Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
2.3. Hypoxia-Reoxygenation Procedure
2.4. Incubation of Polyphenol Compounds
2.5. Analytical Techniques
2.5.1. Lactate Dehydrogenase (LDH) Efflux
2.5.2. Lipid Peroxidation
2.5.3. 3-Nitrotyrosine
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED Investigators. Benefits of the Mediterranean diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Lasa, A.; Miranda, J.; Bulló, M.; Casas, R.; Salas-Salvadó, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutiérrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [Green Version]
- De La Cruz, J.P.; Del Río, S.; Arrebola, M.M.; López-Villodres, J.A.; Jebrouni, N.; González-Correa, J.A. Effect of virgin olive oil plus acetylsalicylic acid on brain slices damage after hypoxia-reoxygenation in rats with type 1-like diabetes mellitus. Neurosci. Lett. 2010, 471, 89–93. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Muñoz-Marín, J.; Arrebola, M.M.; Guerrero, A.; Narbona, F.; López-Villodres, J.A.; De La Cruz, J.P. Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia-reoxygenation. Lipids 2007, 42, 921–929. [Google Scholar] [CrossRef]
- Garcia-Martinez, O.; Ruiz, C.; Gutierrez-Ibanez, A.; Illescas-Montes, R.; Melguizo-Rodriguez, L. Benefits of olive oil phenolic compounds in disease prevention. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 333–340. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, S.; De La Cruz, J.P.; López-Villodres, J.A.; Muñoz-Marín, J.; Guerrero, A.; Reyes, J.J.; Labajos, M.T.; González-Correa, J.A. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J. Nutr. Biochem. 2013, 24, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Borges, T.H.; Serna, A.; López, L.C.; Lara, L.; Nieto, R.; Seiquer, I. Composition and antioxidant properties of Spanish extra virgin olive oil regarding cultivar, harvest year and crop stage. Antioxidants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil by-product alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in large quantities of highly purified hidroxitirosol from liquid-solid waste of two-phase olive oil processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [Green