Neurosonological Findings Related to Non-Motor Features of Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
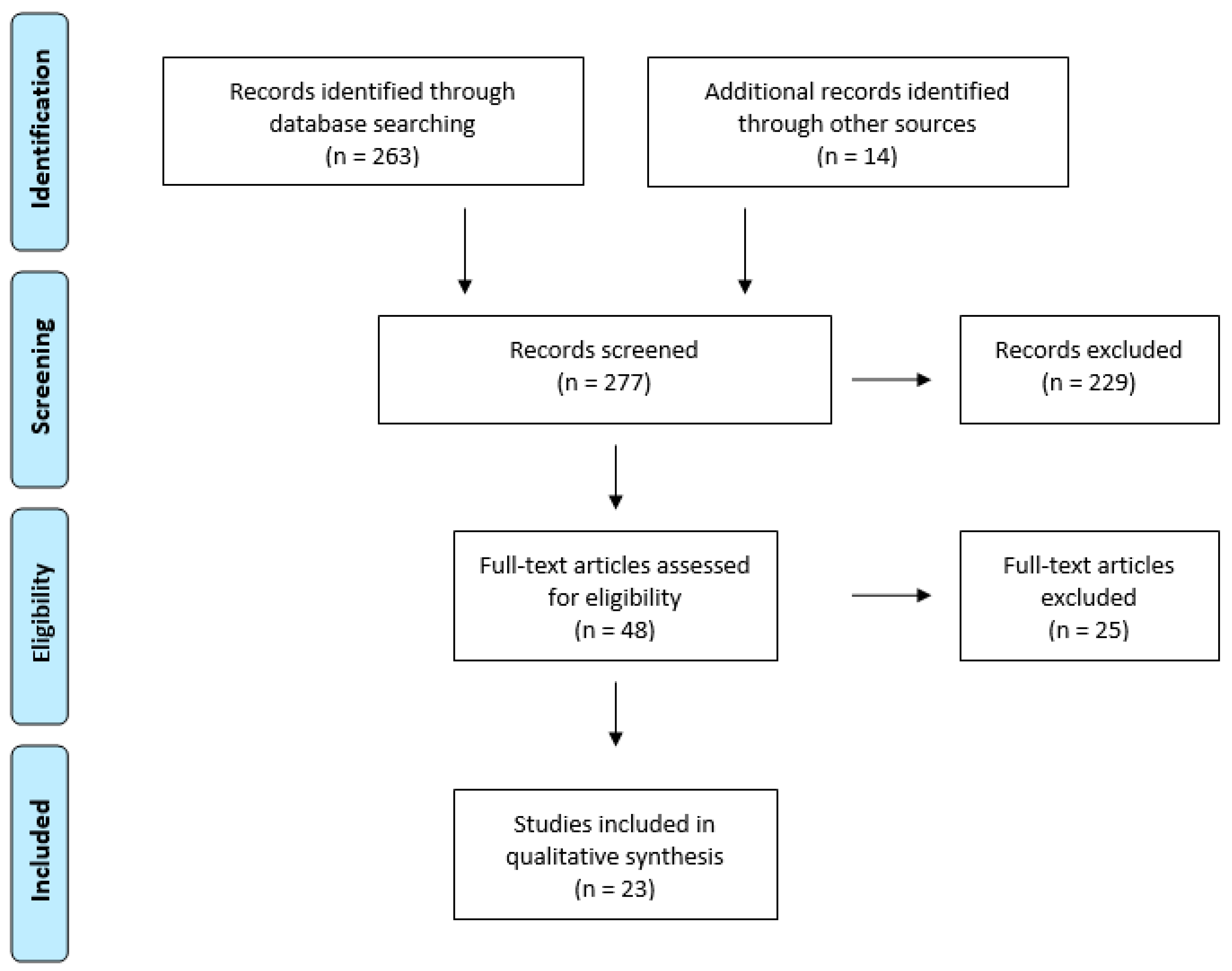
2.2. Selection of Studies
2.3. Data Extraction
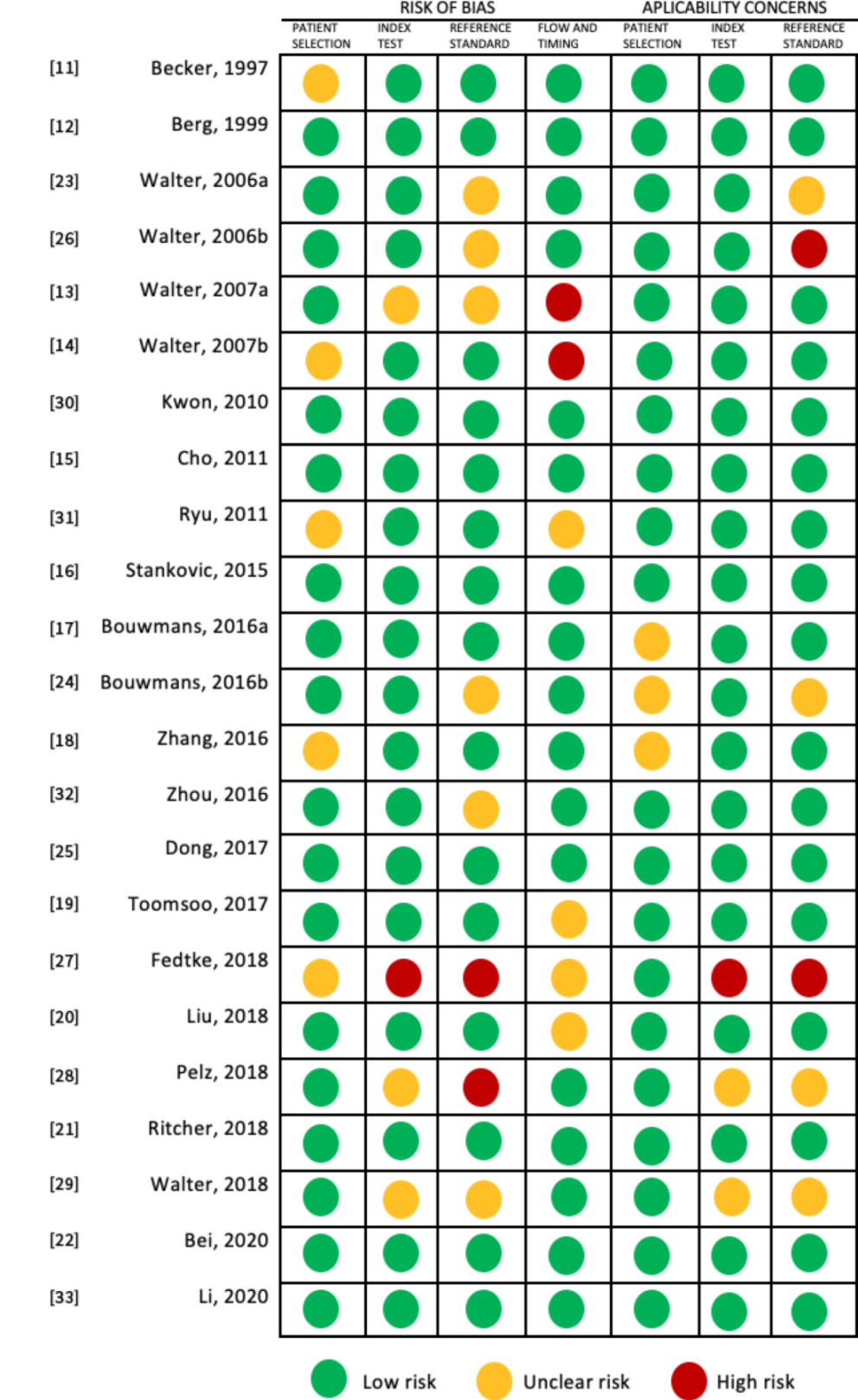
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Main Findings
3.3.1. Depression
3.3.2. Dementia
3.3.3. Autonomic Dysfunction
3.3.4. Restless Leg Syndrome
3.3.5. Hallucinations and Psychosis
4. Discussion
4.1. Brainstem Raphe
4.2. Substantia Nigra
4.3. Lateral and Third Ventricle (Width)
4.4. Vagus Nerve Atrophy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Trascranial Ultrasound Images Identifying the Main Referred Structures
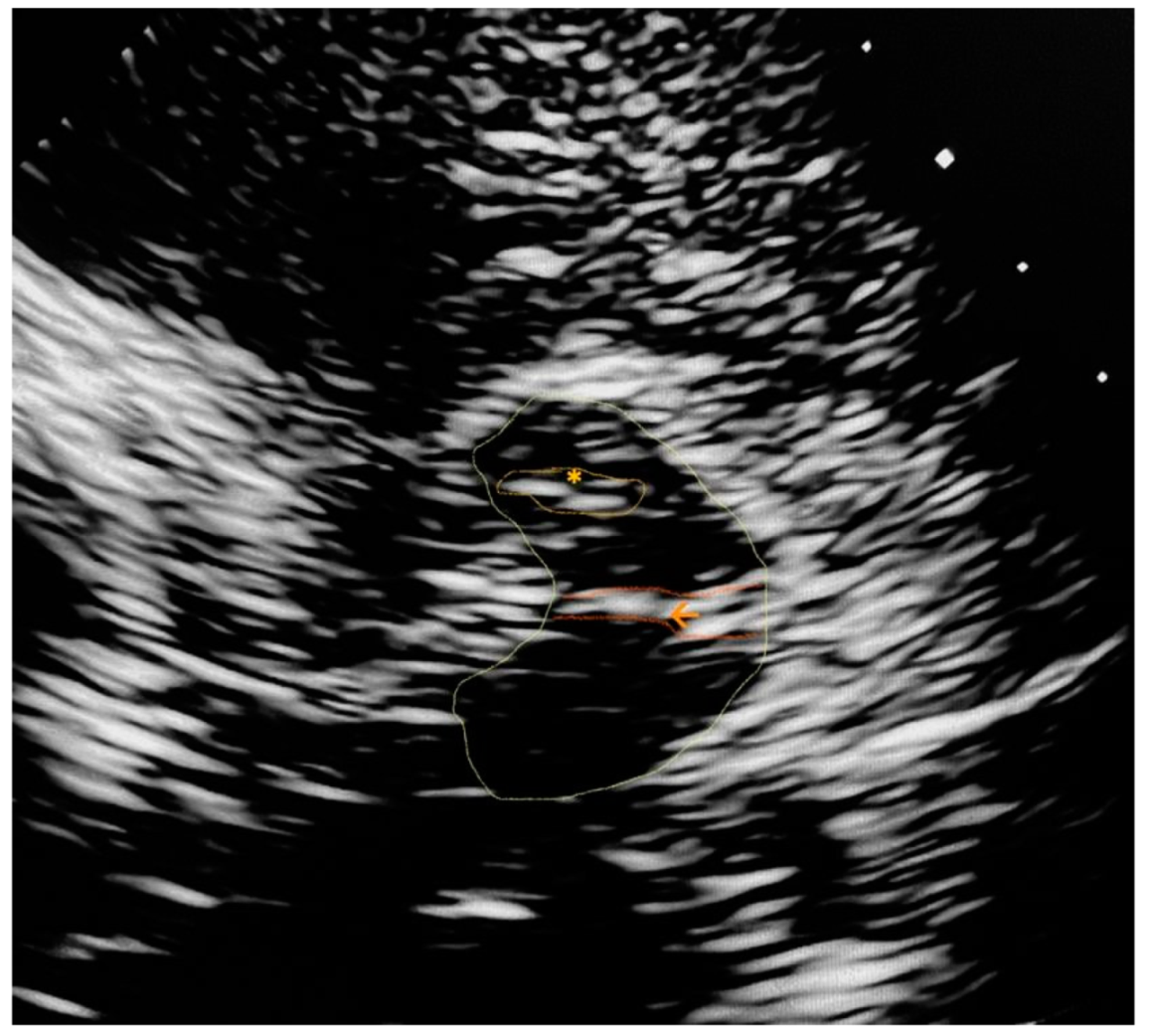

References
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Rela Disord. 2016, 22, S119–S122. Available online: https://pubmed.ncbi.nlm.nih.gov/26372623/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Weerkamp, N.J.; Tissingh, G.; Poels, P.J.E.; Zuidema, S.U.; Munneke, M.; Koopmans, R.T.C.M.; Bloem, B.R. Nonmotor symptoms in nursing home residents with Parkinson’s disease: Prevalence and effect on quality of life. J. Am. Geriat. Soc. 2013, 61, 1714–1721. Available online: https://pubmed.ncbi.nlm.nih.gov/24117286/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Reijnders, J.S.A.M.; Ehrt, U.; Weber, W.E.J.; Aarsland, D.; Leentjens, A.F.G. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189. Available online: https://pubmed.ncbi.nlm.nih.gov/17987654/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Den Brok, M.G.H.E.; van Dalen, J.W.; van Gool, W.A.; Moll van Charante, E.P.; de Bie, R.M.A.; Richard, E. Apathy in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2015, 30, 759–769. Available online: https://pubmed.ncbi.nlm.nih.gov/25787145/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Alonso-Cánovas, A.; López-Sendón, J.L.; Buisán, J.; DeFelipe-Mimbrera, A.; Guillán, M.; García-Barragán, N.; Corral, I.; Matute-Lozano, M.C.; Masjuan, J.; Martínez-Castrillo, J.C.; et al. Sonography for diagnosis of parkinson disease-From theory to practice: A study on 300 participants. J. Ultrasound Med. 2014, 33, 2069–2074. Available online: https://pubmed.ncbi.nlm.nih.gov/25425362/ (accessed on 22 May 2021). [CrossRef]
