Selecting the Most Relevant Brain Regions to Classify Children with Developmental Dyslexia and Typical Readers by Using Complex Magnocellular Stimuli and Multiple Kernel Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessment
- (1)
- IQ, as estimated by the vocabulary and block design subscales of the WISC-III [107];
- (2)
- (3)
- Verbal working memory (VWM), as assessed by the Single Digit Forward Span, Single Digit Backward Span, Single Letter Forward Span, and Single Letter Backward Span tasks [112];
- (4)
- Phonological skills, as assessed by the nonword repetition test (NWR) [113];
- (5)
- Hand preference, as assessed by the Briggs and Nebes Inventory (BNI) [114];
- (6)
2.3. MRI Acquisition Protocol
2.4. fMRI Task Design
2.4.1. Full-Field Sinusoidal Gratings
2.4.2. CM Detection
2.5. Anatomical MRI Data Analysis
2.6. fMRI Data Processing
2.7. Multivariate Analyses
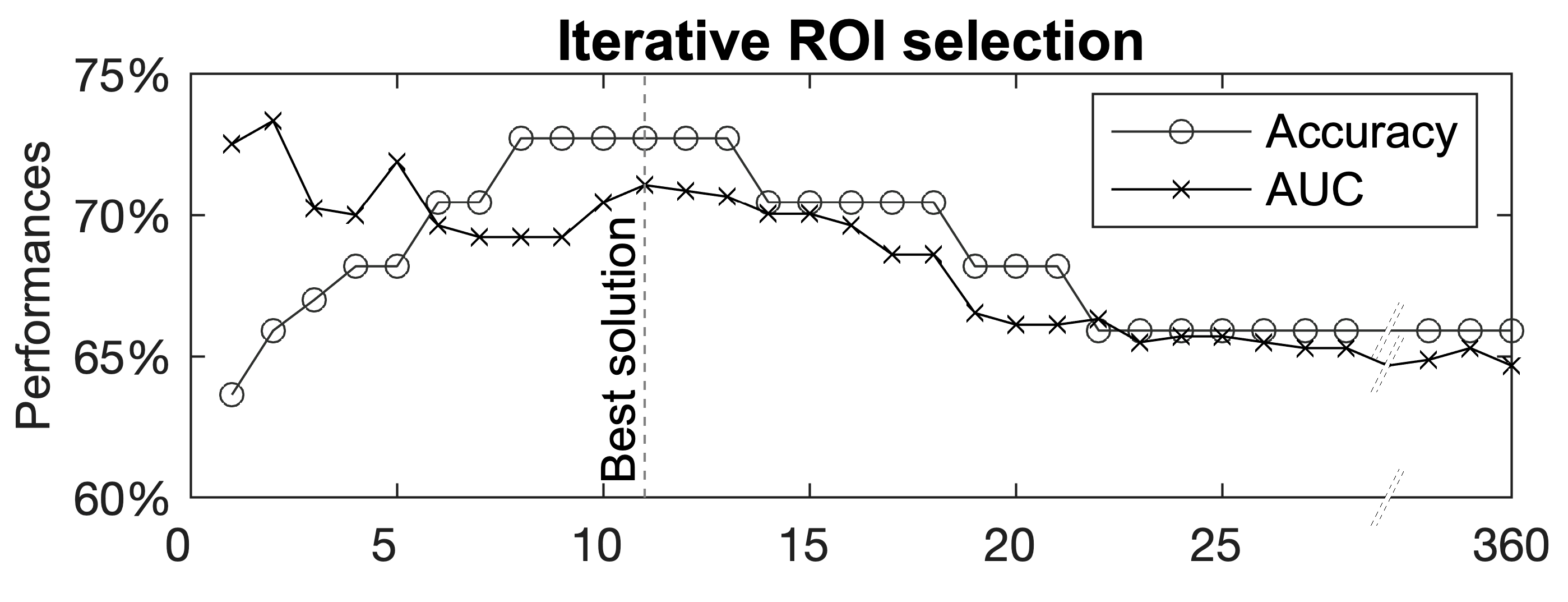
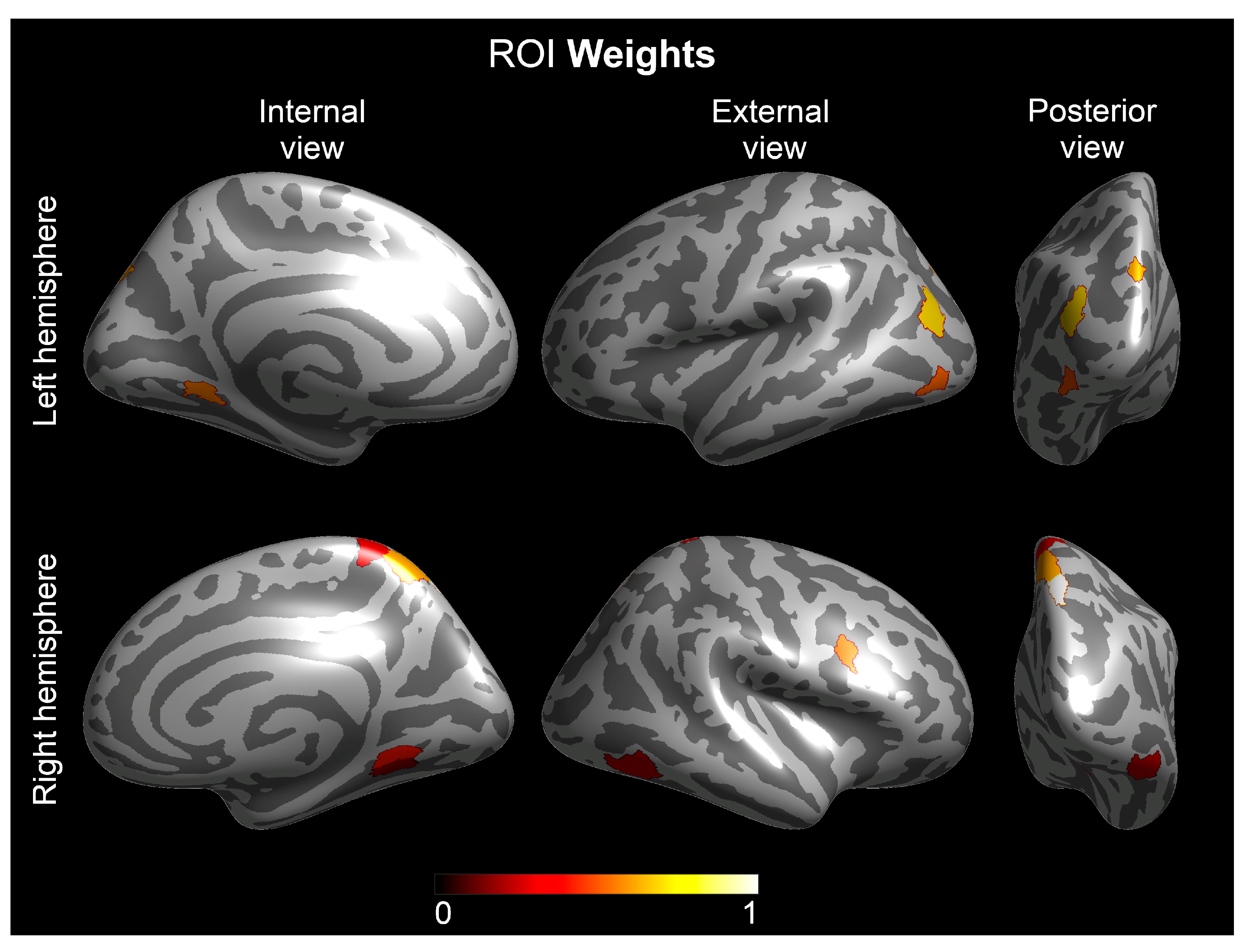
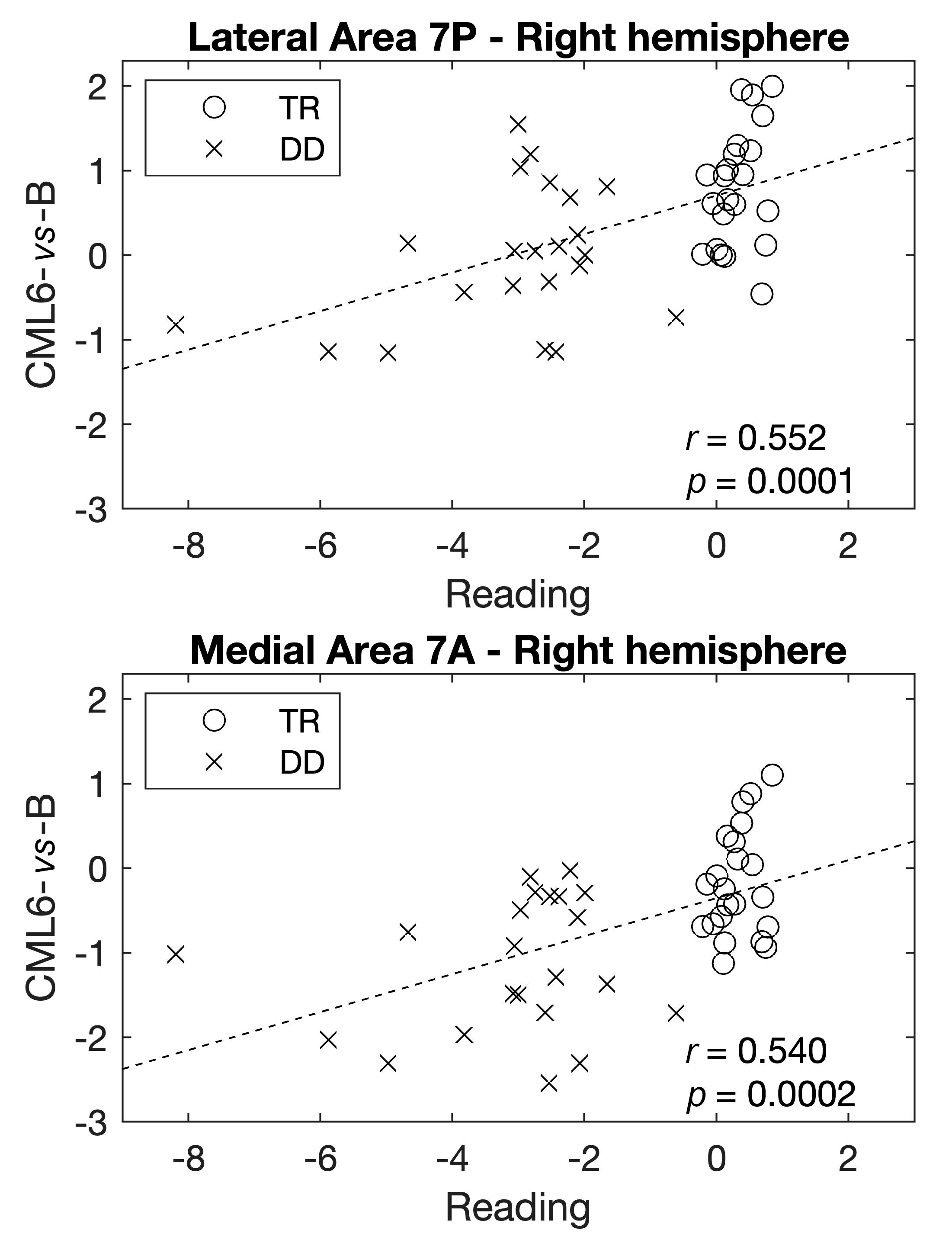
3. Results
Multivariate Analyses—The Group Lasso–Multiple Kernel Learning Algorithm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. DSM-V Manuale Diagnostico e Statistico dei Disturbi Mentali, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- Peterson, R.L.; Pennington, B.F. Developmental Dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307. [Google Scholar] [CrossRef]
- Lyon, G.R.; Shaywitz, S.E.; Shaywitz, B.A. A definition of dyslexia. Ann. Dyslexia 2003, 53, 1–14. [Google Scholar] [CrossRef]
- Bailey, S.K.; Aboud, K.S.; Nguyen, T.Q.; Cutting, L.E. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018, 10, 37. [Google Scholar] [CrossRef]
- Norton, E.S.; Wolf, M. Rapid Automatized Naming (RAN) and Reading Fluency: Implications for Understanding and Treatment of Reading Disabilities. Annu. Rev. Psychol. 2012, 63, 427–452. [Google Scholar] [CrossRef]
- Wylie, J.; Thomson, J.; Leppänen, P.H.; Ackerman, R.; Kanniainen, L.; Prieler, T. Chapter 3. Cognitive processes and digital reading. In Studies in Written Language and Literacy; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2018; Volume 17, pp. 57–90. [Google Scholar]
- D’Mello, A.M.; Gabrieli, J.D.E. Cognitive Neuroscience of Dyslexia. Lang. Speech Hear. Serv. Sch. 2018, 49, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Vellutino, F.R.; Fletcher, J.M.; Snowling, M.J.; Scanlon, D.M. Specific reading disability (dyslexia): What have we learned in the past four decades? J. Child Psychol. Psychiatry 2004, 45, 2–40. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Lancet 2012, 379, 1997–2007. [Google Scholar] [CrossRef]
- Pugh, K.R.; Mencl, W.E.; Jenner, A.R.; Katz, L.; Frost, S.J.; Lee, J.R.; Shaywitz, B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 207–213. [Google Scholar] [CrossRef]
- Norton, E.S.; Beach, S.D.; DE Gabrieli, J. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.I.; Fawcett, A.J. Developmental dyslexia, learning and the cerebellum. J. Neural Trasm Suppl. 2005, 69, 19–36. [Google Scholar] [CrossRef]
- Pugh, K.R.; Landi, N.; Preston, J.L.; Mencl, W.E.; Austin, A.C.; Sibley, D.; Fulbright, R.K.; Seidenberg, M.S.; Grigorenko, E.L.; Constable, R.T.; et al. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013, 125, 173–183. [Google Scholar] [CrossRef]
- Ullman, M.T.; Earle, F.S.; Walenski, M.; Janacsek, K. The Neurocognition of Developmental Disorders of Language. Annu. Rev. Psychol. 2020, 71, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [PubMed]
