Differences in Subjective Memory Impairment, Depressive Symptoms, Sleep, and Physical Activity in African American and Asian American Elderly
Abstract
1. Introduction
2. Methods
2.1. Participants and Recruitment
2.2. Measures
2.3. Data Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Ethnic Differences in SMI
3.3. Sleep, Physical Activity, and Depressive Symptoms by Ethnic Groups
3.4. Association of SMI with Depressive Symptoms, Sleep, and Physical Activity by Ethnic Groups
3.5. Interaction between Ethnicity and Independent Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A Conceptual Framework for Research on Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Abdulrab, K.; Heun, R. Subjective Memory Impairment. A Review of Its Definitions Indicates the Need for a Comprehensive Set of Standardised and Validated Criteria. Eur. Psychiatry 2008, 23, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.C.; Whitfield, K.E.; Ayotte, B.J.; Gamaldo, A.A.; Edwards, C.L.; Allaire, J.C. Subjective Memory in Older African Americans. Exp. Aging Res. 2011, 37, 220–240. [Google Scholar] [CrossRef]
- Reisberg, B.; Prichep, L.; Mosconi, L.; John, E.R.; Glodzik-Sobanska, L.; Boksay, I.; Monteiro, I.; Torossian, C.; Vedvyas, A.; Ashraf, N.; et al. The Pre–Mild Cognitive Impairment, Subjective Cognitive Impairment Stage of Alzheimer’s Disease. Alzheimer’s Dement. 2008, 4, S98–S108. [Google Scholar] [CrossRef]
- Jacinto, A.F.; Brucki, S.M.D.; Porto, C.S.; de Arruda Martins, M.; Nitrini, R. Subjective Memory Complaints in the Elderly: A Sign of Cognitive Impairment? Clinics 2014, 69, 194–197. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M.; et al. Prediction of Dementia by Subjective Memory Impairment: Effects of Severity and Temporal Association with Cognitive Impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef]
- Reisberg, B.; Shulman, M.B.; Torossian, C.; Leng, L.; Zhu, W. Outcome over Seven Years of Healthy Adults with and without Subjective Cognitive Impairment. Alzheimer’s Dement. 2010, 6, 11–24. [Google Scholar] [CrossRef]
- Hessen, E.; Eckerström, M.; Nordlund, A.; Selseth Almdahl, I.; Stålhammar, J.; Bjerke, M.; Eckerström, C.; Göthlin, M.; Fladby, T.; Reinvang, I.; et al. Subjective Cognitive Impairment Is a Predominantly Benign Condition in Memory Clinic Patients Followed for 6 Years: The Gothenburg-Oslo MCI Study. Dement. Geriatr. Cogn. Dis. Extra 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Rönnlund, M.; Sundström, A.; Adolfsson, R.; Nilsson, L.-G. Subjective Memory Impairment in Older Adults Predicts Future Dementia Independent of Baseline Memory Performance: Evidence from the Betula Prospective Cohort Study. Alzheimer’s Dement. 2015, 11, 1385–1392. [Google Scholar] [CrossRef]
- Hao, L.; Xing, Y.; Li, X.; Mu, B.; Zhao, W.; Wang, G.; Wang, T.; Jia, J.; Han, Y. Risk Factors and Neuropsychological Assessments of Subjective Cognitive Decline (plus) in Chinese Memory Clinic. Front. Neurosci. 2019, 13, 846. [Google Scholar] [CrossRef]
- Weuve, J.; Barnes, L.L.; Mendes de Leon, C.F.; Rajan, K.B.; Beck, T.; Aggarwal, N.T.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Evans, D.A. Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology 2018, 29, 151. [Google Scholar] [CrossRef]
- Heyman, A.; Fillenbaum, G.; Prosnitz, B.; Raiford, K.; Burchett, B.; Clark, C. Estimated Prevalence of Dementia Among Elderly Black and White Community Residents. Arch. Neurol. 1991, 48, 594–598. [Google Scholar] [CrossRef]
- Zsembik, B.A.; Peek, M.K. Race Differences in Cognitive Functioning Among Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2001, 56, S266–S274. [Google Scholar] [CrossRef]
- Vásquez, E.; Botoseneanu, A.; Bennett, J.M.; Shaw, B.A. Racial/Ethnic Differences in Trajectories of Cognitive Function in Older Adults: Role of Education, Smoking, and Physical Activity. J. Aging Health 2016, 28, 1382–1402. [Google Scholar] [CrossRef]
- Yaffe, K.; Falvey, C.; Harris, T.B.; Newman, A.; Satterfield, S.; Koster, A.; Ayonayon, H.; Simonsick, E. Effect of Socioeconomic Disparities on Incidence of Dementia among Biracial Older Adults: Prospective Study. BMJ 2013, 347, f7051. [Google Scholar] [CrossRef] [PubMed]