- Pilotto, A.; Yilmaz, R.; Berg, D. Developments in the Role of Transcranial Sonography for the Differential Diagnosis of Parkinsonism. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–10. Available online: https://link.springecom/article/10.1007/s11910-015-0566-9 (accessed on 22 May 2021). [CrossRef]
- Walter, U.; Wittstock, M.; Benecke, R.; Dressler, D. Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. J. Neural Transm. 2002, 109, 191–196. Available online: https://pubmed.ncbi.nlm.nih.gov/12075859/ (accessed on 22 May 2021). [CrossRef]
- Walter, U.; Školoudík, D.; Berg, D. Transcranial sonography findings related to non-motor features of Parkinson’s diseas. J. Neurol. Sci. 2010, 289, 123–127. Available online: https://pubmed.ncbi.nlm.nih.gov/19735925/ (accessed on 22 May 2021). [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, 1–25. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. Am. Coll. Physicians 2011, 155, 529–536. Available online: https://pubmed.ncbi.nlm.nih.gov/22007046/ (accessed on 4 May 2021). [CrossRef]
- Becker, T.; Becker, G.; Seufert, J.; Hofmann, E.; Lange, K.W.; Naumann, M.; Lindner, A.; Reichmann, H.; Riederer, P.; Beckmann, H.; et al. Parkinson’s disease and depression: Evidence for an alteration of the basal limbic system detected by transcranial sonography. J. Neurol. Neurosurg. Psychiatry 1997, 63, 590–596. Available online: https://jnnp.bmj.com/content/63/5/590 (accessed on 4 May 2021). [CrossRef]
- Berg, D.; Supprian, T.; Hofmann, E.; Zeiler, B.; Jäger, A.; Lange, K.W.; Reiners, K.; Becker, T.; Becker, G. Depression in Parkinson’s disease: Brainstem midline alteration on transcranial sonography and magnetic resonance imaging. J. Neurol. 1999, 246, 1186–1193. Available online: https://pubmed.ncbi.nlm.nih.gov/10653314/ (accessed on 4 May 2021). [CrossRef]
