Coenzyme a Biochemistry: From Neurodevelopment to Neurodegeneration
Abstract
1. Introduction
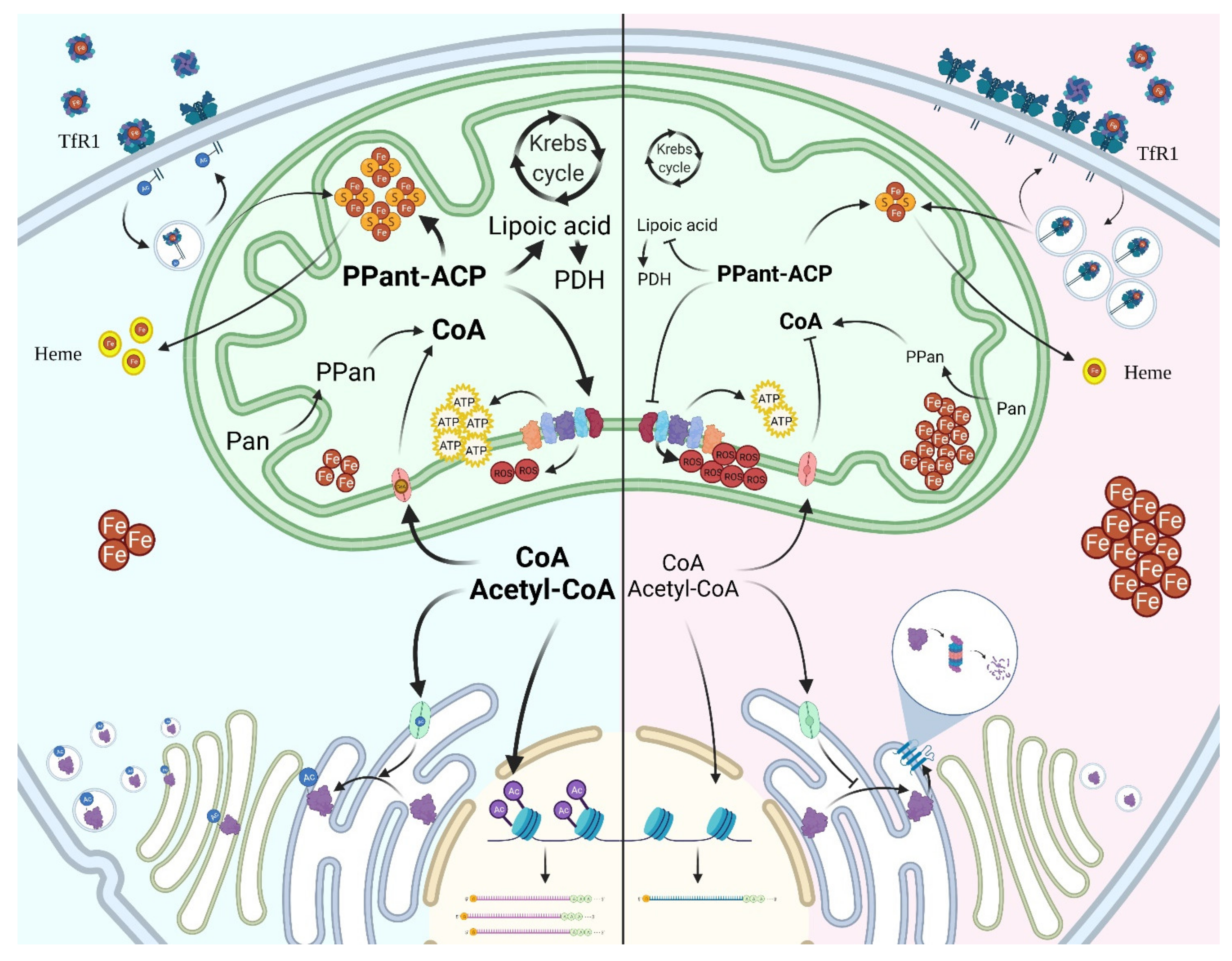
2. Coa Homeostasis
3. De Novo CoA Biosynthesis
4. CoA Degradation
5. CoA Distribution and Usage in the Cell
6. CoA Biosynthesis and Neurodegeneration
7. PKAN
8. CoPAN
9. The Search of Therapeutic Approaches
9.1. Correction of CoA Intracellular Content
9.2. Boosting Residual PANK2 Expression/Activity
Pantothenate
9.3. Bypassing the Blockage in the Biosynthetic Pathway
9.3.1. Pantethine
9.3.2. Fosmetpantotenate
9.3.3. Phosphopantetheine and Acetyl-4′-Phosphopantetheine
9.4. Stimulating Other PANK Isoforms
9.5. Iron Chelation
10. Defects in CoA/Acetyl-CoA Intracellular Compartmentalization
10.1. CoA Transport to the Mitochondria
10.2. CoA Transport to Peroxisomes
10.3. CoA Transport to the ER
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S.; Coenzyme, A. Back in action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Begley, T.P.; Kinsland, C.; Strauss, E. The biosynthesis of coenzyme A in bacteria. Vitam. Horm. 2001, 61, 157–171. [Google Scholar]
- Martinez, D.L.; Tsuchiya, Y.; Gout, I. Coenzyme A biosynthetic machinery in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.; Polanuyer, B.; Farrell, M.; Scholle, M.; Lykidis, A.; de Crécy-Lagard, V.; Osterman, A. Complete reconstitution of the human coenzyme A biosynthetic pathway via comparative genomics. J. Biol. Chem. 2002, 277, 21431–21439. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, A.G.; Beinlich, C.J. Pantothenic acid in health and disease. Vitam. Horm. 1991, 46, 165–228. [Google Scholar] [PubMed]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- Srinivasan, B.; Baratashvili, M.; van der Zwaag, M.; Kanon, B.; Colombelli, C.; Lambrechts, R.A.; Schaap, O.; Nollen, E.A.; Podgoršek, A.; Kosec, G.; et al. Extracellular 4′-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 2015, 11, 784–792. [Google Scholar] [CrossRef]
- Shibata, K.; Gross, C.J.; Henderson, L.M. Hydrolysis and absorption of pantothenate and its coenzymes in the rat small intestine. J. Nutr. 1983, 113, 2107–2115. [Google Scholar] [CrossRef]
- Coxon, K.M.; Chakauya, E.; Ottenhof, H.H.; Whitney, H.M.; Blundell, T.L.; Abell, C.; Smith, A.G. Pantothenate biosynthesis in higher plants. Biochem. Soc. Trans. 2005, 33, 743–746. [Google Scholar] [CrossRef]
- Said, H.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 2011, 437, 357–372. [Google Scholar] [CrossRef]
- Rock, C.O.; Calder, R.B.; Karim, M.A.; Jackowski, S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J. Biol. Chem. 2000, 275, 1377–1383. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O.; Jackowski, S. Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters. J. Biol. Chem. 2005, 280, 32594–32601. [Google Scholar] [CrossRef] [PubMed]
- Zhyvoloup, A.; Nemazanyy, I.; Panasyuk, G.; Valovka, T.; Fenton, T.; Rebholz, H.; Wang, M.-L.; Foxon, R.; Lyzogubov, V.; Usenko, V.; et al. Subcellular localization and regulation of coenzyme A synthase. J. Biol. Chem. 2003, 278, 50316–50321. [Google Scholar] [CrossRef] [PubMed]
- Skrede, S.; Halvorsen, O. Increased biosynthesis of CoA in the liver of rats treated with clofibrate. Eur. J. Biochem. 1979, 98, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Pathophysiological Role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Alfonso-Pecchio, A.; Garcia, M.; Leonardi, R.; Jackowski, S. Compartmentalization of mammalian pantothenate kinases. PLoS ONE 2012, 7, e49509. [Google Scholar] [CrossRef]
- Brunetti, D.; Dusi, S.; Morbin, M.; Uggetti, A.; Moda, F.; D’Amato, I.; Giordano, C.; d’ Amati, G.; Cozzi, A.; Levi, S.; et al. Pantothenate kinase-associated neurodegeneration: Altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum. Mol. Genet. 2012, 21, 5294–5305. [Google Scholar] [CrossRef]
- Johnson, M.; Kuo, Y.; Westaway, S.; Parker, S.; Ching, K.; Gitschier, J.; Haflick, S.J. Mitochondrial localization of human PANK2 and hypotheses of secondary iron accumulation in pantothenate kinase-associated neurodegeneration. Ann. N. Y. Acad. Sci. 2004, 1012, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Dansie, L.E.; Reeves, S.; Miller, K.; Zano, S.P.; Frank, M.; Pate, C.; Wang, J.; Jackowski, S. Physiological roles of the pantothenate kinases. Biochem. Soc. Trans. 2014, 42, 1033–1036. [Google Scholar] [CrossRef]
- Kuo, Y.; Duncan, J.; Westaway, S.; Yang, H.; Nune, G.; Xu, E.; Hayflick, S.J.; Gitschier, J. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum. Mol. Genet. 2005, 14, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Rehg, J.E.; Rock, C.O.; Jackowski, S. Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PLoS ONE 2010, 5, e11107. [Google Scholar] [CrossRef]