Version]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolics compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Guerrero, A.; De la Cruz, J.P.; Muñoz-Marín, J.; López-Villodres, J.A.; Madrona, A.; Espartero, J.L.; González-Correa, J.A. Neuroprotective effect of alkyl hydroxytyrosyl ethers in rat brain slices subjected to a hypoxia-reoxygenation model. Food Chem. 2012, 134, 2176–2183. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Arrebola, M.M.; Cansino, A.L.; Muñoz-Marín, J.; Ruiz-Villafranca, D.; Guerrero, A.; Sánchez de la Cuesta, F.; De La Cruz, J.P. Effects of hypoxia reoxygenation in brain slices from rats with type 1-like diabetes mellitus. Diabetes Metab. Res. Rev. 2006, 22, 390–400. [Google Scholar] [CrossRef]
- Reyes, J.J.; Villanueva, B.; López-Villodres, J.A.; De La Cruz, J.P.; Romero, L.; Rodríguez-Pérez, M.D.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Neuroprotective effect of hydroxytyrosol in experimental diabetes mellitus. J. Agric. Food Chem. 2017, 65, 4378–4383. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and cytoprotection: A projection for clinical interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Pan, S.; Liu, L.; Pan, H.; Ma, Y.; Wang, D.; Kang, K.; Wang, J.; Sun, B.; Sun, X.; Jiang, H. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol. Nutr. Food Res. 2013, 57, 1218–1227. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Beccari, T.; Michelini, S. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; López-Villodres, J.A.; Reyes, J.J.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; Reyes, J.J.; Benitez-Guerrero, A.; Espartero, J.L.; González-Correa, J.A. Differences in the neuroprotective effect of orally administered virgin olive oil (Olea europaea) polyphenols tyrosol and hydroxytyrosol in rats. J. Agric. Food Chem. 2015, 63, 5957–5963. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Angeloni, C.; Barbalace, M.C.; Lacerenza, S.; Ciregia, F.; Ronci, M.; Urbani, A.; Manera, C.; Digiacomo, M.; Macchia, M.; et al. A proteomic approach to uncover neuroprotective mechanisms of oleocanthal against oxidative stress. Int. J. Mol. Sci. 2018, 19, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, R.; Reutzel, M.; Dilberger, B.; Hein, H.; Zotzel, J.; Marx, S.; Tretzel, J.; Sarafeddinov, A.; Fuchs, C.; Eckert, G.P. Purified oleocanthal and ligstroside protect against mitochondrial dysfunction in models of early Alzheimer’s disease and brain ageing. Exp. Neurol. 