- Zuelsdorff, M.; Gleason, C.E.; Kind, A.J.; Koscik, R.L.; Johnson, S.C.; Okonkwo, O.C. Lifetime Stressful Experiences, Racial Disparities, and Cognitive Performance: Findings from the Wisconsin Registry for Alzheimer’s Prevention (Wrap) Study. Alzheimer’s Dement. 2017, 13, P212. [Google Scholar] [CrossRef]
- Díaz-Venegas, C.; Downer, B.; Langa, K.M.; Wong, R. Racial and Ethnic Differences in Cognitive Function among Older Adults in the USA: Cognition of US Older Adults by Race/Ethnicity. Int. J. Geriatr. Psychiatry 2016, 31, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Rosenberger, K.J.; Kulshreshtha, A.; Ayonayon, H.N.; Yaffe, K.; Goldstein, F.C. Association of JNC-8 and SPRINT Systolic Blood Pressure Levels with Cognitive Function and Related Racial Disparity. JAMA Neurol. 2017, 74, 1199. [Google Scholar] [CrossRef]
- Howell, J.C.; Watts, K.D.; Parker, M.W.; Wu, J.; Kollhoff, A.; Wingo, T.S.; Dorbin, C.D.; Qiu, D.; Hu, W.T. Race Modifies the Relationship between Cognition and Alzheimer’s Disease Cerebrospinal Fluid Biomarkers. Alzheimer’s Res. Ther. 2017, 9, 88. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Use of Genetic Variation as Biomarkers for Mild Cognitive Impairment and Progression of Mild Cognitive Impairment to Dementia. J. Alzheimer’s Dis. 2010, 19, 229–251. [Google Scholar] [CrossRef]
- Fan, J.; Tao, W.; Li, X.; Li, H.; Zhang, J.; Wei, D.; Chen, Y.; Zhang, Z. The Contribution of Genetic Factors to Cognitive Impairment and Dementia: Apolipoprotein E Gene, Gene Interactions, and Polygenic Risk. Int. J. Mol. Sci. 2019, 20, 1177. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yu, G.; Wu, B. Self-Reported Cognitive Impairment across Racial/Ethnic Groups in the United States, National Health Interview Survey, 1997–2015. Prev. Chronic Dis. 2018, 15, 170338. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Siddarth, P.; Ercoli, L.M.; Merrill, D.A.; Torres-Gil, F.; Small, G.W. Modifiable Risk Factors for Alzheimer Disease and Subjective Memory Impairment across Age Groups. PLoS ONE 2014, 9, e98630. [Google Scholar] [CrossRef]
- Yates, J.A.; Clare, L.; Woods, R.T.; CFAS, M. Subjective Memory Complaints, Mood and MCI: A Follow-up Study. Aging Ment. Health 2017, 21, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Tobiansky, R.; Blizard, R.; Livingston, G.; Mann, A. The Gospel Oak Study Stage IV: The Clinical Relevance of Subjective Memory Impairment in Older People. Psychol. Med. 1995, 25, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Tsapanou, A.; Gu, Y.; O’Shea, D.M.; Yannakoulia, M.; Kosmidis, M.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Stern, Y.; Scarmeas, N. Sleep Quality and Duration in Relation to Memory in the Elderly: Initial Results from the Hellenic Longitudinal Investigation of Aging and Diet. Neurobiol. Learn. Mem. 2017, 141, 217–225. [Google Scholar] [CrossRef]
- Tsapanou, A.; Vlachos, G.S.; Cosentino, S.; Gu, Y.; Manly, J.J.; Brickman, A.M.; Schupf, N.; Zimmerman, M.E.; Yannakoulia, M.; Kosmidis, M.H.; et al. Sleep and Subjective Cognitive Decline in Cognitively Healthy Elderly. Results from Two Cohorts. J. Sleep Res. 2019, 28, e12759. [Google Scholar] [CrossRef]
- Bubbico, G.; Di Iorio, A.; Lauriola, M.; Sepede, G.; Salice, S.; Spina, E.; Brondi, G.; Esposito, R.; Perrucci, M.G.; Tartaro, A. Subjective Cognitive Decline and Nighttime Sleep Alterations, a Longitudinal Analysis. Front. Aging Neurosci. 2019, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Yoon, I.-Y.; Lee, S.D.; Kim, T.; Lee, C.S.; Han, J.W.; Kim, K.W.; Kim, C.-H. Subjective Memory Complaints in an Elderly Population with Poor Sleep Quality. Aging Ment. Health 2017, 21, 532–536. [Google Scholar] [CrossRef]
- Stocker, R.P.J.; Khan, H.; Henry, L.; Germain, A. Effects of Sleep Loss on Subjective Complaints and Objective Neurocognitive Performance as Measured by the Immediate Post-Concussion Assessment and Cognitive Testing. Arch. Clin. Neuropsychol. 2017, 32, 349–368. [Google Scholar] [CrossRef]