- McCandliss, B.D.; Cohen, L.; Dehaene, S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003, 7, 293–299. [Google Scholar] [CrossRef]
- Pennington, B.F. From single to multiple deficit models of developmental disorders. Cognition 2006, 101, 385–413. [Google Scholar] [CrossRef]
- Castles, A.; Coltheart, M. Is there a causal link from phonological awareness to success in learning to read? Cognition 2004, 91, 77–111. [Google Scholar] [CrossRef]
- Tallal, P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980, 9, 182–198. [Google Scholar] [CrossRef]
- McAnally, K.I.; Stein, J.F. Auditory temporal coding in dyslexia. Proc. Biol. Sci. 1996, 263, 961–965. [Google Scholar]
- Hämäläinen, J.A.; Guttorm, T.K.; Richardson, U.; Alku, P.; Lyytinen, H.; Leppänen, P.H.T. Auditory Event-Related Potentials Measured in Kindergarten Predict Later Reading Problems at School Age. Dev. Neuropsychol. 2013, 38, 550–566. [Google Scholar] [CrossRef]
- Hornickel, J.; Kraus, N. Unstable Representation of Sound: A Biological Marker of Dyslexia. J. Neurosci. 2013, 33, 3500–3504. [Google Scholar] [CrossRef]
- Vandermosten, M.; Boets, B.; Luts, H.; Poelmans, H.; Golestani, N.; Wouters, J.; Ghesquiere, P. Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proc. Natl. Acad. Sci. USA 2010, 107, 10389–10394. [Google Scholar] [CrossRef]
- Vandermosten, M.; Boets, B.; Luts, H.; Poelmans, H.; Wouters, J.; Ghesquière, P. Impairments in speech and nonspeech sound categorization in children with dyslexia are driven by temporal processing difficulties. Res. Dev. Disabil. 2011, 32, 593–603. [Google Scholar] [CrossRef]
- Molfese, D.L. Predicting Dyslexia at 8 Years of Age Using Neonatal Brain Responses. Brain Lang. 2000, 72, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, P.H.; Hamalainen, J.; Salminen, H.K.; Eklund, K.M.; Guttorm, T.K.; Lohvansuu, K.; Puolakanaho, A.; Lyytinen, H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex 2010, 46, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Van Der Leij, A.; Van Bergen, E.; Van Zuijen, T.L.; De Jong, P.; Maurits, N.; Maassen, B.A.M. Precursors of Developmental Dyslexia: An Overview of the Longitudinal Dutch Dyslexia Programme Study. Dyslexia 2013, 19, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Cantiani, C.; Riva, V.; Piazza, C.; Bettoni, R.; Molteni, M.; Choudhury, N.; Marino, C.; Benasich, A.A. Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Dev. Cogn. Neurosci. 2016, 20, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.A.; Helland, T.; Specht, K.; Narr, K.L.; Manis, F.R.; Toga, A.W.; Hugdahl, K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain 2014, 137, 3136–3141. [Google Scholar] [CrossRef]
- Lervåg, A.; Hulme, C. Rapid Automatized Naming (RAN) Taps a Mechanism That Places Constraints on the Development of Early Reading Fluency. Psychol. Sci. 2009, 20, 1040–1048. [Google Scholar] [CrossRef]
- Hari, R.; Renvall, H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn. Sci. 2001, 5, 525–532. [Google Scholar] [CrossRef]
- Renvall, H.; Hari, R. Auditory Cortical Responses to Speech-Like Stimuli in Dyslexic Adults. J. Cogn. Neurosci. 2002, 14, 757–768. [Google Scholar] [CrossRef]
- Franceschini, S.; Gori, S.; Ruffino, M.; Pedrolli, K.; Facoetti, A. A Causal Link between Visual Spatial Attention and Reading Acquisition. Curr. Biol. 2012, 22, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Gori, S.; Ruffino, M.; Viola, S.; Molteni, M.; Facoetti, A. Action Video Games Make Dyslexic Children Read Better. Curr. Biol. 2013, 23, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Lallier, M.; Donnadieu, S.; Valdois, S. Investigating the role of visual and auditory search in reading and developmental dyslexia. Front. Hum. Neurosci. 2013, 7, 597. [Google Scholar] [CrossRef]