- Garcia, M.; Leonardi, R.; Zhang, Y.M.; Rehg, J.E.; Jackowski, S. Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS ONE 2012, 7, e40871. [Google Scholar] [CrossRef] [PubMed]
- Dusi, S.; Valletta, L.; Haack, T.B.; Tsuchiya, Y.; Venco, P.; Pasqualato, S.; Goffrini, P.; Tigano, M.; Demchenko, N.; Wieland, T.; et al. Exome sequence reveals mutations in CoA synthase as a cause of neurodegeneration with brain iron accumulation. Am. J. Hum. Genet. 2014, 94, 11–22. [Google Scholar] [CrossRef]
- Nemazanyy, I.; Panasyuk, G.; Breus, O.; Zhyvoloup, A.; Filonenko, V.; Gout, I.T. Identification of a novel CoA synthase isoform, which is primarily expressed in the brain. Biochem. Biophys. Res. Commun. 2006, 341, 995–1000. [Google Scholar] [CrossRef]
- Jackowski, S.; Rock, C.O. Metabolism of 4’-phosphopantetheine in Escherichia coli. J. Bacteriol. 1984, 158, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sibon, O.C.; Strauss ECoenzyme, A. To make it or uptake it? Nat. Rev. Mol. Cell Biol. 2016, 17, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Hogarth, P.; Placzek, A.; Gregory, A.M.; Fox, R.; Zhen, D.; Hamada, J.; van der Zwaag, M.; Lambrechts, R.; Jin, H.; et al. 4′-Phosphopantetheine corrects CoA, iron, and dopamine metabolic defects in mammalian models of PKAN. EMBO Mol. Med. 2019, 11, e10489. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.O.; Karim, M.A.; Zhang, Y.M.; Jackowski, S. The murine pantothenate kinase (Pank1) gene encodes two differentially regulated pantothenate kinase isozymes. Gene 2002, 291, 35–43. [Google Scholar] [CrossRef]
- Leonardi, R.; Rock, C.; Jackowski, S.; Zhang, Y. Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc. Natl. Acad. Sci. USA 2007, 104, 1494–1499. [Google Scholar] [CrossRef]
- Goding, J.W.; Grobben, B.; Slegers, H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim. Biophys. Acta. 2003, 1638, 1–19. [Google Scholar] [CrossRef]
- Martin, F.; Malergue, F.; Pitari, G.; Philippe, J.M.; Philips, S.; Chabret, C.; Granjeaud, S.; Mattei, M.G.; Mungall, A.J.; Naquet, P.; et al. Vanin genes are clustered (human 6q22-24 and mouse 10A2B1) and encode isoforms of pantetheinase ectoenzymes. Immunogenetics 2001, 53, 296–306. [Google Scholar] [CrossRef]
- Bartucci, R.; Salvati, A.; Olinga, P.; Boersma, Y.L. Vanin 1: Its Physiological Function and Role in Diseases. Int. J. Mol. Sci. 2019, 20, 3891. [Google Scholar] [CrossRef] [PubMed]
- Gout, I.; Coenzyme, A. Protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef]
- Fiermonte, G.; Paradies, E.; Todisco, S.; Marobbio, C.M.; Palmieri, F. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J. Biol. Chem. 2009, 284, 18152–18159. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; De Leonardis, F.; Paradies, E.; De Grassi, A.; Pierri, C.L.; Parisi, G.; Marya, C.; Marobbio, T.; Lasorsa, F.M.; Muto, L.; et al. Biochemical characterization of a new mitochondrial transporter of dephosphocoenzyme A in Drosophila melanogaster. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 137–146. [Google Scholar] [CrossRef]
- Agrimi, G.; Russo, A.; Scarcia, P.; Palmieri, F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A., FAD and NAD+. Biochem. J. 2012, 443, 241–247. [Google Scholar] [CrossRef]
- Lin, P.; Li, J.; Liu, Q.; Mao, F.; Li, J.; Qiu, R.; Hu, H.; Song, Y.; Yang, Y.; Gao, G.; et al. A Missense Mutation in SLC33A1, which Encodes the Acetyl-CoA Transporter, Causes Autosomal-Dominant Spastic Paraplegia (SPG42). Am. J. Hum. Genet. 2008, 83, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, I.A.; Cui, Y.; Braun, M.M.; Lawton, A.J.; Robinson, N.H.; Peotter, J.L.; Yu, Q.; Casler, J.C.; Glick, B.S.; Audhya, A.; et al. Acetyl-CoA flux from the cytosol to the ER regulates engagement and quality of the secretory pathway. Sci. Rep. 2021, 11, 2013. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Sibon, O.C.; Jackowski, S.; Gout, I. Coenzyme A and its derivatives: Renaissance of a textbook classic. Biochem. Soc. Trans. 2014, 42, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Pedro, J.M.B.-S.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Invest. 2012, 122, 1958–1959. [Google Scholar] [CrossRef]