- Hokett, E.; Duarte, A. Age and Race-Related Differences in Sleep Discontinuity Linked to Associative Memory Performance and Its Neural Underpinnings. Front. Hum. Neurosci. 2019, 13, 176. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep Quality and Preclinical Alzheimer Disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- You, J.C.; Jones, E.; Cross, D.E.; Lyon, A.C.; Kang, H.; Newberg, A.B.; Lippa, C.F. Association of β-Amyloid Burden with Sleep Dysfunction and Cognitive Impairment in Elderly Individuals with Cognitive Disorders. JAMA Netw. Open 2019, 2, e1913383. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.H.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C.; Rocca, W.A. Physical Exercise, Aging, and Mild Cognitive Impairment: A Population-Based Study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Silber, T.C.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.H.; Pankratz, V.S.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C. Computer Activities, Physical Exercise, Aging, and Mild Cognitive Impairment: A Population-Based Study. Mayo Clin. Proc. 2012, 87, 437–442. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.; Kurz, A.F. Physical Activity and Mild Cognitive Impairment and Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 352–358. [Google Scholar] [CrossRef]
- Gallaway, P.J.; Miyake, H.; Buchowski, M.S.; Shimada, M.; Yoshitake, Y.; Kim, A.S.; Hongu, N. Physical Activity: A Viable Way to Reduce the Risks of Mild Cognitive Impairment, Alzheimer’s Disease, and Vascular Dementia in Older Adults. Brain Sci. 2017, 7, 22. [Google Scholar] [CrossRef]
- Balsamo, S.; Willardson, J.M.; de Santana Frederico, S.; Prestes, J.; Balsamo, D.C.; da Dahan, C.N.; dos Santos-Neto, L.; Nobrega, O.T. Effectiveness of Exercise on Cognitive Impairment and Alzheimer’s Disease. Int. J. Gen. Med. 2013, 6, 387–391. [Google Scholar] [CrossRef]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise Is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann. Intern. Med. 2006, 144, 73. [Google Scholar] [CrossRef]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical Activity, Diet, and Risk of Alzheimer Disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef]
- McEwen, S.C.; Siddarth, P.; Abedelsater, B.; Kim, Y.; Mui, W.; Wu, P.; Emerson, N.D.; Lee, J.; Greenberg, S.; Shelton, T.; et al. Simultaneous Aerobic Exercise and Memory Training Program in Older Adults with Subjective Memory Impairments. J. Alzheimer’s Dis. 2018, 62, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A. Physical Activity, Exercise, Depression and Anxiety Disorders. J. Neural. Transm. 2008, 116, 777. [Google Scholar] [CrossRef] [PubMed]
- Camacho, T.C.; Roberts, R.E.; Lazarus, N.B.; Kaplan, G.A.; Cohen, R.D. Physical Activity and Depression: Evidence from the Alameda County Study. Am. J. Epidemiol. 1991, 134, 220–231. [Google Scholar] [CrossRef]
- Strawbridge, W.J.; Deleger, S.; Roberts, R.E.; Kaplan, G.A. Physical Activity Reduces the Risk of Subsequent Depression for Older Adults. Am. J. Epidemiol. 2002, 156, 328–334. [Google Scholar] [CrossRef]
- Lee, M.; Bhimla, A.; Lu, W.; Ma, G.X. Correlates of Mental Health Treatment Receipt among Asian Americans with Perceived Mental Health Problems. J. Behav. Health Serv. Res. 2021, 48, 199–212. [Google Scholar] [CrossRef]
- Zhu, L. Depression Risks and Correlates among Different Generations of Chinese Americans: The Effects of Relationships with Friends and Relatives. Soc. Sci. 2017, 6, 56. [Google Scholar] [CrossRef]
- Lee, M.; Lu, W.; Mann-Barnes, T.; Nam, J.-H.; Nelson, J.; Ma, G.X. Mental Health Screening Needs and Preference in Treatment Types and Providers in African American and Asian American Older Adults. Brain Sci. 2021, 11, 597. [Google Scholar] [CrossRef]
- Kalibatseva, Z.; Leong, F.T.L. Depression among Asian Americans: Review and Recommendations. Available online: https://www.hindawi.com/journals/drt/2011/320902/ (accessed on 13 January 2020).
