Development of Motor Imagery in School-Aged Children with Autism Spectrum Disorder: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Outcome Variables
2.4. Statistics
2.4.1. Response Times
2.4.2. Error Rates
3. Results
3.1. Criterion Tasks
3.2. Motor Imagery Task Outcomes
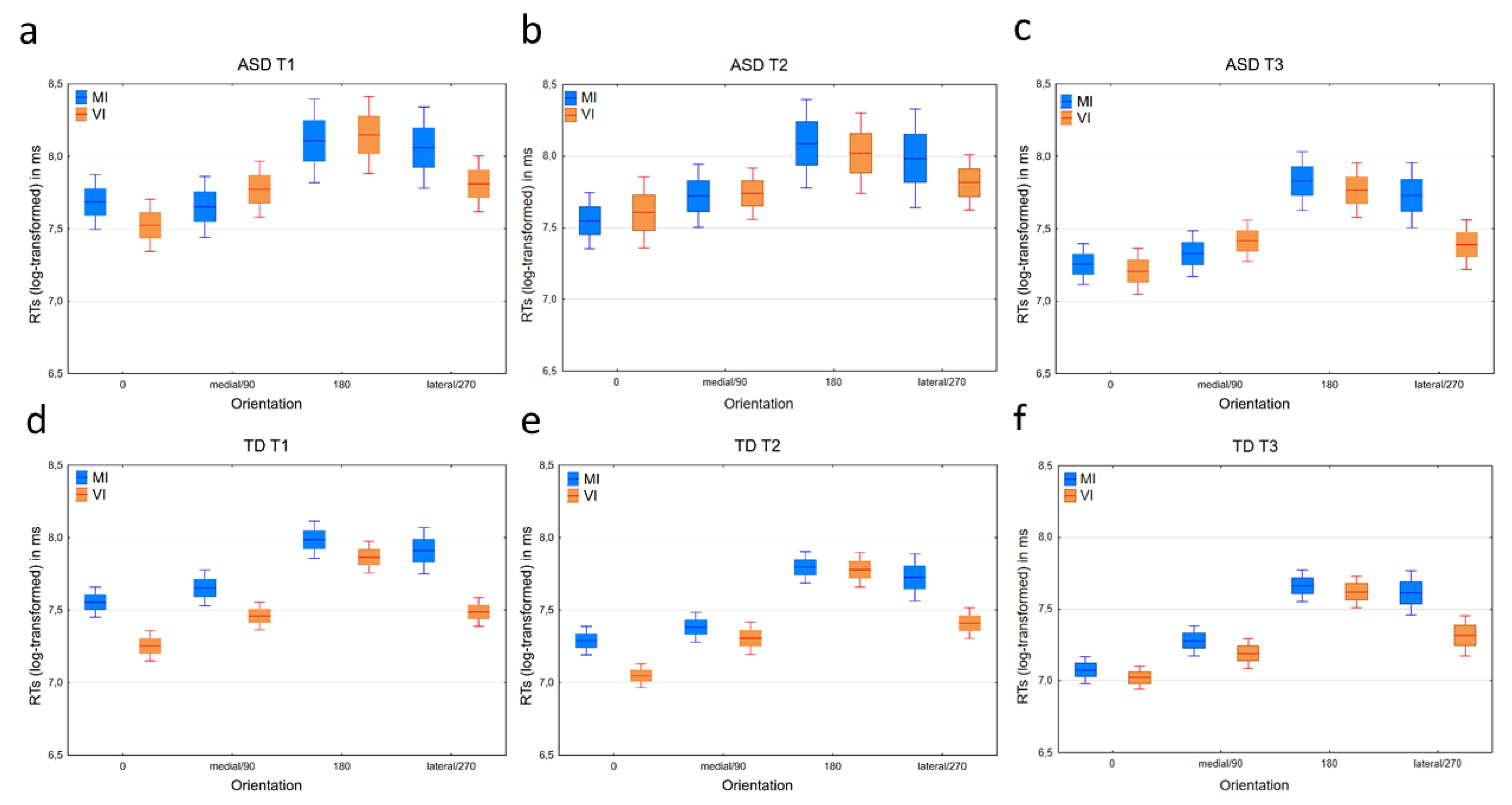
3.2.1. Response Times
3.2.2. Error Rates
Effects of Age
Within Group Effects
3.3. Visual Imagery Task Outcomes
3.3.1. Response Times
3.3.2. Error Rates
Effects of Age Level
Within Group Effects
3.4. Biomechanical Effects
3.4.1. Age Level 1
Within Group Differences
Between Group Differences
3.4.2. Age Level 2
Within Group Differences
Between Group Differences
3.4.3. Age Level 3
Main Effects
Within Group Differences
Between Group Differences
4. Discussion
4.1. Task Success and Error Rates
4.2. Response Times and the Biomechanical Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association Publishing: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Bhat, A.N. Is Motor Impairment in Autism Spectrum Disorder Distinct From Developmental Coordination Disorder? A Report From the SPARK Study. Phys. Ther. 2020, 100, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child Neurol. 2009, 51, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N. Motor Impairment Increases in Children with Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Res. 2021, 14, 202–219. [Google Scholar] [CrossRef]
- Lee, C.M.; Bo, J. Visuomotor adaptation and its relationship with motor ability in children with and without autism spectrum disorder. Hum. Mov. Sci. 2021, 78, 102826. [Google Scholar] [CrossRef] [PubMed]
- Sparaci, L.; Formica, D.; Lasorsa, F.R.; Mazzone, L.; Valeri, G.; Vicari, S. Untrivial Pursuit: Measuring Motor Procedures Learning in Children with Autism. Autism Res. 2015, 8, 398–411. [Google Scholar] [CrossRef]
- Neely, K.A.; Mohanty, S.; Schmitt, L.M.; Wang, Z.; Sweeney, J.A.; Mosconi, M.W. Motor Memory Deficits Contribute to Motor Impairments in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 2675–2684. [Google Scholar] [CrossRef]
- Shafer, R.L.; Wang, Z.; Bartolotti, J.; Mosconi, M.W. Visual and somatosensory feedback mechanisms of precision manual motor control in autism spectrum disorder. J. Neurodev. Disord. 2021, 13, 32. [Google Scholar] [CrossRef]
- van der Fels, I.M.J.; te Wierike, S.C.M.; Hartman, E.; Elferink-Gemser, M.T.; Smith, J.; Visscher, C. The relationship between motor skills and cognitive skills in 4-16 year old typically developing children: A systematic review. J. Sci. Med. Sport 2015, 18, 697–703. [Google Scholar] [CrossRef]