- Plaza, M.; Cohen, H. The contribution of phonological awareness and visual attention in early reading and spelling. Dyslexia 2006, 13, 67–76. [Google Scholar] [CrossRef]
- Vidyasagar, T.R.; Pammer, K. Dyslexia: A deficit in visuo-spatial attention, not in phonological processing. Trends Cogn. Sci. 2010, 14, 57–63. [Google Scholar] [CrossRef]
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychology 2019, 130, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Dehaene, S.; Vinckier, F.; Jobert, A.; Montavont, A. Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. NeuroImage 2008, 40, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.J.; Bryan, R.N.; Miki, A.; Woo, J.H.; Liu, G.T.; Elliott, M.A. Magnocellular and parvocellular visual pathways have different blood oxygen level-dependent signal time courses in human primary visual cortex. Am. J. Neuroradiol. 2006, 27, 1628–1634. [Google Scholar] [PubMed]
- Zhang, P.; Zhou, H.; Wen, W.; He, S. Layer-specific response properties of the human lateral geniculate nucleus and superior colliculus. NeuroImage 2015, 111, 159–166. [Google Scholar] [CrossRef]
- Denison, R.N.; Vu, A.T.; Yacoub, E.; Feinberg, D.A.; Silver, M.A. Functional mapping of the magnocellular and parvocellular subdivisions of human LGN. NeuroImage 2014, 102, 358–369. [Google Scholar] [CrossRef]
- Eden, G.F.; VanMeter, J.W.; Rumsey, J.M.; Maisog, J.M.; Woods, R.P.; Zeffiro, T.A. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature 1996, 382, 66–69. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Matelli, M. Two different streams form the dorsal visual system: Anatomy and functions. Exp. Brain Res. 2003, 153, 146–157. [Google Scholar] [CrossRef]
- Galletti, C.; Gamberini, M.; Kutz, D.F.; Fattori, P.; Luppino, G.; Matelli, M. The cortical connections of area V6: An occipito-parietal network processing visual information. Eur. J. Neurosci. 2001, 13, 1572–1588. [Google Scholar] [CrossRef]
- Gori, S.; Facoetti, A. Perceptual learning as a possible new approach for remediation and prevention of developmental dyslexia. Vis. Res. 2014, 99, 78–87. [Google Scholar] [CrossRef]
- Galletti, C.; Fattori, P. The dorsal visual stream revisited: Stable circuits or dynamic pathways? Cortex 2018, 98, 203–217. [Google Scholar] [CrossRef]
- Pammer, K.; Hansen, P.; Holliday, I.; Cornelissen, P. Attentional shifting and the role of the dorsal pathway in visual word recognition. Neuropsychology 2006, 44, 2926–2936. [Google Scholar] [CrossRef]
- Vidyasagar, T.R. Reading into neuronal oscillations in the visual system: Implications for developmental dyslexia. Front. Hum. Neurosci. 2013, 7, 811. [Google Scholar] [CrossRef] [PubMed]
- Braddick, O.; Atkinson, J. Development of human visual function. Vis. Res. 2011, 51, 1588–1609. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J. The Davida Teller Award lecture, 2016: Visual Brain development: A review of dorsal stream vulnerability-motion, mathematics, amblyopia, actions, and attention. J. Vis. 2017, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.S.; Rosen, G.D.; Drislane, F.W.; Galaburda, A.M. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc. Natl. Acad. Sci. USA 1991, 88, 7943–7947. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Walsh, V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997, 20, 147–152. [Google Scholar] [CrossRef]