- Leong, F.T.L.; Lau, A.S.L. Barriers to Providing Effective Mental Health Services to Asian Americans. Ment. Health Serv. Res. 2001, 3, 201–214. [Google Scholar] [CrossRef]
- Kung, W.W. Cultural and Practical Barriers to Seeking Mental Health Treatment for Chinese Americans. J. Community Psychol. 2004, 32, 27–43. [Google Scholar] [CrossRef]
- Zhu, L. Complementary and Alternative Medical Service Use for Mental Health Problems among Chinese Americans: The Roles of Acculturation-Related Factors. Soc. Ment. Health 2019, 9, 366–387. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Do, D.P. Racial Differences in Self-Reports of Sleep Duration in a Population-Based Study. Sleep 2007, 30, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.P.; Grandner, M.A.; Xie, D.; Branas, C.C.; Gooneratne, N. “Sleep Disparity” in the Population: Poor Sleep Quality Is Strongly Associated with Poverty and Ethnicity. BMC Public Health 2010, 10, 475. [Google Scholar] [CrossRef]
- Grandner, M.A.; Petrov, M.E.R.; Rattanaumpawan, P.; Jackson, N.; Platt, A.; Patel, N.P. Sleep Symptoms, Race/Ethnicity, and Socioeconomic Position. J. Clin. Sleep Med. 2013, 9, 897–905. [Google Scholar] [CrossRef]
- Krueger, P.M.; Friedman, E.M. Sleep Duration in the United States: A Cross-Sectional Population-Based Study. Am. J. Epidemiol. 2009, 169, 1052–1063. [Google Scholar] [CrossRef]
- Gamaldo, A.A.; Wright, R.S.; Aiken-Morgan, A.T.; Allaire, J.C.; Thorpe, R.J.; Whitfield, K.E. The Association between Subjective Memory Complaints and Sleep within Older African American Adults. J. Gerontol. Ser. B 2019, 74, 202–211. [Google Scholar] [CrossRef]
- Marshall, S.J.; Jones, D.A.; Ainsworth, B.E.; Reis, J.P.; Levy, S.S.; Macera, C.A. Race/Ethnicity, Social Class, and Leisure-Time Physical Inactivity. Med. Sci. Sports Exerc. 2007, 39, 44. [Google Scholar] [CrossRef]
- Powell, L.M.; Slater, S.; Chaloupka, F.J.; Harper, D. Availability of Physical Activity–Related Facilities and Neighborhood Demographic and Socioeconomic Characteristics: A National Study. Am. J. Public Health 2006, 96, 1676–1680. [Google Scholar] [CrossRef]
- Wilson-Frederick, S.M.; Thorpe, R.J.; Bell, C.N.; Bleich, S.N.; Ford, J.G.; LaVeist, T.A. Examination of Race Disparities in Physical Inactivity among Adults of Similar Social Context. Ethn. Dis. 2014, 24, 363–369. [Google Scholar]
- Boslaugh, S.E.; Luke, D.A.; Brownson, R.C.; Naleid, K.S.; Kreuter, M.W. Perceptions of Neighborhood Environment for Physical Activity: Is It “Who You Are” or “Where You Live?”. J. Urban Health 2004, 81, 671–681. [Google Scholar] [CrossRef][Green Version]
- Lui, P.P.; Rollock, D. Acculturation and Psychosocial Adjustment among Southeast Asian and Chinese Immigrants: The Effects of Domain-Specific Goals. Asian Am. J. Psychol. 2012, 3, 79–90. [Google Scholar] [CrossRef]