- Walter, U.; Dressler, D.; Wolters, A.; Wittstock, M.; Benecke, R. Transcranial brain sonography findings in clinical subgroups of idiopathic Parkinson’s disease. Mov. Disord. 2007, 22, 48–54. [Google Scholar] [CrossRef]
- Walter, U.; Hoeppner, J.; Prudente-Morrissey, L.; Horowski, S.; Herpertz, S.C.; Benecke, R. Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain 2007, 130, 1799–1807. Available online: https://pubmed.ncbi.nlm.nih.gov/17329323/ (accessed on 22 May 2021). [CrossRef]
- Cho, J.W.; Baik, J.S.; Lee, M.S. Mesencephalic midline change on transcranial sonography in early Parkinson’s disease patients with depression. J. Neurol. Sci. 2011, 310, 50–52. Available online: https://pubmed.ncbi.nlm.nih.gov/21862038/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Stanković, I.; Stefanova, E.; Žiropadja, L.; Mijajlović, M.; Pavlović, A.; Kostić, V.S. Transcranial midbrain sonography and depressive symptoms in patients with Parkinson’s diseas. J. Neurol. 2015, 262, 689–695. Available online: https://pubmed.ncbi.nlm.nih.gov/25557281/ (accessed on 22 May 2021). [CrossRef]
- Bouwmans, A.E.P.; Weber, W.E.J.; Leentjens, A.F.G.; Mess, W.H. Transcranial sonography findings related to depression in parkinsonian disorders: Cross-sectional study in 126 patients. PeerJ 2016, 2016, e2037. Available online: https://peerj.com/articles/2037 (accessed on 22 May 2021). [CrossRef]
- Zhang, Y.C.; Hu, H.; Luo, W.F.; Sheng, Y.J.; Chen, X.F.; Mao, C.J.; Xiong, K.P.; Yu, L.F.; Zhang, Y.; Liu, C.F. Alteration of brainstem raphe measured by transcranial sonography in depression patients with or without Parkinson’s disease. Neurol. Sci. 2016, 37, 45–50. Available online: https://europepmc.org/article/med/26253340 (accessed on 22 May 2021). [CrossRef]
- Toomsoo, T.; Randver, R.; Liepelt-Scarfone, I.; Kadastik-Eerme, L.; Asser, T.; Rubanovits, I.; Berg, D.; Taba, P. Prevalence of depressive symptoms and their association with brainstem raphe echogenicity in patients with Parkinson’s disease and non-PD controls. Psychiatry Res. Neuroimaging 2017, 268, 45–49. Available online: https://europepmc.org/article/med/28865346 (accessed on 22 May 2021). [CrossRef] [PubMed]