- Domellöf, E.; Johansson, A.-M.; Farooqi, A.; Domellöf, M.; Rönnqvist, L. Relations among upper-limb movement organization and cognitive function at school age in children born preterm. J. Dev. Behav. Pediatr. 2013, 34, 344–352. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Flanagan, J.R. Motor prediction. Curr. Biol. 2001, 11, R729–R732. [Google Scholar] [CrossRef]
- Jeannerod, M.; Decety, J. Menal motor imagery: A window into the representational stages of action. Curr. Opin. Neurobiol. 1995, 5, 727–732. [Google Scholar] [CrossRef]
- Jeannerod, M. Motor Cognition: What Actions Tell the Self; Oxford University Press: Kings Lynn, UK, 2006. [Google Scholar] [CrossRef]
- Munzert, J.; Lorey, B.; Zentgraf, K. Cognitive motor processes: The role of motor imagery in the study of motor representations. Brain Res. Rev. 2009, 60, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Flanagan, J.R. Motor learning. Curr. Biol. 2010, 20, R467–R472. [Google Scholar] [CrossRef]
- Rosenbaum, D.A.; Chapman, K.M.; Weigelt, M.; Weiss, D.J.; van der Wel, R. Cognition, action, and object manipulation. Psychol. Bull. 2012, 138, 924–946. [Google Scholar] [CrossRef] [PubMed]
- von Hofsten, C. An action perspective on motor development. Trends Cogn. Sci. 2004, 8, 266–272. [Google Scholar] [CrossRef]
- von Hofsten, C.; Rosander, K. Perception-action in children with ASD. Front. Integr. Neurosci. 2012, 6, 1–6. [Google Scholar] [CrossRef]
- Cavallo, A.; Romeo, L.; Ansuini, C.; Battaglia, F.; Nobili, L.; Pontil, M.; Panzeri, S.; Becchio, C. Identifying the signature of prospective motor control in children with autism. Sci. Rep. 2021, 11, 3165. [Google Scholar] [CrossRef]
- Bäckström, A.; Johansson, A.-M.; Rudolfsson, T.; Rönnqvist, L.; von Hofsten, C.; Rosander, K.; Domellöf, E. Motor planning and movement execution during goal-directed sequential manual movements in 6-year-old children with autism spectrum disorder: A kinematic analysis. Res. Dev. Disabil. 2021, 115, 104014. [Google Scholar] [CrossRef]
- Trevarthen, C.; Delafield-Butt, J.T. Autism as a developmental disorder in intentional movement and affective engagement. Front. Integr. Neurosci. 2013, 7, 1–31. [Google Scholar] [CrossRef]
- Cannon, J.; O’Brien, A.M.; Bungert, L.; Sinha, P. Prediction in Autism Spectrum Disorder: A Systematic Review of Empirical Evidence. Autism Res. 2021, 14, 604–630. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M.; Prablanc, C. The timing of mentally represented actions. Behav. Brain Res. 1989, 34, 35–42. [Google Scholar] [CrossRef]