- Royle, J.; Lincoln, N.B. The Everyday Memory Questionnaire-Revised: Development of a 13-Item Scale. Disabil. Rehabil. 2008, 30, 114–121. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of Sleep Duration in Middle and Old Age with Incidence of Dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Anderson, N.B.; Bulatao, R.A.; Cohen, B.; National Research Council (U.S.) (Eds.) Critical Perspectives on Racial and Ethnic Differences in Health in Late Life; National Academies Press: Washington, DC, USA, 2004; ISBN 978-0-309-09211-1. [Google Scholar]
- McDougall, G.J.J.; Vaughan, P.W.; Acee, T.W.; Becker, H. Memory Performance and Mild Cognitive Impairment in Black and White Community Elders. Ethn. Dis. 2007, 17, 381–388. [Google Scholar] [PubMed]
- Sumner, J.A.; Hagan, K.; Grodstein, F.; Roberts, A.L.; Harel, B.; Koenen, K.C. Posttraumatic Stress Disorder Symptoms and Cognitive Function in a Large Cohort of Middle-Aged Women. Depress. Anxiety 2017, 34, 356–366. [Google Scholar] [CrossRef]
- Clouston, S.A.P.; Pietrzak, R.H.; Kotov, R.; Richards, M.; Spiro, A.; Scott, S.B.; Deri, Y.; Mukherjee, S.; Stewart, C.; Bromet, E.J.; et al. Traumatic Exposures, Posttraumatic Stress Disorder, and Cognitive Functioning in World Trade Center Responders. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 593–602. [Google Scholar] [CrossRef]
- Wolf, E.J.; Morrison, F.G. Traumatic Stress and Accelerated Cellular Aging: From Epigenetics to Cardiometabolic Disease. Curr. Psychiatry Rep. 2017, 19, 75. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective Memory Complaints in Elders: Depression, Anxiety, or Cognitive Decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.K.; Troyer, A.K.; Maione, A.M.; Murphy, K.J. The Impact of Memory Change on Daily Life in Normal Aging and Mild Cognitive Impairment. Gerontologist 2016, 56, 877–885. [Google Scholar] [CrossRef]
- Hill, N.L.; Mogle, J.; Wion, R.; Munoz, E.; DePasquale, N.; Yevchak, A.M.; Parisi, J.M. Subjective Cognitive Impairment and Affective Symptoms: A Systematic Review. Gerontologist 2016, 56, e109–e127. [Google Scholar] [CrossRef]
- Montejo, P.; Montenegro, M.; Fernández, M.A.; Maestú, F. Subjective Memory Complaints in the Elderly: Prevalence and Influence of Temporal Orientation, Depression and Quality of Life in a Population-Based Study in the City of Madrid. Aging Ment. Health 2011, 15, 85–96. [Google Scholar] [CrossRef]
- Weaver Cargin, J.; Collie, A.; Masters, C.; Maruff, P. The Nature of Cognitive Complaints in Healthy Older Adults with and without Objective Memory Decline. J. Clin. Exp. Neuropsychol. 2008, 30, 245–257. [Google Scholar] [CrossRef]
- Burke, D.M.; Shafto, M.A. Aging and Language Production. Curr. Dir. Psychol. Sci. 2004, 13, 21–24. [Google Scholar] [CrossRef]