- Liu, X.J.; Zhang, L.; Zhang, Y.F.; Xu, W.; Hu, Y.; Liu, Y.; Bai, J. Echogenic alteration in the raphe nuclei measured by transcranial sonography in patients with Parkinson disease and depression. Medicine 2018, 7, e13524. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Woitalla, D.; Muhlack, S.; Gold, R.; Tönges, L.; Krogias, C. Brainstem raphe alterations in TCS: A biomarker for depression and apathy in parkinson’s disease patients. Front. Neurol. 2018, 9, 645. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2018.00645/full (accessed on 22 May 2021). [CrossRef]
- Bei, H.Z.; Chen, J.P.; Mao, C.J.; Zhang, Y.C.; Chen, J.; Du, Q.Q.; Xue, F.; He, P.C.; Jin, H.; Wang, F.Y.; et al. Echogenicity Changes in Brainstem Raphe Detected by Transcranial Parenchymal Sonography and Clinical Characteristics in Parkinson’s Disease. Front. Neurol. 2020, 11, 821. Available online: www.frontiersiorg (accessed on 22 May 2021). [CrossRef] [PubMed]
- Walter, U.; Dressler, D.; Wolters, A.; Wittstock, M.; Greim, B.; Benecke, R. Sonographic discrimination of dementia with Lewy bodies and Parkinson’s disease with dementia. J. Neurol. 2006, 253, 448–454. Available online: https://pubmed.ncbi.nlm.nih.gov/16267638/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Bouwmans, A.E.; Leentjens, A.F.; Mess, W.H.; Weber, W.E. Abnormal echogenicity of the substantia nigra, raphe nuclei, and third-ventricle width as markers of cognitive impairment in parkinsonian disorders: A cross-sectional study. Parkinsons Dis. 2016, 2016, 4058580. [Google Scholar] [CrossRef][Green Version]
- Dong, Z.F.; Wang, C.S.; Zhang, Y.C.; Zhang, Y.; Sheng, Y.J.; Hu, H.; Luo, W.F.; Liu, C.F. Transcranial sonographic alterations of substantia Nigra and third ventricle in Parkinson’s disease with or without dementia. Chin. Med. J. 2017, 130, 2291–2295. Available online: https://pubmed.ncbi.nlm.nih.gov/28937033/ (accessed on 22 May 2021).