- de Lange, F.P.; Hagoort, P.; Toni, I. Neural topography and content of movement representations. J. Cogn. Neurosci. 2005, 17, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, S.; van der Kamp, J.; Steenbergen, B. Current insights in the development of childrens motor imagery ability. Front. Psychol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Sekiyama, K.; Kinoshita, T.; Soshi, T. Strong biomechanical constraints on young children’s mental imagery of hands. R. Soc. Open Sci. 2014, 1, 140118. [Google Scholar] [CrossRef]
- Conson, M.; Mazzarella, E.; Trojano, L. Developmental changes of the biomechanical effect in motor imagery. Exp. Brain Res. 2013, 226, 441–449. [Google Scholar] [CrossRef]
- Hirata, S.; Okuzumi, H.; Kitajima, Y.; Hosobuchi, T.; Nakai, A.; Kokubun, M. Relationship between Motor Skill Impairments and Motor Imagery Ability in Children with Autism Spectrum Disorders: A Pilot Study Using the Hand Rotation Task. Psychology 2015, 06, 752–759. [Google Scholar] [CrossRef]
- Chen, Y.T.; Tsou, K.S.; Chen, H.L.; Wong, C.C.; Fan, Y.T.; Wu, C.T. Functional but Inefficient Kinesthetic Motor Imagery in Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 784–795. [Google Scholar] [CrossRef]
- Conson, M.; Mazzarella, E.; Esposito, D.; Grossi, D.; Marino, N.; Massagli, A.; Frolli, A. ‘Put Myself Into Your Place’: Embodied Simulation and Perspective Taking in Autism Spectrum Disorders. Autism Res. 2015, 8, 454–466. [Google Scholar] [CrossRef]
- Conson, M.; Mazzarella, E.; Frolli, A.; Esposito, D.; Marino, N.; Trojano, L.; Massagli, A.; Gison, G.; Aprea, N.; Grossi, D. Motor Imagery in Asperger Syndrome: Testing Action Simulation by the Hand Laterality Task. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Conson, M.; Hamilton, A.; De Bellis, F.; Errico, D.; Improta, I.; Mazzarella, E.; Trojano, L.; Frolli, A. Body Constraints on Motor Simulation in Autism Spectrum Disorders. J Autism Dev Disord. 2016, 46, 1051–1060. [Google Scholar] [CrossRef]
- Wechsler. Wechsler Intelligence Scale for Children, 5th ed.; Pearson: San Antonio, TX, USA, 2014. [Google Scholar]
- Spruijt, S.; Jongsma, M.L.A.; van der Kamp, J.; Steenbergen, B. Predictive Models to Determine Imagery Strategies Employed by Children to Judge Hand Laterality. PLoS ONE 2015, 10, e0126568. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.O.; Cruz, T.K.F.; Fontes, P.L.B.; Batista, R.C.; Haase, V.G. Motor Imagery Development in Children: Changes in Speed and Accuracy With Increasing Age. Front. Pediatr. 2020, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- IBM CORP. IBM SPSS Statistics for Windows; IBM CORP: Armonk, NY, USA, 2020. [Google Scholar]
- The Jamovi Jamovi Project. Jamovi. (Version 2.25) [Computer Software]. 2021. Available online: https://www.jamovi.org (accessed on 10 September 2018).