- Allfrey, V.G.; Faulkner, R.; Mirsky, A.E. Acetylation and methylation of histones and their possible role in the regulation of rna synthesis. Proc. Natl. Acad Sci. USA 1964, 51, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.E.; English, D.M.; Cowley, S.M. Acetylation & Co: An expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 2019, 63, 97–107. [Google Scholar]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of Cellular Metabolism by Protein Lysine Acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Davaapil, H.; Tsuchiya, Y.; Gout, I. Signalling functions of coenzyme A and its derivatives in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1056–1062. [Google Scholar] [CrossRef]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, C.; Tang, M.C.; Wang, Y.; Wang, S.P.; Allard, P.; Furtos, A.; Mitchell, G.A. Inborn errors of mitochondrial acyl-coenzyme a metabolism: Acyl-CoA biology meets the clinic. Mol. Genet. Metab. 2019, 128, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Beld, J.; Sonnenschein, E.; Vickery, C.R.; Noel, J.P.; Burkart, M.D. The phosphopantetheinyl transferases: Catalysis of a post-translational modification crucial for life. Nat. Prod. Rep. 2014, 31, 61–108. [Google Scholar] [CrossRef] [PubMed]
- Masud, A.J.; Kastaniotis, A.J.; Rahman, M.T.; Autio, K.J.; Hiltunen, J.K. Mitochondrial acyl carrier protein (ACP) at the interface of metabolic state sensing and mitochondrial function. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 118540. [Google Scholar] [CrossRef]
- Flugel, R.S.; Hwangbo, Y.; Lambalot, R.H.; Cronan, J.E., Jr.; Walsh, C.T. Holo-(Acyl Carrier Protein) Synthase and Phosphopantetheinyl Transfer in Escherichia coli. J. Biol. Chem. 2000, 275, 959–968. [Google Scholar] [CrossRef]
- Lambrechts, R.A.; Schepers, H.; Yu, Y.; van der Zwaag, M.; Autio, K.J.; Vieira-Lara, M.A.; Hayflick, S.; Grzeschik, N.A.; Sibon, O.C. CoA-dependent activation of mitochondrial acyl carrier protein links four neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10488. [Google Scholar] [CrossRef]
- Di Meo, I.; Carecchio, M.; Tiranti, V. Inborn errors of coenzyme A metabolism and neurodegeneration. J. Inherit. Metab. Dis. 2019, 42, 49–56. [Google Scholar] [CrossRef]
- Iuso, A.; Wiersma, M.; Schüller, H.J.; Pode-Shakked, B.; Marek-Yagel, D.; Grigat, M.; Schwarzmayr, T.; Berutti, R.; Alhaddad, B.; Kanon, B.; et al. Mutations in PPCS, Encoding Phosphopantothenoylcysteine Synthetase, Cause Autosomal-Recessive Dilated Cardiomyopathy. Am. J. Hum. Genet. 2018, 102, 1018–1030. [Google Scholar] [CrossRef]
- Iuso, A.; Alhaddad, B.; Weigel, C.; Kotzaeridou, U.; Mastantuono, E.; Schwarzmayr, T.; Graf, E.; Terrile, C.; Prokisch, H.; Strom, T.M.; et al. A Homozygous Splice Site Mutation in SLC25A42, Encoding the Mitochondrial Transporter of Coenzyme A, Causes Metabolic Crises and Epileptic Encephalopathy. JIMD Rep. 2019, 44, 1–7. [Google Scholar]
- Almannai, M.; Alasmari, A.; Alqasmi, A.; Faqeih, E.; Al Mutairi, F.; Alotaibi, M.; Samman, M.; Eyaid, W.; Aljadhai, Y.; Shamseldin, H.; et al. Expanding the phenotype of SLC25A42 -associated mitochondrial encephalomyopathy. Clin. Genet. 2018, 93, 1097–1102. [Google Scholar] [CrossRef]
- Zhou, B.; Westaway, S.; Levinson, B.; Johnson, M.; Gitschier, J.; Hayflick, S. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat. Genet. 2001, 28, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, S.J. Unraveling the Hallervorden-Spatz syndrome: Pantothenate kinase-associated neurodegeneration is the name. Curr. Opin. Pediatr. 2003, 15, 572–577. [Google Scholar] [CrossRef]
- Annesi, G.; Gagliardi, M.; Iannello, G.; Quattrone, A. Mutational analysis of COASY in an Italian patient with NBIA. Park. Relat. Disord. 2016, 28, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Brezavar, D.; Bonnen, P.E. Incidence of PKAN determined by bioinformatic and population-based analysis of ~140,000 humans. Mol. Genet. Metab. 2019, 128, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Finazzi, D. Neurodegeneration with brain iron accumulation: Update on pathogenic mechanisms. Front. Pharmacol. 2014, 5, 99. [Google Scholar] [CrossRef]
- Hartig, M.B.; Hörtnagel, K.; Garavaglia, B.; Zorzi, G.; Kmiec, T.; Klopstock, T.; Rostasy, K.; Svetel, M.; Kostic, V.S.; Schuelke, M.; et al. Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann. Neurol. 2006, 59, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Kotzbauer, P.T.; Truax, A.C.; Trojanowski, J.Q.; Lee, V.M.-Y. Altered Neuronal Mitochondrial Coenzyme A Synthesis in Neurodegeneration with Brain Iron Accumulation Caused by Abnormal Processing, Stability, and Catalytic Activity of Mutant Pantothenate Kinase 2. J. Neurosci. 2005, 25, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Rock, C.O.; Jackowski, S. Biochemical Properties of Human Pantothenate Kinase 2 Isoforms and Mutations Linked to Pantothenate Kinase-associated Neurodegeneration. J. Biol. Chem. 2006, 281, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Córdoba, M.; Khoury, A.F.; Villanueva-Paz, M.; Gómez-Navarro, C.; Villalón-García, I.; Suárez-Rivero, J.M.; Povea-Cabello, S.; DE LA Mata, M.; Cotán, D.; Talaverón-Rey, M.; et al. Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation. Mol. Neurobiol. 2019, 56, 3638–3656. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, C.; Lv, S.; Zhou, B. Pantothenate kinase-associated neurodegeneration: Insights from a Drosophila model. Hum. Mol. Genet. 2009, 18, 3659–3672. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, J.; Jiang, Y.; Wang, J.; Wu, Y. Natural history and genotype-phenotype correlation of pantothenate kinase-associated neurodegeneration. CNS Neurosci. Ther. 2020, 26, 754–761. [Google Scholar] [CrossRef]
- Hayflick, S.J.; Westaway, S.K.; Levinson, B.; Zhou, B.; Johnson, M.A.; Ching, K.H.L.; Gitschier, J. Genetic, Clinical, and Radiographic Delineation of Hallervorden–Spatz Syndrome. N. Engl. J. Med. 2003, 348, 33–40. [Google Scholar] [CrossRef]
- Kruer, M.C.; Hiken, M.; Gregory, A.; Malandrini, A.; Clark, D.; Hogarth, P.; Grafe, M.; Hayflick, S.J.; Woltjer, R.L. Novel histopathologic findings in molecularly-confirmed pantothenate kinase-associated neurodegeneration. Brain 2011, 134, 947–958. [Google Scholar] [CrossRef]
- Woltjer, R.L.; Reese, L.C.; Richardson, B.E.; Tran, H.; Green, S.; Pham, T.; Chalupsky, M.; Gabriel, I.; Light, T.; Sanford, L.; et al. Pallidal neuronal apolipoprotein E in pantothenate kinase-associated neurodegeneration recapitulates ischemic injury to the globus pallidus. Mol. Genet. Metab. 2015, 116, 289–297. [Google Scholar] [CrossRef][Green Version]
- Levi, S.; Tiranti, V. Neurodegeneration with Brain Iron Accumulation Disorders: Valuable Models Aimed at Understanding the Pathogenesis of Iron Deposition. Pharmaceuticals 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Drecourt, A.; Babdor, J.; Dussiot, M.; Petit, F.; Goudin, N.; Garfa-Traoré, M.; Habarou, F.; Bole-Feysot, C.; Nitschké, P.; Ottolenghi, C.; et al. Impaired Transferrin Receptor Palmitoylation and Recycling in Neurodegeneration with Brain Iron Accumulation. Am. J. Hum. Genet. 2018, 102, 266–277. [Google Scholar] [CrossRef]
- Bosveld, F.; Rana, A.; Van Der Wouden, P.E.; Lemstra, W.; Ritsema, M.; Kampinga, H.; Sibon, O.C.M. De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum. Mol. Genet. 2008, 17, 2058–2069. [Google Scholar] [CrossRef]
- Zizioli, D.; Tiso, N.; Guglielmi, A.; Saraceno, C.; Busolin, G.; Giuliani, R.; Khatri, D.; Monti, E.; Borsani, G.; Argenton, F.; et al. Knock-down of pantothenate kinase 2 severely affects the development of the nervous and vascular system in zebrafish, providing new insights into PKAN disease. Neurobiol. Dis. 2016, 85, 35–48. [Google Scholar] [CrossRef]
- Ganz, J.; Kaslin, J.; Freudenreich, D.; Machate, A.; Geffarth, M.; Brand, M. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 2012, 520, 633–655. [Google Scholar] [CrossRef]
- Khatri, D.; Zizioli, D.; Trivedi, A.; Borsani, G.; Monti, E.; Finazzi, D. Overexpression of Human Mutant PANK2 Proteins Affects Development and Motor Behavior of Zebrafish Embryos. NeuroMolecular Med. 2018, 21, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.; Trivedi, A.; Khatri, D.; Zizioli, D.; Garrafa, E.; Mitola, S.; Finazzi, D. Silencing of pantothenate kinase 2 reduces endothelial cell angiogenesis. Mol. Med. Rep. 2018, 18, 4739–4746. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Hayflick, S.J.; Gitschier, J. Deprivation of pantothenic acid elicits a movement disorder and azoospermia in a mouse model of pantothenate kinase-associated neurodegeneration. J. Inherit. Metab. Dis. 2007, 30, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Subramanian, C.; Yun, M.-K.; Frank, M.W.; White, S.W.; Rock, C.O.; Lee, R.E.; Jackowski, S. A therapeutic approach to pantothenate kinase associated neurodegeneration. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Shumar, S.A.; Fagone, P.; Alfonso-Pecchio, A.; Gray, J.T.; Rehg, J.E.; Jackowski, S.; Leonardi, R. Induction of Neuron-Specific Degradation of Coenzyme A Models Pantothenate Kinase-Associated Neurodegeneration by Reducing Motor Coordination in Mice. PLoS ONE 2015, 10, e0130013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ceccatelli Berti, C.; Gilea, A.I.; De Gregorio, M.A.; Goffrini, P. Exploring Yeast as a Study Model of Pantothenate Kinase-Associated Neurodegeneration and for the Identification of Therapeutic Compounds. Int. J. Mol. Sci. 2020, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Derosas, M.; Luscieti, S.; Cavadini, P.; Campanella, A.; Verardi, R.; Finazzi, D.; Arosio, P. Pantothenate kinase-2 (Pank2) silencing causes cell growth reduction, cell-specific ferroportin upregulation and iron deregulation. Neurobiol. Dis. 2010, 39, 204–210. [Google Scholar] [CrossRef]