- Potter, G.G.; Hartman, M.; Ward, T. Perceived Stress and Everyday Memory Complaints among Older Adult Women. Anxiety Stress Coping 2009, 22, 475–481. [Google Scholar] [CrossRef]
- Trouton, A.; Stewart, R.; Prince, M. Does Social Activity Influence the Accuracy of Subjective Memory Deficit? Findings from a British Community Survey: Social Activity and Subjective Memory Deficit. J. Am. Geriatr. Soc. 2006, 54, 1108–1113. [Google Scholar] [CrossRef]
- Reid, L.M.; MacLullich, A.M.J. Subjective Memory Complaints and Cognitive Impairment in Older People. Dement. Geriatr. Cogn. Disord. 2006, 22, 471–485. [Google Scholar] [CrossRef]
- Rapp, M.A.; Schnaider-Beeri, M.; Grossman, H.T.; Sano, M.; Perl, D.P.; Purohit, D.P.; Gorman, J.M.; Haroutunian, V. Increased Hippocampal Plaques and Tangles in Patients with Alzheimer Disease with a Lifetime History of Major Depression. Arch. Gen. Psychiatry 2006, 63, 161. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, A.; Westman, E.; Lebedev, A.V.; Li, X.; Winblad, B.; Simmons, A.; Wahlund, L.-O.; Aarsland, D. Structural Brain Changes Associated with Depressive Symptoms in the Elderly with Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 930–935. [Google Scholar] [CrossRef]
- Missinne, S.; Bracke, P. Depressive Symptoms among Immigrants and Ethnic Minorities: A Population Based Study in 23 European Countries. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 97–109. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J. Memory Self-Efficacy and Memory Performance among Black and White Elders. Nurs. Res. 2004, 53, 323–331. [Google Scholar] [CrossRef]
- Whitfield, K.E.; Fillenbaum, G.G.; Pieper, C.; Albert, M.S.; Berkman, L.F.; Blazer, D.G.; Rowe, J.W.; Seeman, T. The Effect of Race and Health-Related Factors on Naming and Memory: The MacArthur Studies of Successful Aging. J. Aging Health 2000, 12, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; Jones, K.; Savage, C.R.; Berkman, L.; Seeman, T.; Blazer, D.; Rowe, J.W. Predictors of Cognitive Change in Older Persons: MacArthur Studies of Successful Aging. Psychol. Aging 1995, 10, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Albert, M.S.; Mohs, R.; Sun, K.; Berkman, L.F. Cognitive Performance in a High-Functioning Community-Dwelling Elderly Population. J. Gerontol. 1993, 48, M146–M151. [Google Scholar] [CrossRef]
- Blazer, D.G.; Hays, J.C.; Fillenbaum, G.G.; Gold, D.T. Memory Complaint as a Predictor of Cognitive Decline: A Comparison of African American and White Elders. J. Aging Health 1997, 9, 171–184. [Google Scholar] [CrossRef] [PubMed]