- Gallucci, M. GAMLj: General Analyses for Linear Models. (Version 2.6.1). 2019. [Computer Software]. Available online: https://gamlj.github.io/ (accessed on 10 September 2018).

| ASD (N = 14) | TD (N = 17) | |||||
|---|---|---|---|---|---|---|
| Mean ± S.D. | Median | Range | Mean ± S.D. | Median | Range | |
| FSIQ | 82 ± 15.3 | 86 | 54–110 | 106 ± 9.4 | 104 | 93–126 |
| VeI | 83 ± 17.0 | 81 | 48–106 | 108 ± 7.0 | 106 | 100–127 |
| FI | 93 ± 16.0 | 94 | 72–126 | 102 ± 9.6 | 100 | 88–118 |
| WMI | 84 ± 15.8 | 88 | 65–115 | 103 ± 10.5 | 103 | 88–122 |
| Test | Factor | Contrast | GMD % | 95% CI | F | df | p |
|---|---|---|---|---|---|---|---|
| MI | Group | - | - | - | 3.90 | 1; 113.0 | 0.051 |
| ASD–TD | 17 | −0.1; 37 | - | - | 0.051 | ||
| Age level | - | - | - | 20.33 | 2; 108.5 | 0.0001 | |
| A1–A2 | 25 | 5; 48 | - | - | 0.008 | ||
| A1–A3 | 53 | 29; 81 | - | - | 0.0001 | ||
| A2–A3 | 23 | 7; 40 | - | - | 0.001 | ||
| Orientation | - | - | - | 9.42 | 2; 111.8 | 0.0001 | |
| 0°–medial | −9 | −32; 22 | - | - | 0.955 | ||
| 0°–180° | −40 | −56; −20 | - | - | 0.0001 | ||
| 0°–lateral | −35 | −52, −12 | - | - | 0.001 | ||
| medial–180° | −35 | −51; −12 | - | - | 0.001 | ||
| medial–lateral | −28 | −47; −4 | - | - | 0.020 | ||
| 180°–lateral | 9 | 19; 48 | - | - | 0.960 | ||
| VI | Group | - | - | - | 20.73 | 1; 121.33 | 0.0001 |
| ASD–TD | 34 | 18; 53 | - | - | 0.0001 | ||
| Age level | - | - | - | 27.48 | 2; 117.61 | 0.009 | |
| A1–A2 | 7 | 6; 23 | - | - | 0.521 | ||
| A1–A3 | 35 | 20; 52 | - | - | 0.0001 | ||
| A2–A3 | 26 | 14; 39 | - | - | 0.0001 | ||
| Orientation | - | - | - | 14.66 | 3; 121.76 | 0.0001 | |
| 0°–90° | −20 | −37, 1 | - | - | 0.014 | ||
| 0°–180° | −45 | −57; −30 | - | - | 0.0001 | ||
| 0°–270° | −21 | −38; 0,5 | - | - | 0.058 | ||
| 90°–180° | −31 | −46; −17 | - | - | 0.001 | ||
| 90°–270° | −1 | −22; 26 | - | - | 1.0 |
| Task | Group | Orient- Ation | n cor. (% cor.) | Mdn RT * | IQR RT * | M LnRT | 95% CI LnRT | n cor. (% cor.) | Mdn RT * | IQR RT * | M LnRT | 95% CI LnRT | n cor. (% cor.) | Mdn RT * | IQR RT * | M LnRT | 95% CI LnRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI | ASD | 0° | 48 (89) | 1940 | 1426 | 7.65 | 7.48–7.83 | 43 (90) | 1660 | 1012 | 7.58 | 7.38–7.77 | 53 (84) | 1346 | 778 | 7.21 | 7.07–7.35 |
| Medial | 42 (78) | 1816 | 1461 | 7.57 | 7.41–7.73 | 36 (75) | 2333 | 2012 | 7.8 | 7.59–8.01 | 52 (81) | 1383 | 873 | 7.28 | 7.12–7.43 | ||
| 180° | 34 (63) | 3626 | 3661 | 8.09 | 7.81–8.38 | 34 (71) | 3885 | 5363 | 8.2 | 7.91–8.49 | 44 (70) | 2431 | 1837 | 7.81 | 7.60–8.01 | ||