- Campanella, A.; Privitera, D.; Guaraldo, M.; Rovelli, E.; Barzaghi, C.; Garavaglia, B.; Santambrogio, P.; Cozzi, A.; Levi, S. Skin fibroblasts from pantothenate kinase-associated neurodegeneration patients show altered cellular oxidative status and have defective iron-handling properties. Hum. Mol. Genet. 2012, 21, 4049–4059. [Google Scholar] [CrossRef]
- Santambrogio, P.; Dusi, S.; Guaraldo, M.; Rotundo, L.I.; Broccoli, V.; Garavaglia, B.; Tiranti, V.; Levi, S. Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Neurobiol. Dis. 2015, 81, 144–153. [Google Scholar] [CrossRef]
- Orellana, D.; Santambrogio, P.; Rubio, A.; Yekhlef, L.; Cancellieri, C.; Dusi, S.; Giannelli, S.G.; Venco, P.; Mazzara, P.G.; Cozzi, A.; et al. Coenzyme A corrects pathological defects in human neurons of PANK 2-associated neurodegeneration. EMBO Mol. Med. 2016, 8, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Arber, C.E.; Li, A.; Houlden, H.; Wray, S. Review: Insights into molecular mechanisms of disease in neurodegeneration with brain iron accumulation: Unifying theories. Neuropathol. Appl. Neurobiol. 2015, 42, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, P.; Ripamonti, M.; Paolizzi, C.; Panteghini, C.; Carecchio, M.; Chiapparini, L.; Raimondi, M.; Rubio, A.; Meo, I.D.; Cozzi, A.; et al. Harmful Iron-Calcium Relationship in Pantothenate kinase Associated Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3664. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, F.; Ye, X.; Li, X.; Zhao, Q.; Lin, Z.; Hu, Y.; Wang, J. Basal ganglia calcification and novel compound heterozygous mutations in the PANK2 gene in a Chinese boy with classic Pantothenate kinase-associated neurodegeneration: A case report. Medicine 2018, 97, e0316. [Google Scholar] [CrossRef]
- Wu, Y.W.; Hess, C.; Singhal, N.S.; Groden, C.; Toro, C. Idiopathic Basal Ganglia Calcifications: An Atypical Presentation of PKAN. Pediatr. Neurol. 2013, 49, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, J.; Kou, L.P.; Feng, H.C.; Deng, Y.H.; Zhang, Z.B.; Zhou, L.; Wang, J.M.; Jiang, Y.W.; Wu, Y. [Phenotypic and genotypic features of twenty children with classic pantothenate kinase-associated neurodegeneration]. Zhonghua Er Ke Za Zhi 2017, 55, 678–682. [Google Scholar]
- Rana, A.; Seinen, E.; Siudeja, K.; Muntendam, R.; Srinivasan, B.; van der Want, J.; Hayflick, S.; Reijngoud, D.-J.; Kayser, O.; Sibon, O.C.M. Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 6988–6993. [Google Scholar] [CrossRef] [PubMed]
- Siudeja, K.; Grzeschik, N.A.; Rana, A.; de Jong, J.; Sibon, O.C. Cofilin/Twinstar phosphorylation levels increase in response to impaired coenzyme a metabolism. PLoS ONE 2012, 7, e43145. [Google Scholar]
- Van Vranken, J.G.; Jeong, M.Y.; Wei, P.; Chen, Y.C.; Gygi, S.P.; Winge, D.R.; Rutter, J. The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. Elife 2016, 5, e17828. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M.; Pastore, A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J. Neurol. 2009, 256, 9–17. [Google Scholar] [CrossRef]
- Leoni, V.; Strittmatter, L.; Zorzi, G.; Zibordi, F.; Dusi, S.; Garavaglia, B.; Venco, P.; Caccia, C.; Souza, A.L.; Deik, A.; et al. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol. Genet. Metab. 2012, 105, 463–471. [Google Scholar] [CrossRef]
- Evers, C.; Seitz, A.; Assmann, B.; Opladen, T.; Karch, S.; Hinderhofer, K.; Granzow, M.; Paramasivam, N.; Eils, R.; Diessl, N.; et al. Diagnosis of CoPAN by whole exome sequencing: Waking up a sleeping tiger’s eye. Am. J. Med. Genet. A 2017, 173, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, T.; Ferdinandusse, S.; Ruiter, J.P.N.; Alders, M.; Mathijssen, I.B.; Parboosingh, J.S.; Innes, A.M.; Meijers-Heijboer, H.; Poll-The, B.T.; Bernier, F.P.; et al. Biallelic loss of function variants in COASY cause prenatal onset pontocerebellar hypoplasia, microcephaly, and arthrogryposis. Eur. J. Hum. Genet. 2018, 26, 1752–1758. [Google Scholar] [CrossRef]