- John, A.; James, S.-N.; Patel, U.; Rusted, J.; Richards, M.; Gaysina, D. Longitudinal Associations of Affective Symptoms with Mid-Life Cognitive Function: Evidence from a British Birth Cohort. Br. J. Psychiatry 2019, 215, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Glymour, M.M.; Manly, J.J. Lifecourse Social Conditions and Racial and Ethnic Patterns of Cognitive Aging. Neuropsychol. Rev. 2008, 18, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Wang, J. Disparities Among Older Adults in Measures of Cognitive Function by Race or Ethnicity. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005, 60, P242–P250. [Google Scholar] [CrossRef]
- Brewster, P.W.H.; Melrose, R.J.; Marquine, M.J.; Johnson, J.K.; Napoles, A.; MacKay-Brandt, A.; Farias, S.; Reed, B.; Mungas, D. Life Experience and Demographic Influences on Cognitive Function in Older Adults. Neuropsychology 2014, 28, 846–858. [Google Scholar] [CrossRef]


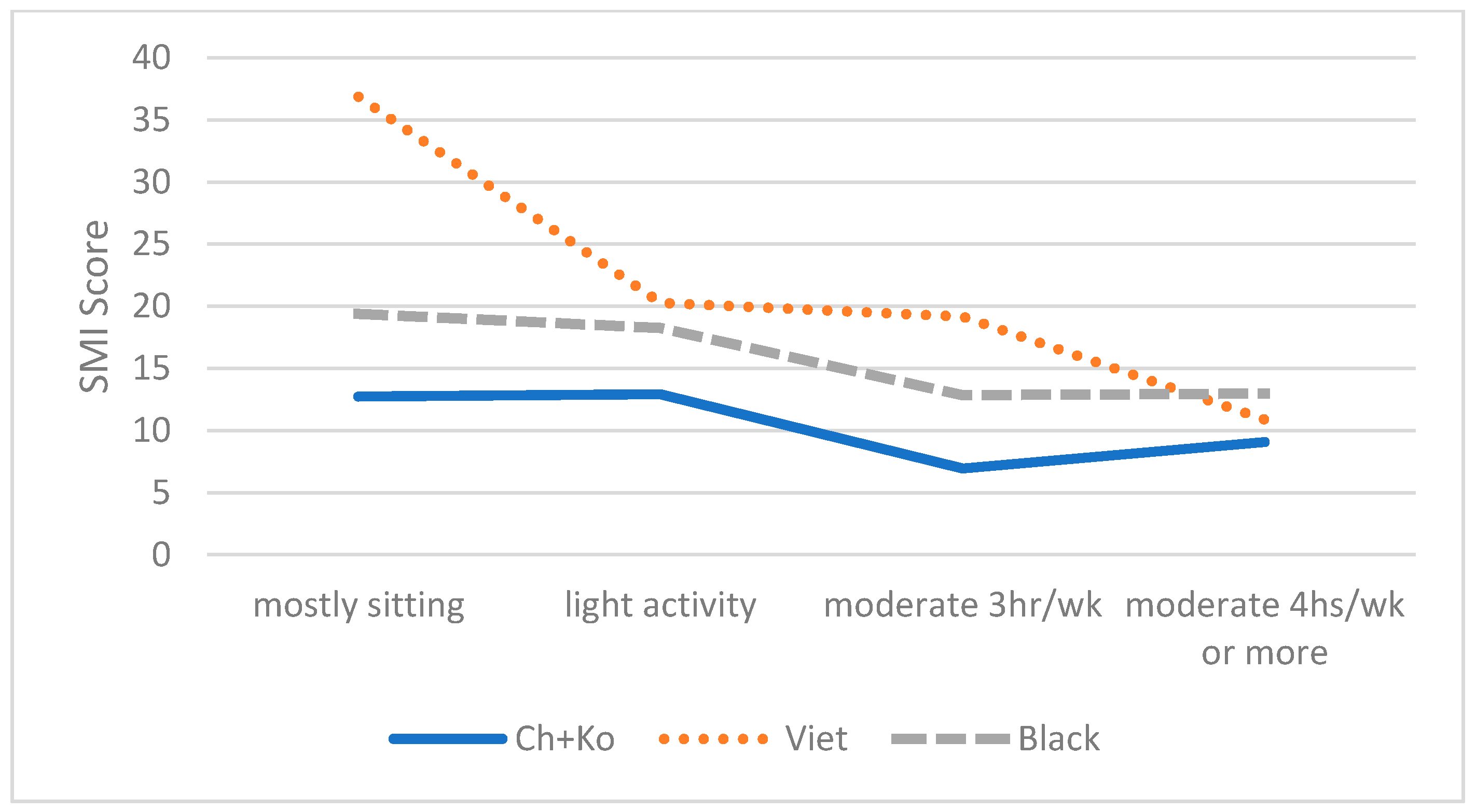
| Total n = 243 | African American (n = 93) | East Asian (Chinese + Korean) (n = 85) | Vietnamese (n = 65) | χ2, F | |
|---|---|---|---|---|---|
| M (SD), n (%) | M (SD), n (%) | M (SD), n (%) | M (SD), n (%) | ||
| DEMOGRAPHICS | |||||
| Age | 67.6 (9.40) | 68.4 (9.67) | 66.5 (9.70) | 67.9 (8.59) | 0.96 |
| Gender | 6.03 * | ||||
| Male | 86 (36.3%) | 25 (28.4%) | 30 (35.7%) | 31 (47.7%) | |
| Female | 151 (63.7%) | 63 (71.6%) | 54 (64.3%) | 34 (52.3%) | |
| Marital Status | 85.32 *** | ||||
| Currently single | 115 (47.5%) | 78 (84.8%) | 16 (18.8%) | 21 (32.3%) | |
| Married | 127 (52.5%) | 14 (15.2%) | 69 (81.2%) | 44 (67.7%) | |
| Education | 4.34 | ||||
| High school or under | 145 (61.4%) | 65 (71.4%) | 42 (50.6%) | 42 (67.7%) | |
| College or over | 91 (38.6%) | 26 (28.6%) | 41 (49.4%) | 20 (32.3%) | |
| Family Income | 5.23 | ||||
| Less than $40,000 | 180 (81.8%) | 66 (82.5%) | 60 (75.0%) | 54 (90.0%) | |
| $40,000 or higher | 26 (18.2%) | 8 (17.5%) | 20 (25.0%) | 6 (10.0%) | |
| Chronic Disease (yes) | 145 (59.7%) | 61 (65.6%) | 36 (42.4%) | 48 (73.8%) | |
| SMI | |||||
| Sx Presence (yes) | 154 (72.0%) | 57 (78.1%) | 40 (50.6%) | 57 (91.1%) | 31.43 *** |
| Total Score | 15.46 (11.65) | 15.84 (10.81) | 10.70 (9.83) | 21.0 (12.49) | 15.35 *** |
| Dep Total Scores | 5.05 (5.96) | 4.99 (6.01) | 1.45 (0.72) | 6.29 (6.57) | 2.31 |
| Depression Levels | 5.20 | ||||
| Minimal (0–4) | 140 (61.9%) | 56 (62.9%) | 51 (68.0%) | 33 (53.2%) | |
| Mild (5–9) | 47 (20.8%) | 15 (16.9%) | 14 (18.7%) | 18 (29.0%) | |
| Moderate/severe (10+) | 39 (17.3%) | 18 (20.2%) | 10 (13.3%) | 11 (17.7%) | |
| Avg Hours of Sleep | 6.42 (2.21) | 4.99 (6.02) | 6.84 (1.25) | 5.86 (1.42) | 8.14 *** |
| Sleep Duration | 49.90 ** | ||||
| ≤6 h sleep | 57 (25.6%) | 29 (36.7%) | 10 (12.5%) | 18 (28.1%) | |
| ≥6 h sleep | 166 (74.4%) | 50 (63.3%) | 70 (87.5%) | 46 (71.9%) | |
| Sleep troubles (yes) | 123 (51.9%) | 37 (40.7%) | 35 (42.2%) | 51 (81.0%) | 16.35 *** |
| Physical Activity Level | 5.72 | ||||