2020, 328, 113248. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Robledo, P.; Tanner, J.A.; Boronat, A.; Pérez-Mañá, C.; Oliver Chen, C.Y.; Tyndale, R.F.; de la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017, 217, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Prior, M.Á.; Fatuarte, J.C.P.; Oria, A.B.; Viera-Alcaide, I.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. New liquid source of antioxidant phenolic compounds in the olive oil industry: Alperujo water. Foods 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; de Roos, B.; Duthie, G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015, 54, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; De La Cruz, J.P.; Muñoz-Marin, J.; Guerrero, A.; Reyes, J.J.; González-Correa, J.A. Cytoprotective effect of nonsteroidal antiinflammatory drugs in rat brain slices subjected to reoxygenation after oxygen-glucose deprivation. Eur. J. Pharm. Sci. 2012, 45, 624–631. [Google Scholar] [CrossRef] [PubMed]



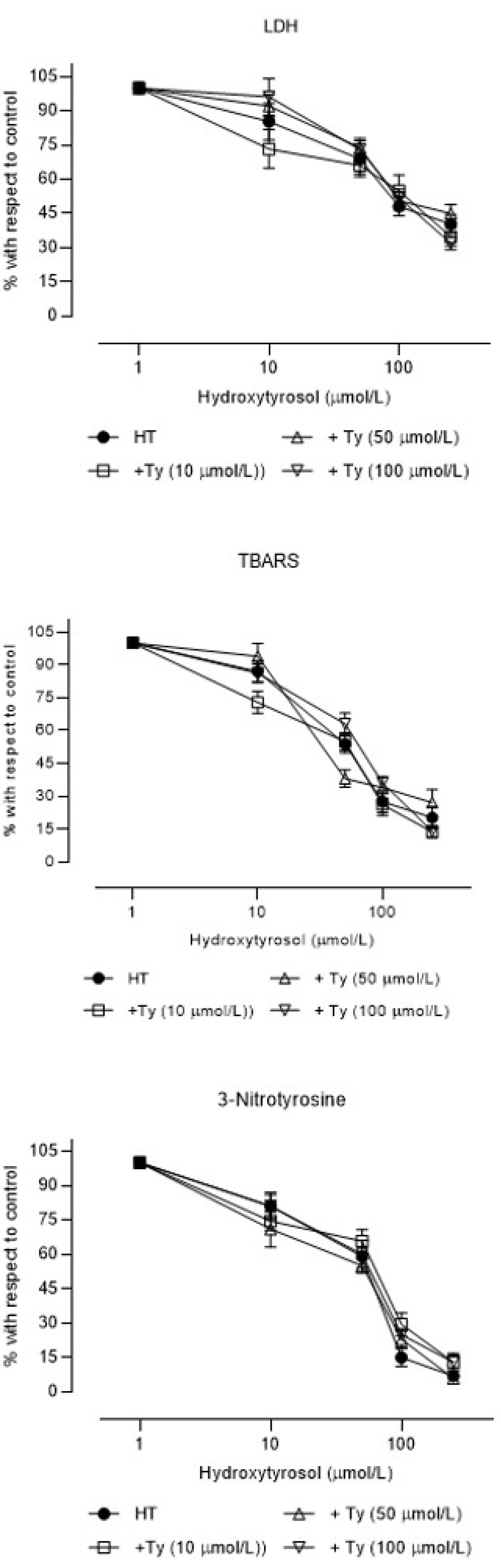
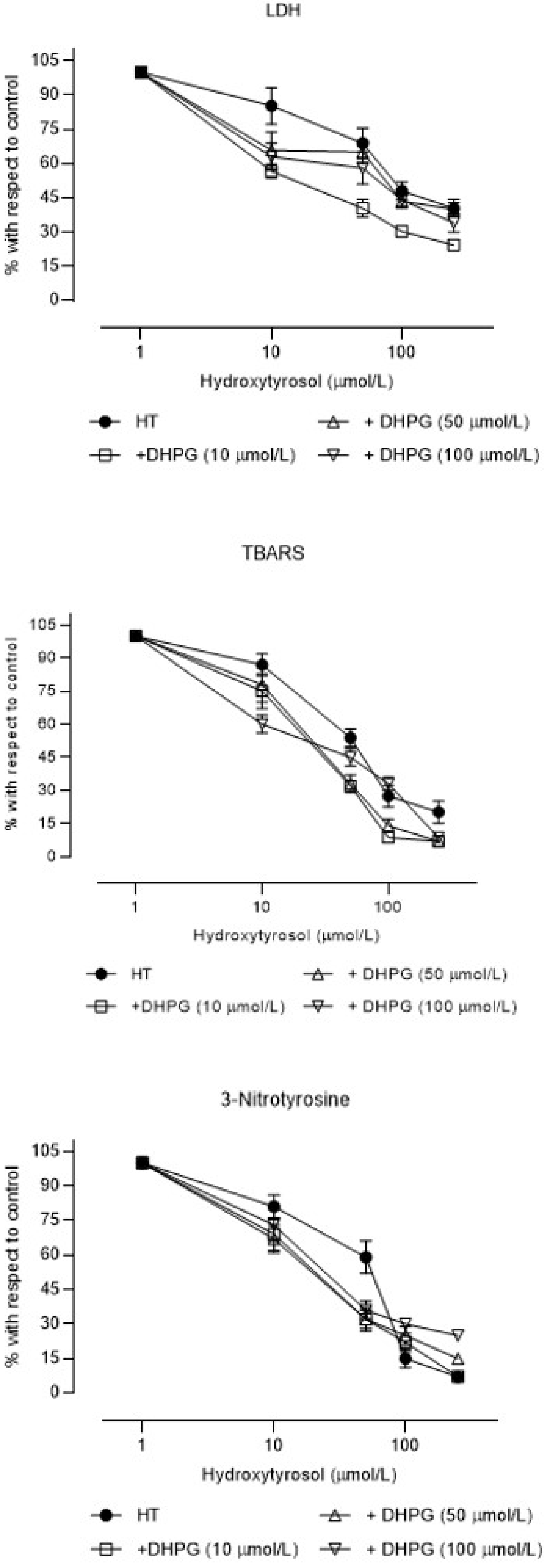
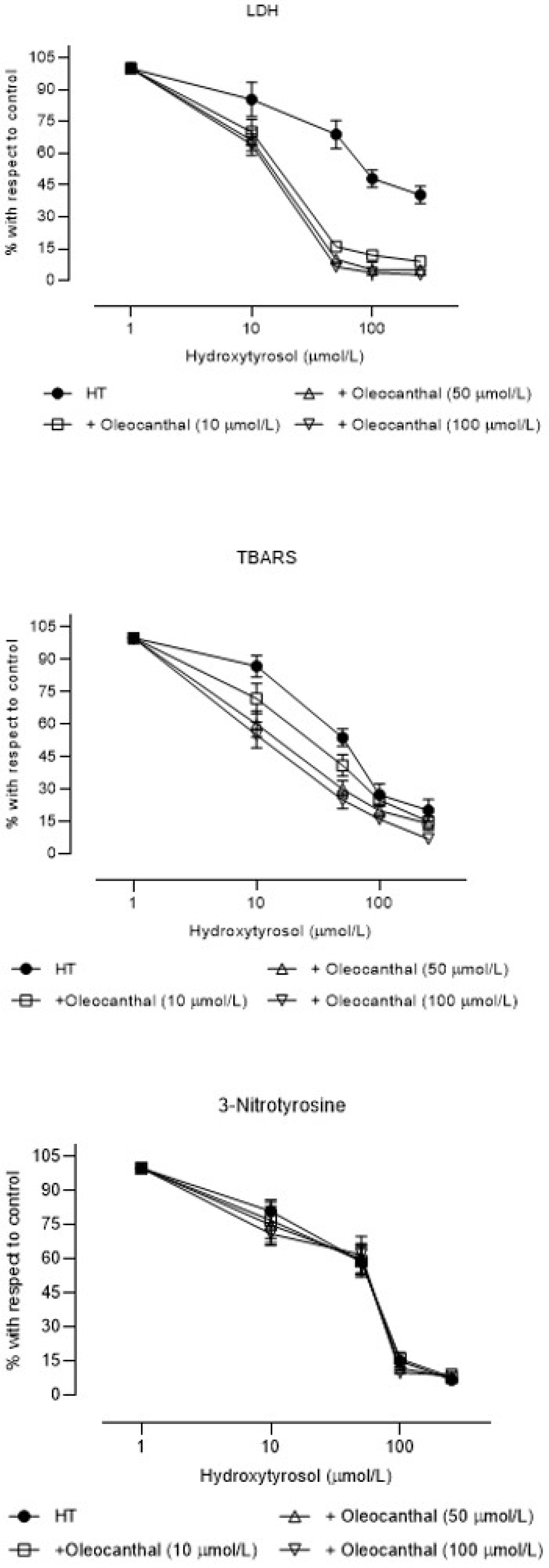
| - | HT | Ty | DHPG | OLC |
|---|---|---|---|---|
| EVOO—LP | - | - | - | - |
| Range | 1.0–5.0 | - | 0.0–0.5 | 10–15 |
| (incubated) | 2.5 | 0.25 | 0.25 | 20 |
| EVOO—MP | - | - | - | - |
| Range | 10–50 | 0.5–1.1 | 1.0–5.0 | 100–200 |
| (incubated) | 30 | 0.8 | 0.3 | 150 |
| EVOO—HP | - | - | - | - |
| Range | 100–300 | 2.0–8.0 | 10–25 | 250–400 |
| (incubated) | 200 | 5.0 | 20 | 325 |
| - | Pre-Hypoxia | Post-Reperfusion |
|---|---|---|
| LDH efflux (OD492–OD600 nm) | 0.7 ± 0.1 | 3.2 ± 0.3 * |
| TBARS (nmol/mg protein) | 0.8 ± 0.06 | 5.9 ± 0.6 * |
| 3-NTy (nmol/mg tissue) | 0.3 ± 0.02 | 2.7 ± 0.2 * |
| Compound | LDH | TBARS | 3-NTy |
|---|---|---|---|
| Hydroxytyrosol (HT) | 81.1 ± 4.7 ***** | 57.1 ± 5.7 ** | 64.4 ± 5.8 |
| Tyrosol (Ty) | 425 ± 25.1 | 154 ± 13.3 *** | 68.9 ± 4.4 |
| DHPG | 314 ± 22.5 | 84.7 ± 6.3 | 40.5 ±3.9 * |