- Walter, U.; Dressler, D.; Wolters, A.; Wittstock, M.; Benecke, R. Overactive bladder in Parkinson’s disease: Alteration of brainstem raphe detected by transcranial sonography. Eur. J. Neurol. 2006, 13, 1291–1297. Available online: https://pubmed.ncbi.nlm.nih.gov/17116210/ (accessed on 22 May 2021). [CrossRef]
- Fedtke, N.; Witte, O.W.; Prell, T. Ultrasonography of the vagus nerve in Parkinson’s disease. Front. Neurol. 2018, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.; Belau, E.; Fricke, C.; Classen, J.; Weise, D. Axonal degeneration of the vagus nerve in Parkinson’s disease-a high-resolution ultrasound study. Front. Neurol. 2018, 9, 951. Available online: https://pubmed.ncbi.nlm.nih.gov/30483212/ (accessed on 22 May 2021). [CrossRef]
- Walter, U.; Tsiberidou, P.; Kersten, M.; Storch, A.; Löhle, M. Atrophy of the Vagus Nerve in Parkinson’s Disease Revealed by High-Resolution Ultrasonography. Front. Neurol. 2018, 9, 805. Available online: https://pubmed.ncbi.nlm.nih.gov/30319534/ (accessed on 22 May 2021). [CrossRef]
- Kwon, D.Y.; Seo, W.K.; Yoon, H.K.; Park, M.H.; Koh, S.B.; Park, K.W. Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Mov. Disord. 2010, 25, 1373–1378. Available online: https://pubmed.ncbi.nlm.nih.gov/20544813/ (accessed on 22 May 2021). [CrossRef]
- Ryu, J.H.; Lee, M.S.; Baik, J.S. Sonographic abnormalities in idiopathic restless legs syndrome (RLS) and RLS in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 201–203. Available online: https://pubmed.ncbi.nlm.nih.gov/21183393/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Zhou, H.Y.; Sun, Q.; Tan, Y.Y.; Hu, Y.Y.; Zhan, W.W.; Li, D.H.; Wang, Y.; Xiao, Q.; Liu, J.; Chen, S.D. Substantia nigra echogenicity correlated with clinical features of Parkinson’s disease. Park. Relat. Disord. 2016, 24, 28–33. Available online: https://pubmed.ncbi.nlm.nih.gov/26842545/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Li, T.; Shi, J.; Qin, B.; Fan, D.; Liu, N.; Ni, J.; Zhang, T.; Zhou, H.; Xu, X.; Wei, M.; et al. Increased substantia nigra echogenicity correlated with visual hallucinations in Parkinson’s disease: A Chinese population-based study. Neurol. Sci. 2020, 41, 661–667. Available online: https://link.springer.com/content/pdf/10.1007/s10072-019-04110-z.pdf (accessed on 22 May 2021). [CrossRef] [PubMed]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; van Hilten, J.J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord. 2004, 19, 1306–1312. Available online: https://pubmed.ncbi.nlm.nih.gov/15390007/ (accessed on 23 May 2021). [CrossRef]
- Laucius, O.; Balnytė, R.; Petrikonis, K.; Matijošaitis, V.; Jucevičiūtė, N.; Vanagas, T.; Danielius, V. Ultrasonography of the Vagus Nerve in the Diagnosis of Parkinson’s Disease. Park. Dis. 2020, 2020, 2627471. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2018.00525/full (accessed on 22 May 2021). [CrossRef]
- Goetz, C.C. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations [Internet]. Vol. 18, Movement Disorders. Mov. Disord. 2003, 18, 738–750. Available online: https://pubmed.ncbi.nlm.nih.gov/12815652/ (accessed on 23 May 2021).
- Chaudhuri, K.R.; Pal, S.; Dimarco, A.; Whately-Smith, C.; Bridgman, K.; Mathew, R.; Pezzela, F.R.; Forbes, A.; Högl, B.; Trenkwalder, C. The Parkinson’s disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 629–635. Available online: www.jnnp.com (accessed on 27 May 2021). [CrossRef] [PubMed]
- Varma, M.G.; Wang, J.Y.; Berian, J.R.; Patterson, T.R.; McCrea, G.L.; Hart, S.L. The constipation severity instrument: A validated measure. Dis. Colon Rectum 2008, 51, 162–172. Available online: https://link.springecom/article/10.1007/s10350-007-9140-0 (accessed on 27 May 2021). [CrossRef]
- Brown, R.G.; Dittner, A.; Findley, L.; Wessely, S.C. The Parkinson fatigue scale. Park. Relat. Disord. 2005, 11, 49–55. Available online: https://pubmed.ncbi.nlm.nih.gov/15619463/ (accessed on 27 May 2021). [CrossRef]
- Becker, G.; Struck, M.; Bogdahn, U.; Becker, T. Echogenicity of the brainstem raphe in patients with major depression. Psychiatry Res. Neuroimaging 1994, 55, 75–84. Available online: https://pubmed.ncbi.nlm.nih.gov/10711796/ (accessed on 22 May 2021). [CrossRef]
- Walter, U.; Prudente-Morrissey, L.; Herpertz, S.C.; Benecke, R.; Hoeppner, J. Relationship of brainstem raphe echogenicity and clinical findings in depressive states. Psychiatry Res. Neuroimaging 2007, 155, 67–73. Available online: https://pubmed.ncbi.nlm.nih.gov/17391931/ (accessed on 22 May 2021). [CrossRef]