- Berti, C.; Dallabona, C.; Lazzaretti, M.; Dusi, S.; Tosi, E.; Tiranti, V.; Goffrini, P. Modeling human Coenzyme A synthase mutation in yeast reveals altered mitochondrial function, lipid content and iron metabolism. Microb. Cell 2015, 2, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Zizioli, D.; Tiso, N.; Facchinello, N.; Vezzoli, S.; Gianoncelli, A.; Memo, M.; Monti, E.; Borsani, G.; Finazzi, D. Down-regulation of coasy, the gene associated with NBIA-VI, reduces Bmp signaling, perturbs dorso-ventral patterning and alters neuronal development in zebrafish. Sci. Rep. 2016, 6, 37660. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, I.; Cavestro, C.; Pedretti, S.; Fu, T.; Ligorio, S.; Manocchio, A.; Munuera-Cabeza, M.; Salas, J.J.; Sánchez-Alcázar, J.A. Neuronal Ablation of CoA Synthase Causes Motor Deficits, Iron Dyshomeostasis, and Mitochondrial Dysfunctions in a CoPAN Mouse Model. Int. J. Mol. Sci. 2020, 21, 9707. [Google Scholar] [CrossRef]
- Álvarez-Córdoba, M.; Talaverón-Rey, M.; Villalón-García, I.; Povea-Cabello, S.; Suárez-Rivero, J.M.; Suárez-Carrillo, A. Down regulation of the expression of mitochondrial phosphopantetheinyl-proteins in pantothenate kinase-associated neurodegeneration: Pathophysiological consequences and therapeutic perspectives. Orphanet J. Rare. Dis. 2021, 16, 201. [Google Scholar] [CrossRef]
- Werning, M.; Müllner, E.W.; Mlynek, G.; Dobretzberger, V.; Djinovic-Carugo, K.; Baron, D.M.; Prokisch, H.; Büchner, B.; Klopstock, T.; Salzer, U. PKAN neurodegeneration and residual PANK2 activities in patient erythrocytes. Ann. Clin. Transl. Neurol. 2020, 7, 1340–1351. [Google Scholar] [CrossRef]
- Siudeja, K.; Srinivasan, B.; Xu, L.; Rana, A.; De Jong, J.; Nollen, E.A.A.; Jackowski, S.; Sanford, L.; Hayflick, S.; Sibon, O.C.M. Impaired Coenzyme A metabolism affects histone and tubulin acetylation in Drosophila and human cell models of pantothenate kinase associated neurodegeneration. EMBO Mol. Med. 2011, 3, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, D.; Dusi, S.; Giordano, C.; Lamperti, C.; Morbin, M.; Fugnanesi, V.; Marchet, S.; Fagiolari, G.; Sibon, O.; Moggio, M.; et al. Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model. Brain 2014, 137, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, J.; Jiang, Y.; Yao, B.; Wang, J.; Wu, Y. Pilot trial on the efficacy and safety of pantethine in children with pantothenate kinase-associated neurodegeneration: A single-arm, open-label study. Orphanet. J. Rare Dis. 2020, 15, 248. [Google Scholar] [CrossRef]
- Elbaum, D.; Beconi, M.G.; Monteagudo, E.; Di Marco, A.; Quinton, M.S.; Lyons, K.A.; Vaino, A.; Harper, S. Fosmetpantotenate (RE-024), a phosphopantothenate replacement therapy for pantothenate kinase-associated neurodegeneration: Mechanism of action and efficacy in nonclinical models. PLoS ONE 2018, 13, e0192028. [Google Scholar] [CrossRef] [PubMed]
- Christou, Y.-P.; Tanteles, G.A.; Kkolou, E.; Ormiston, A.; Konstantopoulos, K.; Beconi, M.; Marshall, R.D.; Plotkin, H.; Kleopa, K.A. Open-Label Fosmetpantotenate, a Phosphopantothenate Replacement Therapy in a Single Patient with Atypical PKAN. Case Rep. Neurol. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Klopstock, T.; Videnovic, A.; Bischoff, A.T.; Bonnet, C.; Cif, L.; Comella, C.; Correa-Vela, M.; Escolar, M.L.; Fraser, J.L.; Gonzalez, V.; et al. Fosmetpantotenate Randomized Controlled Trial in Pantothenate Kinase–Associated Neurodegeneration. Mov. Disord. 2021, 36, 1342–1352. [Google Scholar] [CrossRef]
- Di Meo, I.; Colombelli, C.; Srinivasan, B.; de Villiers, M.; Hamada, J.; Jeong, S.Y.; Fox, R.; Woltjer, R.L.; Tepper, P.G.; Lahaye, L.L.; et al. Acetyl-4′-phosphopantetheine is stable in serum and prevents phenotypes induced by pantothenate kinase deficiency. Sci. Rep. 2017, 7, 11260. [Google Scholar] [CrossRef]
- Jackowski, S. Proposed Therapies for Pantothenate-Kinase-Associated Neurodegeneration. J. Exp. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Sharma, L.K.; Leonardi, R.; Lin, W.; Boyd, V.A.; Goktug, A.; Shelat, A.; Chen, T.; Jackowski, S.; Rock, C.O. A High-Throughput Screen Reveals New Small-Molecule Activators and Inhibitors of Pantothenate Kinases. J. Med. Chem. 2015, 58, 1563–1568. [Google Scholar] [CrossRef]
- Boddaert, N.; Sang, K.-H.L.Q.; Rotig, A.; Leroy-Willig, A.; Gallet, S.; Brunelle, F.; Sidi, D.; Thalabard, J.-C.; Munnich, A.; Cabantchik, Z.I. Selective iron chelation in Friedreich ataxia: Biologic and clinical implications. Blood 2007, 110, 401–408. [Google Scholar] [CrossRef]
- Forni, G.L.; Balocco, M.; Cremonesi, L.; Abbruzzese, G.; Parodi, R.C.; Marchese, R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov. Disord. 2008, 23, 904–907. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Ryckewaert, G.; Tchofo, P.J.; Moreau, C.; Vuillaume, I.; Chinnery, P.; Destée, A.; Defebvre, L.; Devos, D. Long-term improvement under deferiprone in a case of neurodegeneration with brain iron accumulation. Park. Relat. Disord. 2012, 18, 110–112. [Google Scholar] [CrossRef]