| Oleocanthal (OLC) | 78.9 ± 5.8 ***** | 82.0 ± 6.3 | 75.3 ± 6.4 |
| - | - | - | - |
| HT + Ty (10 µM) | 185 ± 12.6 **** | 51.3 ± 6.5 | 71.5 ± 5.7 |
| HT + Ty (50 µM) | 227 ± 8.2 **** | 41.7 ± 3.9 | 57.6 ± 4.2 |
| HT + Ty (100 µM) | 125 ± 11.2 **** | 91.4 ± 5.0 **** | 84.2 ± 8.4 |
| - | - | - | - |
| HT + DHPG (10 µM) | 28.3 ± 3.4 **** | 56.0 ± 5.2 | 27.7 ± 4.8 **** |
| HT + DHPG (50 µM) | 85.9 ± 8.3 | 30.1 ± 3.8 **** | 29.4 ± 4.7 **** |
| HT + DHPG (100 µM) | 84.3 ± 8.2 | 49.8 ± 5.6 **** | 32.9 ± 4.5 **** |
| - | - | - | - |
| HT + OLC (10 µM) | 19.1 ± 2.6 **** | 34.8 ± 2.9 **** | 67.2 ± 7.1 |
| HT + OLC (50 µM) | 21.2 ± 1.8 **** | 26.0 ± 2.8 **** | 65.3 ± 6.6 |
| HT + OLC (100 µM) | 18.3 ± 1.1 **** | 21.2 ± 2.7 **** | 68.0 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Cruz Cortés, J.P.; Pérez de Algaba, I.; Martín-Aurioles, E.; Arrebola, M.M.; Ortega-Hombrados, L.; Rodríguez-Pérez, M.D.; Fernández-Prior, M.Á.; Bermúdez-Oria, A.; Verdugo, C.; González-Correa, J.A. Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain. Brain Sci. 2021, 11, 1133. https://doi.org/10.3390/brainsci11091133
De La Cruz Cortés JP, Pérez de Algaba I, Martín-Aurioles E, Arrebola MM, Ortega-Hombrados L, Rodríguez-Pérez MD, Fernández-Prior MÁ, Bermúdez-Oria A, Verdugo C, González-Correa JA. Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain. Brain Sciences. 2021; 11(9):1133. https://doi.org/10.3390/brainsci11091133
Chicago/Turabian StyleDe La Cruz Cortés, José Pedro, Inmaculada Pérez de Algaba, Esther Martín-Aurioles, María Monsalud Arrebola, Laura Ortega-Hombrados, María Dolores Rodríguez-Pérez, María África Fernández-Prior, Alejandra Bermúdez-Oria, Cristina Verdugo, and José Antonio González-Correa. 2021. "Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain" Brain Sciences 11, no. 9: 1133. https://doi.org/10.3390/brainsci11091133
APA StyleDe La Cruz Cortés, J. P., Pérez de Algaba, I., Martín-Aurioles, E., Arrebola, M. M., Ortega-Hombrados, L., Rodríguez-Pérez, M. D., Fernández-Prior, M. Á., Bermúdez-Oria, A., Verdugo, C., & González-Correa, J. A. (2021). Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain. Brain Sciences, 11(9), 1133. https://doi.org/10.3390/brainsci11091133