- Vidyasagar, T.R. Gating of neuronal responses in macaque primary visual cortex by an attentional spotlight. NeuroReport 1998, 9, 1947–1952. [Google Scholar] [CrossRef]
- Stein, J. Dyslexia: The Role of Vision and Visual Attention. Curr. Dev. Disord. Rep. 2014, 1, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Baudewig, J.; Paulus, W.; Dechent, P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis. Neurosci. 2008, 25, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Grande, M.; Pape-Neumann, J.; van Ermingen, M.; Meffert, E.; Grabowska, A.; Huber, W.; Amunts, K. Interaction of phonological awareness and ‘magnocellular’ processing during normal and dyslexic reading: Behavioural and fMRI investigations. Dyslexia 2010, 16, 258–282. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Weidner, R.; Von Overheidt, A.-C.; Tholen, N.; Grande, M.; Amunts, K. Experimental induction of reading difficulties in normal readers provides novel insights into the neurofunctional mechanisms of visual word recognition. Brain Struct. Funct. 2013, 219, 461–471. [Google Scholar] [CrossRef]
- Costagli, M.; Ueno, K.; Sun, P.; Gardner, J.L.; Wan, X.; Ricciardi, E.; Pietrini, P.; Tanaka, K.; Cheng, K. Functional Signalers of Changes in Visual Stimuli: Cortical Responses to Increments and Decrements in Motion Coherence. Cereb. Cortex 2012, 24, 110–118. [Google Scholar] [CrossRef]
- Csete, G.; Szabó, N.; Rokszin, A.; Tóth, E.; Braunitzer, G.; Benedek, G.; Vécsei, L.; Kincses, Z.T. An investigation of the white matter microstructure in motion detection using diffusion MRI. Brain Res. 2014, 1570, 35–42. [Google Scholar] [CrossRef]
- Danelli, L.; Berlingeri, M.; Bottini, G.; Ferri, F.; Vacchi, L.; Sberna, M.; Paulesu, E. Neural intersections of the phonological, visual magnocellular and motor/cerebellar systems in normal readers: Implications for imaging studies on dyslexia. Hum. Brain Mapp. 2012, 34, 2669–2687. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, Y.; Zhao, J.; Bi, H.-Y. Magnocellular-dorsal pathway function is associated with orthographic but not phonological skill: fMRI evidence from skilled Chinese readers. Neuropsychology 2015, 71, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Smith, A.T.; Greenlee, M. Spatiotemporal Frequency and Direction Sensitivities of Human Visual Areas Measured Using fMRI. NeuroImage 2000, 12, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Demb, J.B.; Boynton, G.M.; Best, M.; Heeger, D.J. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vis. Res. 1998, 38, 1555–1559. [Google Scholar] [CrossRef]
- Demb, J.; Boynton, G.M.; Heeger, D.J. Brain activity in visual cortex predicts individual differences in reading performance. Proc. Natl. Acad. Sci. USA 1997, 94, 13363–13366. [Google Scholar] [CrossRef]
- Olulade, O.A.; Napoliello, E.M.; Eden, G.F. Abnormal Visual Motion Processing Is Not a Cause of Dyslexia. Neuron 2013, 79, 180–190. [Google Scholar] [CrossRef]
- Ferrera, V.P.; Nealey, T.A.; Maunsell, J.H.R. Mixed parvocellular and magnocellular geniculate signals in visual area V4. Nat. Cell Biol. 1992, 358, 756–758. [Google Scholar] [CrossRef]
- Boden, C.; Giaschi, D. M-stream deficits and reading-related visual processes in developmental dyslexia. Psychol. Bull. 2007, 133, 346–366. [Google Scholar] [CrossRef]