- Walter, U.; Krolikowski, K.; Tarnacka, B.; Benecke, R.; Czlonkowska, A.; Dressler, D. Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology 2005, 64, 1726–1732. Available online: https://pubmed.ncbi.nlm.nih.gov/15911799/ (accessed on 22 May 2021). [CrossRef]
- Krogias, C.; Strassburger, K.; Eyding, J.; Gold, R.; Norra, C.; Juckel, G.; Saft, C.; Ninphius, D. Depression in patients with Huntington disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J. Psychiatry Neurosci. 2011, 36, 187–194. Available online: https://pubmed.ncbi.nlm.nih.gov/21138658/ (accessed on 22 May 2021). [CrossRef]
- Becker, G.; Berg, D.; Lesch, K.P.; Becker, T. Basal limbic system alteration in major depression: A hypothesis supported by transcranial sonography and MRI findings. International Journal of Neuropsychopharmacology. Int. J. Neuropsychopharmacol. 2001, 4, 21–31. Available online: https://pubmed.ncbi.nlm.nih.gov/11343626/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Berg, D.; Supprian, T.; Thomae, J.; Warmuth-Metz, M.; Horowski, A.; Zeiler, B.; Magnus, T.; Rieckmann, P.; Becker, G. Lesion pattern in patients with multiple sclerosis and depression. Mult. Scler. J. 2000, 6, 156–162. Available online: https://pubmed.ncbi.nlm.nih.gov/10871826/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Brooks, D.J.; Piccini, P. Imaging in Parkinson’s Disease: The Role of Monoamines in Behavior. Biol. Psychiatry 2006, 59, 908–918. Available online: https://pubmed.ncbi.nlm.nih.gov/16581032/ (accessed on 22 May 2021). [CrossRef]
- Mayeux, R.; Stern, Y.; Sano, M.; Williams, J.B.W.; Cote, L.J. The relationship of serotonin to depression in Parkinson’s disease. Mov. Disord. 1988, 3, 237–244. Available online: https://pubmed.ncbi.nlm.nih.gov/2461509/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Zhuang, X.; Xu, H.; Fang, Z.; Xu, C.; Xue, C.; Hong, X. Platelet serotonin and serotonin transporter as peripheral surrogates in depression and anxiety patients. Eur. J. Pharmacol. 2018, 834, 213–220. Available online: https://europepmc.org/article/med/30031795 (accessed on 22 May 2021). [CrossRef] [PubMed]
- Andersson, K.E.; Pehrson, R. CNS Involvement in Overactive Bladder: Pathophysiology and Opportunities for Pharmacological Intervention Drugs. Drugs 2003, 63, 2595–2611. Available online: https://pubmed.ncbi.nlm.nih.gov/14636079/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Cheng, S.; Lin, D.; Hu, T.; Cao, L.; Liao, H.; Mou, X.; Zhang, Q.; Liu, J.; Wu, T. Association of urinary incontinence and depression or anxiety: A meta-analysis. J. Int. Med. Res. 2020, 48, 300060520931348. Available online: https://pubmed.ncbi.nlm.nih.gov/32552169/ (accessed on 22 May 2021). [CrossRef]
- Roy, H.A.; Griffiths, D.J.; Aziz, T.Z.; Green, A.L.; Menke, R.A.L. Investigation of urinary storage symptoms in Parkinson’s disease utilizing structural MRI techniques. Neurourol. Urodyn. 2019, 38, 1168–1175. Available online: https://pubmed.ncbi.nlm.nih.gov/30869824/ (accessed on 22 May 2021). [CrossRef]
- Nilsson, F.M.; Kessing, L.V.; Bolwig, T.G. Increased risk of developing Parkinson’s disease for patients with major affective disorder: A register study. Acta Psychiatr. Scand. 2001, 104, 380–386. Available online: https://pubmed.ncbi.nlm.nih.gov/11722320/ (accessed on 22 May 2021). [CrossRef]
- Schuurman, A.G.; Van den Akker, M.; Ensinck, K.T.J.L.; Metsemakers, J.F.M.; Knottnerus, J.A.; Leentjens, A.F.G.; Buntinx, F. Increased risk of Parkinson’s disease after depression: A retrospective cohort study. Neurology 2002, 58, 1501–1504. Available online: https://pubmed.ncbi.nlm.nih.gov/12034786/ (accessed on 22 May 2021). [CrossRef]
- Leentjens, A.F.G.; Van den Akker, M.; Metsemakers, J.F.M.; Lousberg, R.; Verhey, F.R.J. Higher incidence of depression preceding the onset of parkinson’s disease: A register study. Mov. Disord. 2003, 18, 414–418. Available online: https://pubmed.ncbi.nlm.nih.gov/12671948/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Lenka, A.; Jhunjhunwala, K.R.; Saini, J.; Pal, P.K. Structural and functional neuroimaging in patients with Parkinson’s disease and visual hallucinations: A critical review. Parkinsonism Relat. Disord. 2015, 21, 683–691. Available online: https://pubmed.ncbi.nlm.nih.gov/25920541/ (accessed on 22 May 2021). [CrossRef]