| Lateral | 33 (61) | 2780 | 4212 | 8.03 | 7.76–8.31 | 25 (52) | 3268 | 5443 | 8.05 | 7.70–8.41 | 42 (68) | 2163 | 1871 | 7.71 | 7.50–7.39 | ||
| Overall | 157 (73) | 2281 | 2603 | 7.8 | 7.69–7.92 | 138 (78) | 2365 | 2713 | 7.87 | 7.74–8.01 | 191 (76) | 1645 | 1434 | 7.48 | 7.38–7.57 | ||
| TD | 0° | 95 (93) | 1947 | 1322 | 7.55 | 7.45–7.66 | 98 (96) | 1342 | 998 | 7.29 | 7.19–7.39 | 101 (99) | 1075 | 554 | 7.05 | 6.97–7.13 | |
| Medial | 96 (94) | 1853 | 1466 | 7.65 | 7.53–7.77 | 92 (90) | 1441 | 890 | 7.58 | 7.28–7.48 | 100 (98) | 1304 | 655 | 7.21 | 7.17–7.36 | ||
| 180° | 94 (92) | 2491 | 2254 | 7.99 | 7.86–8.11 | 87 (85) | 2491 | 1733 | 7.8 | 7.69–7.90 | 91 (89) | 1904 | 1198 | 7.63 | 7.53–7.73 | ||
| Lateral | 87 (85) | 2311 | 3697 | 7.55 | 7.75–8.07 | 87 (85) | 1910 | 2686 | 7.73 | 7.57–7.89 | 99 (97) | 1681 | 1390 | 7.91 | 7.41–7.68 | ||
| Overall | 372 (91) | 2135 | 1833 | 7.77 | 7.71–7.84 | 364 (89) | 2274 | 1471 | 7.54 | 7.47–7.60 | 391 (96) | 1514 | 1041 | 7.37 | 7.31–7.42 | ||
| VI | ASD | 0° | 52 (72) | 1684 | 1244 | 7.52 | 7.35–7.70 | 48 (80) | 1607 | 1459 | 7.61 | 7.36–7.85 | 66 (92) | 1091 | 971 | 7.21 | 7.05–7.36 |
| 90° | 46 (64) | 2191 | 1627 | 7.77 | 7.59–7.96 | 43 (72) | 2265 | 1358 | 7.74 | 7.56–7.91 | 64 (86) | 1555 | 1217 | 7.46 | 7.31–7.61 | ||
| 180° | 39 (54) | 3007 | 4293 | 8.15 | 7.89–8.40 | 29 (48) | 2855 | 2010 | 8.02 | 7.75–8.29 | 49 (68) | 2455 | 1462 | 7.77 | 7.58–7.95 | ||
| 270° | 49 (68) | 2234 | 2676 | 7.81 | 7.62–8.00 | 47 (78) | 2372 | 1299 | 7.82 | 7.63–8.00 | 67 (89) | 1448 | 991 | 7.41 | 7.25–7.58 | ||
| Overall | 186 (35) | 2175 | 2129 | 7.79 | 7.69–7.90 | 167 (69) | 2274 | 1789 | 7.77 | 7.66–7.88 | 246 (84) | 1514 | 1495 | 7.44 | 7.36–7.52 | ||
| TD | 0° | 102 (0) | 1286 | 944 | 7.25 | 7.15–7.36 | 102 (100) | 1099 | 661 | 7.05 | 6.97–7.13 | 102 (100) | 1064 | 565 | 7.03 | 6.96–7.11 | |
| 90° | 100 (98) | 1509 | 1146 | 7.46 | 7.36–7.55 | 93 (91) | 1367 | 839 | 7.3 | 7.19–7.42 | 97 (94) | 1202 | 667 | 7.19 | 7.09–7.28 | ||
| 180° | 92 (90) | 2411 | 2059 | 7.86 | 7.76–7.97 | 88 (86) | 2110 | 1495 | 7.78 | 7.66–7.90 | 100 (98) | 1765 | 1162 | 7.6 | 7.50–7.70 | ||
| 270° | 100 (98) | 1585 | 1052 | 7.49 | 7.39–7.58 | 99 (97) | 1558 | 1225 | 7.41 | 7.30–7.51 | 99 (97) | 1341 | 646 | 7.29 | 7.17–7.41 | ||
| Overall | 394 (97) | 1704 | 1323 | 7.51 | 7.45–7.56 | 382 (94) | 1468 | 1105 | 7.37 | 7.31–7.43 | 398 (97) | 1325 | 781 | 7.28 | 7.22–7.33 |
| ASD | TD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lateral–Medial | 270°–90° | Lateral–Medial | 270°–90° | |||||||||
| GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | |
| A1 | 60 | 21, 111 | 0.001 | 4 | −19, 33 | 0.761 | 29 | 8, 55 | 0.005 | 3 | −13, 22 | 0.753 |
| A2 | 29 | −6, 76 | 0.118 | 8 | −16, 40 | 0.556 | 41 | 18, 69 | 0.0001 | 11 | −7, 32 | 0.243 |
| A3 | 55 | 21, 98 | 0.001 | −4 | −22, 18 | 0.667 | 32 | 12, 56 | 0.001 | 11 | −6, 31 | 0.225 |
| ASD | TD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medial—90° | Lateral—270° | Medial—90° | Lateral—270° | |||||||||
| GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | |
| A1 | −19 | −37, 5 | 0.115 | 25 | −5, 64 | 0.107 | 21 | 2, 44 | 0.027 | 53 | 28, 82 | 0.0001 |
| A2 | 6 | −19, 40 | 0.661 | 30 | −6, 71 | 0.123 | 8 | −10, 29 | 0.400 | 37 | 15, 64 | 0.001 |
| A3 | −17 | −35, −8 | 0.109 | 35 | 7, 71 | 0.012 | 8 | −19, 28 | 0.358 | 29 | 9, 53 | 0.003 |
| ASD-TD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI: Medial | MI: Lateral | VI: 90° | VI: 270° | |||||||||
| GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | GMD% | 95% CI | p | |
| A1 | −8 | −26, 15 | 0.448 | 13 | −12, 45 | 0.325 | 37 | 11, 70 | 0.004 | 38 | 12, 71 | 0.002 |
| A2 | 52 | 20, 93 | 0.001 | 39 | 5, 83 | 0.020 | 54 | 23, 93 | 0.0001 | 50 | 21, 86 | 0.0001 |
| A3 | 6 | −13, 31 | 0.554 | 20 | −3, 50 | 0.098 | 26 | 4, 52 | 0.018 | 11 | −8, 33 | 0.296 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, A.-M.; Rudolfsson, T.; Bäckström, A.; Rönnqvist, L.; von Hofsten, C.; Rosander, K.; Domellöf, E. Development of Motor Imagery in School-Aged Children with Autism Spectrum Disorder: A Longitudinal Study. Brain Sci. 2022, 12, 1307. https://doi.org/10.3390/brainsci12101307
Johansson A-M, Rudolfsson T, Bäckström A, Rönnqvist L, von Hofsten C, Rosander K, Domellöf E. Development of Motor Imagery in School-Aged Children with Autism Spectrum Disorder: A Longitudinal Study. Brain Sciences. 2022; 12(10):1307. https://doi.org/10.3390/brainsci12101307
Chicago/Turabian StyleJohansson, Anna-Maria, Thomas Rudolfsson, Anna Bäckström, Louise Rönnqvist, Claes von Hofsten, Kerstin Rosander, and Erik Domellöf. 2022. "Development of Motor Imagery in School-Aged Children with Autism Spectrum Disorder: A Longitudinal Study" Brain Sciences 12, no. 10: 1307. https://doi.org/10.3390/brainsci12101307
APA StyleJohansson, A.-M., Rudolfsson, T., Bäckström, A., Rönnqvist, L., von Hofsten, C., Rosander, K., & Domellöf, E. (2022). Development of Motor Imagery in School-Aged Children with Autism Spectrum Disorder: A Longitudinal Study. Brain Sciences, 12(10), 1307. https://doi.org/10.3390/brainsci12101307