- Giraldo-Chica, M.; Schneider, K.A. Hemispheric asymmetries in the orientation and location of the lateral geniculate nucleus in dyslexia. Dyslexia 2018, 24, 197–203. [Google Scholar] [CrossRef]
- Giraldo-Chica, M.; Hegarty, J.P.; Schneider, K.A. Morphological differences in the lateral geniculate nucleus associated with dyslexia. NeuroImage Clin. 2015, 7, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, W.; Martin, F.; Bowling, A.; Blackwood, M.; Badcock, D.; Paxton, S. Contrast sensitivity functions and specific reading disability. Neuropsychology 1982, 20, 309–315. [Google Scholar] [CrossRef]
- Martin, F.; Lovegrove, W. The effects of field size and luminance on contrast sensitivity differences between specifically reading disabled and normal children. Neuropsychology 1984, 22, 73–77. [Google Scholar] [CrossRef]
- Martin, F.; Lovegrove, W. Flicker Contrast Sensitivity in Normal and Specifically Disabled Readers. Perception 1987, 16, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.; Drasdo, N.; Richards, I.L. An investigation of some sensory and refractive visual factors in dyslexia. Vis. Res. 1994, 34, 1913–1926. [Google Scholar] [CrossRef]
- Cornelissen, P.; Richardson, A.; Mason, A.; Fowler, S.; Stein, J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vis. Res. 1995, 35, 1483–1494. [Google Scholar] [CrossRef]
- Borsting, E.; Ridder, W.H.; Dudeck, K.; Kelley, C.; Matsui, L.; Motoyama, J. The presence of a magnocellular defect depends on the type of dyslexia. Vis. Res. 1996, 36, 1047–1053. [Google Scholar] [CrossRef][Green Version]
- Hansen, P.C.; Stein, J.F.; Orde, S.R.; Winter, J.L.; Talcott, J.B. Are dyslexics??? visual deficits limited to measures of dorsal stream function? NeuroReport 2001, 12, 1527–1530. [Google Scholar] [CrossRef]
- Meng, X.; Cheng-Lai, A.; Zeng, B.; Stein, J.F.; Zhou, X. Dynamic visual perception and reading development in Chinese school children. Ann. Dyslexia 2011, 61, 161–176. [Google Scholar] [CrossRef]
- Talcott, J.B.; Gram, A.; Van Ingelghem, M.; Witton, C.; Stein, J.F.; Toennessen, F.E. Impaired sensitivity to dynamic stimuli in poor readers of a regular orthography. Brain Lang. 2003, 87, 259–266. [Google Scholar] [CrossRef]
- Talcott, J.B.; Hansen, P.C.; Assoku, E.L.; Stein, J.F. Visual motion sensitivity in dyslexia: Evidence for temporal and energy integration deficits. Neuropsychology 2000, 38, 935–943. [Google Scholar] [CrossRef]
- Witton, C.; Talcott, J.; Hansen, P.; Richardson, A.; Griffiths, T.; Rees, A.; Stein, J.; Green, G. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Curr. Biol. 1998, 8, 791–797. [Google Scholar] [CrossRef]
- Boets, B.; Vandermosten, M.; Cornelissen, P.; Wouters, J.; Ghesquière, P. Coherent Motion Sensitivity and Reading Development in the Transition From Prereading to Reading Stage. Child Dev. 2011, 82, 854–869. [Google Scholar] [CrossRef]
- May, J.G.; Lovegrove, W.J.; Martin, F.; Nelson, P. Pattern-elicited visual evoked potentials in good and poor readers. Clin. Vis. Sci. 1991, 6, 131–136. [Google Scholar]
- Lehmkuhle, S.; Garzia, R.P.; Turner, L.; Hash, T.; Baro, J.A. A Defective Visual Pathway in Children with Reading Disability. N. Engl. J. Med. 1993, 328, 989–996. [Google Scholar] [CrossRef]
- Kubova, Z.; Kuba, M.; Peregrin, J.; Nováková, V. Visual evoked potential evidence for magnocellular system deficit in dyslexia. Physiol. Res. 1996, 45, 87–89. [Google Scholar]
- Merigan, W.H.; Maunsell, J.H.R. How Parallel are the Primate Visual Pathways? Annu. Rev. Neurosci. 1993, 16, 369–402. [Google Scholar] [CrossRef] [PubMed]