- Zorzi, G.; Zibordi, F.; Chiapparini, L.; Bertini, E.; Russo, L.; Piga, A.; Longo, F.; Garavaglia, B.; Aquino, D.; Savoiardo, M.; et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: Results of a phase II pilot trial. Mov. Disord. 2011, 26, 1755–1759. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Cossu, G.; Balocco, M.; Marchese, R.; Murgia, D.; Melis, M.; Galanello, R.; Barella, S.; Matta, G.; Ruffinengo, U.; et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica 2011, 96, 1708–1711. [Google Scholar] [CrossRef]
- Cossu, G.; Abbruzzese, G.; Matta, G.; Murgia, D.; Melis, M.; Ricchi, V.; Galanello, R.; Barella, S.; Origa, R.; Balocco, M.; et al. Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): Results from a four years follow-up. Park. Relat. Disord. 2014, 20, 651–654. [Google Scholar] [CrossRef]
- Klopstock, T.; Tricta, F.; Neumayr, L.; Karin, I.; Zorzi, G.; Fradette, C.; Kmieć, T.; Büchner, B.; Steele, H.E.; Horvath, R.; et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: A randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol. 2019, 18, 631–642. [Google Scholar] [CrossRef]
- Thakur, N.; Klopstock, T.; Jackowski, S.; Kuscer, E.; Tricta, F.; Videnovic, A.; Jinnah, H.A. Rational Design of Novel Therapies for Pantothenate Kinase-Associated Neurodegeneration. Mov. Disord. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, H.E.; Smith, L.L.; Kentab, A.; Alkhalidi, H.; Summers, B.; Alsedairy, H.; Xiong, Y.; Gupta, V.A.; Alkuraya, F.S. Mutation of the mitochondrial carrier SLC25A42 causes a novel form of mitochondrial myopathy in humans. Qual. Life Res. 2015, 135, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nam, I.; Lee, D.; Bhandari, S.; Charton, L.; Kwak, S.; Lim, J.; Hong, K.; Kim, S.; Lee, J.N.; et al. Slc25a17 acts as a peroxisomal coenzyme A transporter and regulates multiorgan development in zebrafish. J. Cell. Physiol. 2020, 235, 151–165. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P.; de Schryver, E.; Young, S.G.; Zwijsen, A.; Fransen, M.; Espeel, M.; Baes, M. Gene Trapped Mice: PMP34 Plays a Role in the Peroxisomal Degradation of Phytanic and Pristanic Acid. Front. Cell Dev. Biol. 2020, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Pehar, M.; Lehnus, M.; Karst, A.; Puglielli, L. Proteomic assessment shows that many endoplasmic reticulum (ER)-resident proteins are targeted by N(epsilon)-lysine acetylation in the lumen of the organelle and predicts broad biological impact. J. Biol. Chem. 2012, 287, 22436–22440. [Google Scholar] [CrossRef]
- Butor, C.; Diaz, S.; Varki, A. High level O-acetylation of sialic acids on N-linked oligosaccharides of rat liver membranes. Differential subcellular distribution of 7- and 9-O-acetyl groups and of enzymes involved in their regulation. J. Biol. Chem. 1993, 268, 10197–10206. [Google Scholar] [CrossRef]
- Kanamori, A.; Nakayama, J.; Fukuda, M.N.; Stallcup, W.B.; Sasaki, K.; Fukuda, M.; Hirabayashi, Y. Expression cloning and characterization of a cDNA encoding a novel membrane protein required for the formation of O-acetylated ganglioside: A putative acetyl-CoA transporter. Proc. Natl. Acad. Sci. USA 1997, 94, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Nomura, K.H.; Nomura, K. The acetyl-CoA transporter family SLC33. Mol Asp. Med. 2013, 34, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.C.; Pehar, M.; Puglielli, L. AT-1 is the ER membrane acetyl-CoA transporter and is essential for cell viability. J. Cell Sci. 2010, 123, 3378–3388. [Google Scholar] [CrossRef]
- Chiplunkar, S.; Bindu, P.S.; Nagappa, M.; Bineesh, C.; Govindaraj, P.; Gayathri, N.; Bharath, M.M.S.; Arvinda, H.R.; Mathuranath, P.S.; Sinha, S.; et al. Huppke-Brendel syndrome in a seven months old boy with a novel 2-bp deletion in SLC33A1. Metab. Brain Dis. 2016, 31, 1195–1198. [Google Scholar] [CrossRef]
- Huppke, P.; Brendel, C.; Kalscheuer, V.; Korenke, G.C.; Marquardt, I.; Freisinger, P.; Christodoulou, J.; Hillebrand, M.; Pitelet, G.; Wilson, C.; et al. Mutations in SLC33A1 cause a lethal autosomal-recessive disorder with congenital cataracts, hearing loss, and low serum copper and ceruloplasmin. Am. J. Hum. Genet. 2012, 90, 61–68. [Google Scholar] [CrossRef]
- Sanders, S.J.; Ercan-Sencicek, A.G.; Hus, V.; Luo, R.; Murtha, M.T.; Moreno-De-Luca, D.; Chu, S.H.; Moreau, M.P.; Gupta, A.R.; Thomson, S.A.; et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011, 70, 863–885. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Li, Z.; Zhao, B.; Lin, P.; Liu, P.; Zhai, M.; Liu, Q.; Shao, C.; Sun, W.; Gong, Y. Identification and Functional Analysis of a SLC33A1: c.339T>G (p.Ser113Arg) Variant in the Original SPG42 Family. Hum. Mutat. 2015, 36, 240–249. [Google Scholar] [CrossRef]