| Mostly sedentary | 36 (15.9%) | 12 (14.5%) | 13 (16.7%) | 11 (16.9%) | |
| Light activity/housework | 76 (33.6%) | 30 (36.1%) | 29 (37.2%) | 17 (26.2%) | |
| Moderate act. for 3hrs/wk. | 68 (30.1%) | 21 (25.3%) | 21 (26.9%) | 26 (40.0%) | |
| Moderate act. for 4hrs/wk., active sports | 46 (20.4%) | 20 (24.1%) | 15 (19.2%) | 11 (16.9%) |
| Variables | Total | African American | East Asian | Vietnamese |
|---|---|---|---|---|
| Ethnicity | ||||
| East Asian | −0.202 | |||
| Vietnamese | 0.165 | |||
| Age | 0.055 | 0.117 | 0.149 | −0.017 |
| Gender | 0.061 | 0.017 | 0.028 | 0.08 |
| Marital Status | 0.047 | −0.074 | 0.041 | −0.012 |
| Education | −0.081 | −0.21 | −0.145 | −0.102 |
| Family Income | 0.065 | −0.005 | 0.181 | −0.022 |
| Chronic Dis. (yes) | −0.065 | −0.066 | −0.001 | −0.139 |
| Sleep hours | −0.189 ** | −0.329* | −0.220 | −0.015 |
| Trouble in sleep | −0.202 ** | −0.316 | 0.074 | −0.317 * |
| Physical Activity | −0.182 ** | −0.091 | −0.240 | −0.248 * |
| Depressive Sx | 0.503 *** | 0.385 * | 0.361 * | 0.651 *** |
| Adjusted R2 | 0.44 | 0.16 | 0.23 | 0.6 |
| Sources | SS | df | MS | F | η2 |
|---|---|---|---|---|---|
| Ethnicity × Dep symptoms | 2386.86 | 4 | 596.71 | 7.81 *** | 0.15 |
| error | 13,291.51 | 204 | 76.39 | ||
| Ethnicity × Sleep hours | 355.79 | 2 | 177.89 | 1.74 | 0.02 |
| error | 17,924.08 | 205 | 102.42 | ||
| Ethnicity × Sleep trouble | 436.77 | 2 | 218.39 | 1.87 | 0.02 |
| error | 21,733.85 | 216 | 116.85 | ||
| Ethnicity × Physical activity | 1882.83 | 6 | 313.68 | 3.29 ** | 0.11 |
| error | 16,287.83 | 201 | 95.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Nam, J.-H.; Yi, E.; Bhimla, A.; Nelson, J.; Ma, G.X. Differences in Subjective Memory Impairment, Depressive Symptoms, Sleep, and Physical Activity in African American and Asian American Elderly. Brain Sci. 2021, 11, 1155. https://doi.org/10.3390/brainsci11091155
Lee M, Nam J-H, Yi E, Bhimla A, Nelson J, Ma GX. Differences in Subjective Memory Impairment, Depressive Symptoms, Sleep, and Physical Activity in African American and Asian American Elderly. Brain Sciences. 2021; 11(9):1155. https://doi.org/10.3390/brainsci11091155
Chicago/Turabian StyleLee, Minsun, Jin-Hyeok Nam, Elizabeth Yi, Aisha Bhimla, Julie Nelson, and Grace X. Ma. 2021. "Differences in Subjective Memory Impairment, Depressive Symptoms, Sleep, and Physical Activity in African American and Asian American Elderly" Brain Sciences 11, no. 9: 1155. https://doi.org/10.3390/brainsci11091155
APA StyleLee, M., Nam, J.-H., Yi, E., Bhimla, A., Nelson, J., & Ma, G. X. (2021). Differences in Subjective Memory Impairment, Depressive Symptoms, Sleep, and Physical Activity in African American and Asian American Elderly. Brain Sciences, 11(9), 1155. https://doi.org/10.3390/brainsci11091155