- Danelli, L.; Berlingeri, M.; Bottini, G.; Borghese, N.A.; Lucchese, M.; Sberna, M.; Price, C.J.; Paulesu, E. How many deficits in the same dyslexic brains? A behavioural and fMRI assessment of comorbidity in adult dyslexics. Cortex 2017, 97, 125–142. [Google Scholar] [CrossRef]
- Taylor, C.; Olulade, O.; Luetje, M.; Eden, G. An fMRI study of coherent visual motion processing in children and adults. NeuroImage 2018, 173, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Laycock, R.; Crewther, S. Towards an understanding of the role of the ‘magnocellular advantage’ in fluent reading. Neurosci. Biobehav. Rev. 2008, 32, 1494–1506. [Google Scholar] [CrossRef]
- Skottun, B.C. A few remarks on the utility of visual motion perception to assess the integrity of the magnocellular system or the dorsal stream. Cortex 2016, 79, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Peiker, I.; David, N.; Schneider, T.R.; Nolte, G.; Schöttle, D.; Engel, A.K. Perceptual Integration Deficits in Autism Spectrum Disorders Are Associated with Reduced Interhemispheric Gamma-Band Coherence. J. Neurosci. 2015, 35, 16352–16361. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Thomas, C.; Kravitz, D.J.; Wallace, G.; Baron-Cohen, S.; Martin, A.; Baker, C.I. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain 2014, 137, 2588–2599. [Google Scholar] [CrossRef]
- Buchel, C.; Josephs, O.; Rees, G.; Turner, R.; Frith, C.D.; Friston, K.J. The functional anatomy of attention to visual motion. A functional MRI study. Brain 1998, 121, 1281–1294. [Google Scholar] [CrossRef]
- Cheng, K.; Fujita, H.; Kanno, I.; Miura, S.; Tanaka, K. Human cortical regions activated by wide-field visual motion: An H2(15)O PET study. J. Neurophysiol. 1995, 74, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Culham, J.C.; Brandt, S.A.; Cavanagh, P.; Kanwisher, N.G.; Dale, A.M.; Tootell, R.B.H. Cortical fMRI Activation Produced by Attentive Tracking of Moving Targets. J. Neurophysiol. 1998, 80, 2657–2670. [Google Scholar] [CrossRef]
- Könönen, M.; Pääkkönen, A.; Pihlajamäki, M.; Partanen, K.; Karjalainen, P.A.; Soimakallio, S.; Aronen, H.J. Visual processing of coherent rotation in the central visual field: An fMRI study. Perception 2003, 32, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Van Schouwenburg, M.R.; Ouden, H.E.M.D.; Cools, R. The Human Basal Ganglia Modulate Frontal-Posterior Connectivity during Attention Shifting. J. Neurosci. 2010, 30, 9910–9918. [Google Scholar] [CrossRef]
- Van Schouwenburg, M.R.; Ouden, H.E.M.D.; Cools, R. Selective Attentional Enhancement and Inhibition of Fronto-Posterior Connectivity by the Basal Ganglia during Attention Switching. Cereb. Cortex 2015, 25, 1527–1534. [Google Scholar] [CrossRef]
- Patel, S.S.; Bedell, H.E.; Tsang, D.K.; Ukwade, M.T. Relationship between threshold and suprathreshold perception of position and stereoscopic depth. J. Opt. Soc. Am. A 2009, 26, 847–861. [Google Scholar] [CrossRef]
- Fechner, G.T. Elemente der Psychophysik [Elements of Psychophysics], 2nd ed.; Breitkopf und Härtel: Leipzig, Germany, 1860. [Google Scholar]
- Green, D.M.; Swets, J.A. Signal Detection Theory and Psychophysics; A Reprint with Corrections of the Original 1966 ed.; Robert, E., Ed.; Krieger Publishing, Co: Huntington, NY, USA, 1974. [Google Scholar]
- Peruzzo, D.; PICOS-Veneto Group; Castellani, U.; Perlini, C.; Bellani, M.; Marinelli, V.; Rambaldelli, G.; Lasalvia, A.; Tosato, S.; De Santi, K.; et al. Classification of first-episode psychosis: A multi-modal multi-feature approach integrating structural and diffusion imaging. J. Neural Transm. 2014, 122, 897–905. [Google Scholar] [CrossRef]