- Liepelt, I.; Wendt, A.; Schweitzer, K.J.; Wolf, B.; Godau, J.; Gaenslen, A.; Bruessel, T.; Berg, D. Substantia nigra hyperechogenicity assessed by transcranial sonography is related to neuropsychological impairment in the elderly population. J. Neural. Transm. 2008, 115, 993–999. Available online: https://pubmed.ncbi.nlm.nih.gov/18368284/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Yilmaz, R.; Behnke, S.; Liepelt-Scarfone, I.; Roeben, B.; Pausch, C.; Runkel, A.; Heinzel, S.; Niebler, R.; Suenkel, U.; Eschweiler, G.W.; et al. Substantia nigra hyperechogenicity is related to decline in verbal memory in healthy elderly adults. Eur. J. Neurol. 2016, 23, 973–978. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/en12974 (accessed on 22 May 2021). [CrossRef] [PubMed]
- Lucero, C.; Campbell, M.C.; Flores, H.; Maiti, B.; Perlmutter, J.S.; Foster, E.R. Cognitive reserve and β-amyloid pathology in Parkinson disease. Park. Relat. Disord. 2015, 21, 899–904. Available online: https://pubmed.ncbi.nlm.nih.gov/26037458/ (accessed on 22 May 2021). [CrossRef]
- Armstrong, R.A. Laminar degeneration of frontal and temporal cortex in Parkinson disease dementia. Neurol. Sci. 2017, 38, 667–671. Available online: https://pubmed.ncbi.nlm.nih.gov/28181068/ (accessed on 22 May 2021). [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. Available online: https://pubmed.ncbi.nlm.nih.gov/12721813/ (accessed on 22 May 2021). [CrossRef] [PubMed]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.Y.; Roybon, L.; Melki, R.; Li, J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820. Available online: https://pubmed.ncbi.nlm.nih.gov/25296989/ (accessed on 22 May 2021). [CrossRef]
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529. Available online: https://pubmed.ncbi.nlm.nih.gov/26031848/ (accessed on 22 May 2021). [CrossRef] [PubMed]
| STUDY | Population N, Age/Male | Symptom Evaluation | Ultrasound Evaluation | Main Findings | Other Findings |
|---|---|---|---|---|---|
| Depression | |||||
| Becker, 1997 [11] | 30 PD+. 68,3/25 30 PD– 65/24 | DSM-III HDRS CGI-S | TCS, 2.25 MHz. BR echogenicity * Ventricles Width | PD+, D+: ↓BR echogenicity, ↑lateral ventricles. Correlation: BR echogenicity and D severity. | No differences PD+, D− and healthy controls. |
| Berg, 1999 [12] | 31 PD+ 65,5/16 | DSM-IV HDRS BDI | TCS, 2.5 MHz. BR echogenicity * | PD+, D+: ↓BR echogenicity | MRI: PD+, D+: hyperintense mesencephal ic midline |
| Walter, 2007a [13] | 101 PD+ 66,6/58 | DSM IV | TCS, 2.5 MHz. BR echogenicity ^ | PD+, D+: ↓ BR echogenicity | N.R. |
| Walter, 2007b [14] | 45PD+, D+ 45PD+, D− 55 PD−, D+ 55PD−, D− 61/84 | DSM IV DRS BDI | TCS, 2.5 MHz. SN echogenicity (N < 20 mm2) BR echogenicity ^. | PD+, D+: ↓BR echogenicity. PD+, D+ vs. D−: No difference in SN. ↑SN, ↓BR: Depression prior to PD diagnosis | PD−, D+: ↑SN |
| Cho, 2011 [15] | 61 PD+ 68/38 41 PD−, D– 58/28 | HDRS BDI | TCS, 2.5 MHz. BR echogenicity * | PD+, D+: ↓BR echogenicity, Correlation: ↓ BR echogenicity and ↑Hamilton Depression Scale. | PD + D+: higher motor severity |
| Stankovic, 2015 [16] | 118 PD+ 61/72 | HARS Apathy Scale MADRS | TCS, 2.5 MHz SN echogenicity (N < 19 mm2) BR echogenicity * | PD+, D+: ↓BR echogenicity (> sadness, pessimism, >anxiety) | ↓BR echogenicity, ↑L-Dopa motor complications. |
| Bouwmans, 2016a [17] | 72 PD+, 68/N.R. 54 other PK 72/N.R. | HDRS | TCS, 2–4 MHz. SN echogenicity (N < 20 mm2) BR echogenicity ^ 3rd. ventricle Width | No differences (Only 16 D+) | N.R. |
| Zhang, 2016 [18] | 80 PD+ 40 PD− D+ 40 PD– D− 61/97 | HDRS BDI | TCS, 2.5 MHz BR echogenicity † | PD+, D+ and PD−, D+: ↓BR echogenicity. Correlation: ↓ BR echogenicity and ↑HDRS, BDI. | N.R. |
| Toomsoo, 2017 [19] | 266 PD+ 168 PD– 69,7/228 | BDI | TCS, 1.8–3.6 MHz SN echogenicity (N < 20 mm2) BR echogenicity * | PD + D+ and PD− D +: ↓BR echogenicity. Correlation: ↓ BR echogenicity and ↑BDI | Correlation: D and PD duration, motor and cognitive impairment. |
| Liu, 2018 [20] | 30 D+ PD+ 30 D− PD+ 24 D+ PD− | HDRS | TCS, 2.5 MHz BR echogenicity ^ SN echogenicity | PD+, D+ and PD− D+: ↓BR echogenicity. No | Platelet serotonin. |
| 28 D– PD− 55/56 | Platelet serotonin levels | (N < 20 mm2). 3rd. ventricle width | association SN and RN echogenicity. | Levels: no differences. | |
| Ritcher, 2018 [21] | 31 PD+ 16 ET+ 16 PD−, ET− | Lille apathy rating scale. BDI | TCS, 2.5 MHz. SN echogenicity (N < 20 mm2) BR echogenicity * | PD+: ↓BR echogenicity. Correlation: ↓ BR echogenicity and ↑Apathy, Beck Scores. | No difference: SN in ET+ and controls. |
| Bei, 2020 [22] | 135 PD+ 63/83 | HDRS HARS | TCS, 2.5 MHz SN echogenicity (N < 20 mm2) BR echogenicity ^ | D+, Anxiety+: ↓BR echogenicity. Correlation: ↓ BR echogenicity and ↑Hamilton Scores, PDQ-39 | No relation BR and motor symptoms. |
| Dementia | |||||
