar] [CrossRef] [PubMed]
- Pohjalainen, T.; Rinne, J.O.; Någren, K.; Syvälahti, E.; Hietala, J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am. J. Psychiatry 1998, 155, 768–773. [Google Scholar]
- Wong, D.F.; Broussolle, E.P.; Wand, G.; Villemagne, V.; Dannals, R.F.; Links, J.M.; Zacur, H.A.; Harris, J.; Naidu, S.; Braestrup, C.; et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography. Age and sex differences. Ann. N. Y. Acad. Sci. 1988, 515, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Nord, M.; Farde, L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci. Ther. 2011, 17, 97–103. [Google Scholar] [CrossRef]
- Yamamoto, H.; Arimura, S.; Nakanishi, A.; Shimo, Y.; Motoi, Y.; Ishiguro, K.; Murakami, K.; Hattori, N.; Aoki, S. Age-related effects and gender differences in Japanese healthy controls for [123 I] FP-CIT SPECT. Ann. Nucl. Med. 2017, 31, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kaasinen, V.; Joutsa, J.; Noponen, T.; Johansson, J.; Seppänen, M. Effects of aging and gender on striatal and extrastriatal [123I] FP-CIT binding in Parkinson’s disease. Neurobiol. Aging 2015, 36, 1757–1763. [Google Scholar] [CrossRef]
- Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Booij, J.; Kapucu, O.L.; Kluge, A.; Knudsen, G.M.; Koulibaly, P.M.; et al. European multicentre database of healthy controls for [123I] FP-CIT SPECT (ENC-DAT): Age-related effects, gender differences and evaluation of different methods of analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 213–227. [Google Scholar] [CrossRef]
- Laakso, A.; Vilkman, H.; Örgen Bergman, J.; Haaparanta, M.; Solin, O.; Syvälahti, E.; Salokangas, R.K.R.; Hietala, J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol. Psychiatry 2002, 52, 759–763. [Google Scholar] [CrossRef]
- Czoty, P.W.; Riddick, N.V.; Gage, H.D.; Sandridge, M.; Nader, S.H.; Garg, S.; Bounds, M.; Garg, P.K.; Nader, M.A. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 2009, 34, 548–554. [Google Scholar] [CrossRef]
- Loke, H.; Harley, V.; Lee, J. Biological factors underlying sex differences in neurological disorders. Int. J. Biochem. Cell Biol. 2015, 65, 139–150. [Google Scholar] [CrossRef]
- Iversen, T.S.J.; Steen, N.E.; Dieset, I.; Hope, S.; Mørch, R.; Gardsjord, E.S.; Jørgensen, K.N.; Melle, I.; Andreassen, O.A.; Molden, E.; et al. Side effect burden of antipsychotic drugs in real life—Impact of gender and polypharmacy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 263–271. [Google Scholar] [CrossRef]
- Vargas, A.; Ormseth, G.; Seifi, A. Gender and psychotropic poisoning in the USA. J. Neurol. Res. 2020, 10, 220–225. [Google Scholar] [CrossRef]
- Ghahramani, N.M.; Ngun, T.C.; Chen, P.Y.; Tian, Y.; Krishnan, S.; Muir, S.; Rubbi, L.; Arnold, A.P.; de Vries, G.J.; Forger, N.G.; et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol. Sex Differ. 2014, 5, 8. [Google Scholar] [CrossRef]
- Shen, E.Y.; Ahern, T.H.; Cheung, I.; Straubhaar, J.; Dincer, A.; Houston, I.; de Vries, G.J.; Akbarian, S.; Forger, N.G. Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Exp. Neurol. 2015, 268, 21–29. [Google Scholar] [CrossRef]
- Seeman, M.V. Interaction of sex, age, and neuroleptic dose. Compr. Psychiatry 1983, 24, 125–128. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Bernardo, M.; Penadés, R.; Arias, B.; Ruiz Cortés, V.; Seeman, M.V.; Catalán, R. Do FSH/LH ratio and gonadal hormone levels predict clinical improvement in postmenopausal schizophrenia women? Arch. Women Ment. Health 2017, 20, 613–620. [Google Scholar] [CrossRef]
- Bartkowiak-Wieczorek, J.; Wolski, H.; Bogacz, A.; Kujawski, R.; Ożarowski, M.; Majchrzycki, M.; Seremak-Mrozikiewicz, A. Gender-specific implications for pharmacology in childbearing age and in postmenopausal women. Ginekologia Polska 2015, 86, 143–149. [Google Scholar] [CrossRef]
- Huerta-Ramos, E.; Labad, J.; Cobo, J.; Núñez, C.; Creus, M.; García-Parés, G.; Cuadras, D.; Franco, J.; Miquel, E.; Reyes, J.C.; et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: A 24-week double-blind, randomized, parallel, placebo-controlled trial. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Usall, J.; Huerta-Ramos, E.; Labad, J.; Cobo, J.; Núñez, C.; Creus, M.; Parés, G.G.; Cuadras, D.; Franco, J.; Miquel, E.; et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: A 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr. Bull. 2016, 42, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Zheng, W.; Li, X.H.; Cai, D.B.; Yang, X.H.; Ungvari, G.S.; Ng, C.H.; Wang, X.P.; Kulkarni, J.; Grigg, J.; et al. Adjunctive raloxifene for postmenopausal women with schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophr. Res. 2018, 197, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, X.; Wang, Y.; Li, X. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: A meta-analysis of randomized controlled trials. Arch. Womens Ment. Health 2018, 21, 31–41. [Google Scholar] [CrossRef]
- Hoekstra, S.; Bartz-Johannessen, C.; Sinkeviciute, I.; Reitan, S.K.; Kroken, R.; Løberg, E.M.; Larsen, T.K.; Rettenbacher, M.; Johnsen, E.; Sommer, I.E. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. NPJ Schizophr. 2021, 7, 39. [Google Scholar] [CrossRef]
- Labad, J.; Montalvo, I.; González-Rodríguez, A.; García-Rizo, C.; Crespo-Facorro, B.; Monreal, J.A.; Palao, D. Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: A systematic review and meta-analysis. Schizophr. Res. 2020, 222, 88–96. [Google Scholar] [CrossRef]
- Barth, C.; Blokland, G.A.M.; Riecher-Rössler, A. Editorial: Sex and the suffering brain—A call for sex-stratified analyses in psychiatric research. Front. Psychiatry 2022, in press. [Google Scholar] [CrossRef]
- Correll, C.U. Epidemiology and prevention of tardive dyskinesia. J. Clin. Psychiatry 2017, 78, e1426. [Google Scholar] [CrossRef]
- Turrone, P.; Seeman, M.V.; Silvestri, S. Estrogen receptor activation and tardive dyskinesia. Can. J. Psychiatry 2000, 45, 288–290. [Google Scholar] [CrossRef]
- Dion-Albert, L.; Binder, L.B.; Daigle, B.; Hong-Minh, A.; Lebel, M.; Menard, C. Sex differences in the blood-brain barrier: Implications for mental health. Front. Neuroendocrinol. 2022, 65, 100989. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Day, A.J.; Zhang, W.; John, P.; Ng, D.J.; Banov, D. Safety and efficacy of compounded bioidentical hormone therapy (cBHT) in perimenopausal and postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Menopause 2022, 29, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; McCarthy, M.; Raval, A.P. Xenoestrogens impact brain estrogen receptor signaling during the female lifespan: A precursor to neurological disease? Neurobiol. Dis. 2022, 163, 105596. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Seeman, M.V. Pharmacotherapy for schizophrenia in postmenopausal women. Expert Opin. Pharmacother. 2018, 19, 809–821. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).