- Riva, V.; Mozzi, A.; Forni, D.; Trezzi, V.; Giorda, R.; Riva, S.; Villa, M.; Sironi, M.; Cagliani, R.; Mascheretti, S. The influence of DCDC2 risk genetic variants on reading: Testing main and haplotypic effects. Neuropsychology 2019, 130, 52–58. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2006. [Google Scholar]
- Riva, V.; Battaglia, M.; Nobile, M.; Cattaneo, F.; Lazazzera, C.; Mascheretti, S.; Giorda, R.; Mérette, C.; Émond, C.; Maziade, M.; et al. GRIN2B predicts attention problems among disadvantaged children. Eur. Child Adolesc. Psychiatry 2014, 24, 827–836. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 3rd ed.; Organizzazioni Speciali: Firenze, Italy, 2006. [Google Scholar]
- Cornoldi, C. Nuove Prove di Lettura MT per la Scuola Media Inferiore; Organizzazioni Speciali: Firenze, Italy, 1995. [Google Scholar]
- Cornoldi, C.; Colpo, G. Prove di Lettura MT per la Scuola Elementare, 2nd ed.; Organizzazioni Speciali: Firenze, Italy, 1998. [Google Scholar]
- Sartori, G.; Job, R.; Tressoldi, P.E. Batteria per la Valutazione Della Dislessia e Della Disortografia Evolutive; Organizzazioni Speciali: Firenze, Italy, 1995. [Google Scholar]
- Arina, S.; Iervolino, I.; Stella, G. Prima raccolta di dati normativi per la valutazione della dislessia evolutiva negli adolescenti su un campione di scuola secondaria di secondo grado. Dislessia 2013, 10, 9–38. [Google Scholar]
- Reynolds, C.R.; Bigler, E.D. Test of Memory and Learning; Erickson: Trento, Italy, 1994. [Google Scholar]
- Bertelli, B.; Bilancia, G. VAUMeLF Batteria per la Valutazione Dell’attenzione Uditiva e Della Memoria di Lavoro Fonologica Nell’età Evolutiva; Organizzazioni Speciali: Firenze, Italy, 2006. [Google Scholar]
- Briggs, G.G.; Nebes, R.D. Patterns of Hand Preference in a Student Population. Cortex 1975, 11, 230–238. [Google Scholar] [CrossRef]
- Conners, C.K. Manual for Conners’ Rating Scales; Multi-Health Systems: North Tonawanda, NY, USA, 1989. [Google Scholar]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.A.; Epstein, J.N. The Revised Conners’ Parent Rating Scale (CPRS-R): Factor Structure, Reliability, and Criterion Validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef]
- Nobile, M.; Alberti, B.; Zuddas, A. CRS-R. Conners’ Rating Scale—Revised; Organizzazioni Speciali: Firenze, Italy, 2007. [Google Scholar]
- Cunningham, A.E.; Stanovich, K.E. Early reading acquisition and its relation to reading experience and ability 10 years later. Dev. Psychol. 1997, 33, 934–945. [Google Scholar] [CrossRef]
- Ferrer, E.; Shaywitz, B.A.; Holahan, J.M.; Marchione, K.; Shaywitz, S.E. Uncoupling of Reading and IQ Over Time. Psychol. Sci. 2009, 21, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nagy, W.E.; Herman, P.A.; Anderson, R.C. Learning Words from Context. Read. Res. Q. 1985, 20, 233. [Google Scholar] [CrossRef]
- Ramsden, S.; Richardson, F.M.; Josse, G.; Shakeshaft, C.; Seghier, M.L.; Price, C.J. The influence of reading ability on subsequent changes in verbal IQ in the teenage years. Dev. Cogn. Neurosci. 2013, 6, 30–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanovich, K.E.; Cunningham, A.E. Where does knowledge come from? Specific associations between print exposure and information acquisition. J. Educ. Psychol. 1993, 85, 211–229. [Google Scholar] [CrossRef]