| Walter, 2006a [23] | 104 PD+ 14 DLB+ 70/69 | MMSE Addenbrooke cognitive examination | TCS, 2.5 MHz SN echogenicity (N < 20 mm2) Thalami, Caudate, BR echogenicity ^, 3rd. ventricle Width | PD + Dementia+: ↑ lateral frontal (17.3 mm), 3rd ventricle (8.6 mm) widths. | DLB+ vs. PD+ dementia +: Bilateral ↑ SN in DLB+. Similar ventricle widths. |
| Walter, 2007a [13] | 101 PD+ 66,6/58 | DSM IV MMSE | TCS, 2.5 MHz. ventricles width | PD + Dementia+: Lateral frontal horn ≥15.4 mm | ↑Caudate echogenicity: ↑drug- induced psychosis. |
| Bouwmans, 2016b [24] | 72 PD+ 68/70 54 other PK 72/80 | SCOPA-COG: PD cognition Scale. | TCS, 2–4 MHz. SN echogenicity (N < 20 mm2) BR echogenicity ^, 3rd. ventricle width | Larger 3rd ventricle in PD+ and cognitive impairment. SN: Not related to cognition. | Atypical PK + cognitive symptoms: ↓BR echogenicity (not in PD) |
| Dong, 2017 [25] | 98 PD+ 77/68 40 PD– 65/27 | Dementia clinical diagnosis. MMSE MoCA PD-NMSQ | TCS, 2.5 MHz. SN echogenicity (N < 20 mm2) 3rd. ventricle width (Normal < 7/10 mm under/over 60 y.) | Larger 3rd ventricle in PD+ with dementia. Cutoff 6.8 mm (S: 69.6%, Sp: 61.5%). SN: Not related with cognition. | 3rd. ventricle: No differences PD without dementia and controls. |
| Autonomic dysfunction | |||||
| Walter, 2006b [26] | 116 PD+ 66,5/65 | Overactive bladder symptoms (other causes ruled out) | TCS, 2.5 MHz. BR echogenicity * SN echogenicity, thalamus, 3rd.ventricle width | Overactive bladder: ↓BR echogenicity. | N.R. |
| Fedtke, 2018 [27] | 32 PD+ 30 PD− 70/40 | UPDRS I–IV | HRUS Vagus nerve (cervical CSA) | No differences PD+, PD−. No correlation with UPDRS I-IV. | Positive correlation: Right CSA and bradykinesia score. |
| Pelz, 2018 [28] | 35 PD+ 35 PD– 67/34 | PD-NMSQ, MoCA | 15 MHz HRUS Vagus nerve (cervical CSA) | PD+: Smaller bilateral CSA. No | N.R. |
| correlation with PD− NMSQ. | |||||
| Walter, 2018 [29] | 20 PD+ 73/13 61 PD− 45/23 | PD-NMSQ, heart rate variability (R-R) | 15 MHz HRUS Vagus, spinal, accessory, phrenic nerves (cervical CSA) | PD+: Smaller bilateral CSA. Negative Correlation: CSA and PD-NMSQ, autonomic items. Heart rate variability and right CSA in PD + and PD−. No differences in other nerves | Left CSA correlates with motor severity. |
| Restless legs syndrome | |||||
| Kwon, 2010 [30] | 63 PD+ 65/30 40iRLS+ 53/21 40 controls 69/21 | Sleep questionnaire Neurologist assessment | TCS, 2.5 MHz. SN echogenicity (N < 20 mm2) | SN: No differences in PD + with and without RLS. | iRLS+: ↓↓SN size than PD+ and controls. |
| Ryu, 2011 [31] | 44PD+ 41iRLS+ 35 controls 60–71 | RLS Diagnostic criteria | TCS, 2.5 MHz. SN echogenicity (N < 20 mm2) | SN: No differences in PD + with and without RLS. | iRLS +: ↓↓SN size than PD+ and controls. |
| Hallucinations and psychosis | |||||
| Zhou, 2016 [32] | 201 PD+ 92 PD– 60/193 | PD-NMSQ Odor test RBDSQ SCOPA-AUT MMSE HDRS | TCS, 2.5 MHz. SN echogenicity (N < 18 mm2) | No correlation SN and non-motor symptoms | Correlation: SN and UPDRS-II score |
| Li, 2020 [33] | 111 PD+ 61 PD– 66;63/110 | PD-NMSQ Sleep Scale, Constipation, Fatigue, MMSE HDRS, HARS | TCS, 1.82 MHz. SN echogenicity (N < 23.5 mm2) | PD with hallucinations: ↑ SN echo-size | No other differences |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Toro Pérez, C.; Amaya Pascasio, L.; Arjona Padillo, A.; Olivares Romero, J.; Mejías Olmedo, M.V.; Fernández Pérez, J.; Payán Ortiz, M.; Martínez-Sánchez, P. Neurosonological Findings Related to Non-Motor Features of Parkinson’s Disease: A Systematic Review. Brain Sci. 2021, 11, 776. https://doi.org/10.3390/brainsci11060776
del Toro Pérez C, Amaya Pascasio L, Arjona Padillo A, Olivares Romero J, Mejías Olmedo MV, Fernández Pérez J, Payán Ortiz M, Martínez-Sánchez P. Neurosonological Findings Related to Non-Motor Features of Parkinson’s Disease: A Systematic Review. Brain Sciences. 2021; 11(6):776. https://doi.org/10.3390/brainsci11060776
Chicago/Turabian Styledel Toro Pérez, Cristina, Laura Amaya Pascasio, Antonio Arjona Padillo, Jesús Olivares Romero, María Victoria Mejías Olmedo, Javier Fernández Pérez, Manuel Payán Ortiz, and Patricia Martínez-Sánchez. 2021. "Neurosonological Findings Related to Non-Motor Features of Parkinson’s Disease: A Systematic Review" Brain Sciences 11, no. 6: 776. https://doi.org/10.3390/brainsci11060776
APA Styledel Toro Pérez, C., Amaya Pascasio, L., Arjona Padillo, A., Olivares Romero, J., Mejías Olmedo, M. V., Fernández Pérez, J., Payán Ortiz, M., & Martínez-Sánchez, P. (2021). Neurosonological Findings Related to Non-Motor Features of Parkinson’s Disease: A Systematic Review. Brain Sciences, 11(6), 776. https://doi.org/10.3390/brainsci11060776