- Peng, Y.; Li, M.; Clarkson, B.D.; Pehar, M.; Lao, P.J.; Hillmer, A.T.; Barnhart, T.E.; Christian, B.T.; Mitchell, H.A.; Bendlin, B.B.; et al. Deficient import of acetyl-CoA into the ER lumen causes neurodegeneration and propensity to infections, inflammation, and cancer. J. Neurosci. 2014, 34, 6772–6789. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, B.; Ma, J.; Lin, P.; Zhang, Y.; Shao, C.; Sun, W.; Gong, Y. S113R mutation in Slc33a1 leads to neurodegeneration and augmented BMP signaling in a mouse model. Dis. Model. Mech. 2017, 10, 53–62. [Google Scholar] [CrossRef]
- Pehar, M.; Jonas, M.C.; Hare, T.M.; Puglielli, L. SLC33A1/AT-1 protein regulates the induction of autophagy downstream of IRE1/XBP1 pathway. J. Biol. Chem. 2012, 287, 29921–29930. [Google Scholar] [CrossRef]
- Hullinger, R.; Li, M.; Wang, J.; Peng, Y.; Dowell, J.A.; Bomba-Warczak, E.; Mitchell, H.A.; Burger, C.; Chapman, E.; Denu, J.M.; et al. Increased expression of AT-1/SLC33A1 causes an autistic-like phenotype in mice by affecting dendritic branching and spine formation. J. Exp. Med. 2016, 213, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.A.; Puglielli, L. Nε-lysine acetylation in the endoplasmic reticulum – a novel cellular mechanism that regulates proteostasis and autophagy. J. Cell Sci. 2018, 131, jcs221747. [Google Scholar] [CrossRef]
- Dieterich, I.A.; Lawton, A.J.; Peng, Y.; Yu, Q.; Rhoads, T.W.; Overmyer, K.A.; Cui, Y.; Armstrong, E.A.; Howell, P.R.; Burhans, M.S.; et al. Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk. Nat. Commun. 2019, 10, 3929. [Google Scholar] [CrossRef] [PubMed]
- Lipmann, F.; Kaplan, N.O. Coenzyme for acetylation, a pantothenic acid derivative. J. Biol. Chem. 1947, 167, 869. [Google Scholar] [CrossRef]

| Gene (OMIM *) | Protein Function | Disorder (OMIM °) | Inheritance | Clinical Features | Molecules Investigated for Therapeutic Potential |
|---|---|---|---|---|---|
| PANK2 (606157) | PANK2 catalyzes the phosphorylation of pantothenate to 4-phosphopantothenate, first step of CoA biosynthesis. | Pantothenate kinase-associated neurodegeneration -PKAN (234200) | AR | Early (childhood) or late (early adulthood) onset. Dystonia, spasticity, parkinsonism, retinal degeneration, cognitive decline, neuropsychiatric disturbances. | Pantothenate; Pantethine; Fosmetpantotenate; 4′-Phosphopantetheine and Acetyl-4′-Phosphopantetheine; Coenzyme A; Pantazines; Artesunate; Deferiprone |
| COASY (609855) | COASY catalyzes the two final steps of CoA biosynthesis. | COASY Protein-Associated Neurodegeneration–CoPAN (615643) | AR | Early onset gait impairment and learning difficulties, with dystonia, and spasticity. | Coenzyme A |
| SLC25A42 (610823) | Mitochondrial CoA transporter | Recurrent metabolic crises with variable encephalomyopathic features and neurologic regression -MECREN (618416) | AR | Usually childhood onset with episodic lactic acidosis. Possible developmental regression of motor and cognitive skills. | - |
| SLC33A1 (603690) | Endoplasmic Reticulum acetyl-CoA transporter | Congenital cataracts, hearing loss, and neurodegeneration -CCHLNDH-uppke and Brendel syndrome (614482) | AR | Severe psychomotor retardation, congenital cataracts and hearing loss. More variable neurologic features. Low copper and ceruloplasmin serum levels. | - |
| Spastic paraplegia 42 (612539) | AD - rs121909484 (p.S113R) in the Chinese population. | Spastic gait, increased lower limb tone, hyperreflexia, and weakness and atrophy of the lower limb muscles. | - | ||
| Autism-spectrum disorder with intellectual disability | AD (gene duplication) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignani, L.; Gnutti, B.; Zizioli, D.; Finazzi, D. Coenzyme a Biochemistry: From Neurodevelopment to Neurodegeneration. Brain Sci. 2021, 11, 1031. https://doi.org/10.3390/brainsci11081031
Mignani L, Gnutti B, Zizioli D, Finazzi D. Coenzyme a Biochemistry: From Neurodevelopment to Neurodegeneration. Brain Sciences. 2021; 11(8):1031. https://doi.org/10.3390/brainsci11081031
Chicago/Turabian StyleMignani, Luca, Barbara Gnutti, Daniela Zizioli, and Dario Finazzi. 2021. "Coenzyme a Biochemistry: From Neurodevelopment to Neurodegeneration" Brain Sciences 11, no. 8: 1031. https://doi.org/10.3390/brainsci11081031
APA StyleMignani, L., Gnutti, B., Zizioli, D., & Finazzi, D. (2021). Coenzyme a Biochemistry: From Neurodevelopment to Neurodegeneration. Brain Sciences, 11(8), 1031. https://doi.org/10.3390/brainsci11081031