- Swanborn, M.; De Glopper, K. Incidental Word Learning While Reading: A Meta-Analysis. Rev. Educ. Res. 1999, 69, 261–285. [Google Scholar] [CrossRef]
- Hollingshead, A.B. Four Factor Index of Social Status; Yale University: New Haven, CT, USA, 1975. [Google Scholar]
- Cicchini, G.M.; Marino, C.; Mascheretti, S.; Perani, D.; Morrone, M.C. Strong Motion Deficits in Dyslexia Associated with DCDC2 Gene Alteration. J. Neurosci. 2015, 35, 8059–8064. [Google Scholar] [CrossRef] [PubMed]
- Sperling, A.J.; Lu, Z.-L.; Manis, F.R.; Seidenberg, M.S. Deficits in perceptual noise exclusion in developmental dyslexia. Nat. Neurosci. 2005, 8, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, E.Y.K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A multi-modal parcellation of human cerebral cortex. Nat. Cell Biol. 2016, 536, 171–178. [Google Scholar] [CrossRef]
- Bach, F.R.; Lanckriet, G.R.G.; Jordan, M.I. Multiple kernel learning, conic duality, and the SMO algorithm. In Proceedings of the Twenty-first international conference on Machine learning—ICML ’04, Association for Computing Machinery (ACM), New York, NY, USA, 4–8 July 2004; p. 6. [Google Scholar]
- Lanckriet, G.R.G.; Cristianini, N.; Bartlett, P.; El Ghaoui, L.; Jordan, M.I. Learning the kernel matrix with semidefinite programming. J. Mach. Learn. Res. 2004, 5, 27–52. [Google Scholar]
- Xu, Z.; Jin, R.; Yang, H.; King, I.; Lyu, M.R. Simple and efficient multiple kernel learning by group lasso. In Proceedings of the 27th International Conference on Machine Learning (ICML-10), Haifa, Israel, 21–24 June 2010. [Google Scholar]
- Poldrack, R.A.; Huckins, G.; Varoquaux, G. Establishment of best practices for evidence for prediction, a review. JAMA Psychiatry 2019, 77, 534–540. [Google Scholar] [CrossRef]
- Bellotti, T.; Nouretdinov, I.; Yang, M.; Gammerman, A. Feature selection. In Balasubramanian, Conformal Prediction for Reliable Machine Learning: Theory, Adaptations and Applications; Ho, V.N., Vovk, S.-S., Eds.; Morgan Kaufmann Publishers: Burlington, MA, USA, 2014; pp. 116–130. [Google Scholar]
- Haufe, S.; Meinecke, F.; Görgen, K.; Dähne, S.; Haynes, J.-D.; Blankertz, B.; Bießmann, F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage 2014, 87, 96–110. [Google Scholar] [CrossRef]
- Price, C.; Winterburn, D.; Giraud, A.; Moore, C.; Noppeney, U. Cortical localisation of the visual and auditory word form areas: A reconsideration of the evidence. Brain Lang. 2003, 86, 272–286. [Google Scholar] [CrossRef]
- Hoeft, F.; Hernandez, A.; McMillon, G.; Taylor-Hill, H.; Martindale, J.L.; Meyler, A.; Keller, T.A.; Siok, W.T.; Deutsch, G.K.; Just, M.; et al. Neural Basis of Dyslexia: A Comparison between Dyslexic and Nondyslexic Children Equated for Reading Ability. J. Neurosci. 2006, 26, 10700–10708. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; McCandliss, B.D.; Black, J.M.; Gantman, A.; Zakerani, N.; Hulme, C.; Lyytinen, H.; Whitfield-Gabrieli, S.; Glover, G.H.; Reiss, A.L.; et al. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. USA 2011, 108, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Flint, J.; Timpson, N.; Munafò, M. Assessing the utility of intermediate phenotypes for genetic mapping of psychiatric disease. Trends Neurosci. 2014, 37, 733–741. [Google Scholar] [CrossRef]
- Turkeltaub, P.; Gareau, L.; Flowers, D.L.; Zeffiro, A.T.; Eden, G.F. Development of neural mechanisms for reading. Nat. Neurosci. 2003, 6, 767–773. [Google Scholar] [CrossRef]
- Sperling, A.J.; Lu, Z.-L.; Manis, F.R.; Seidenberg, M.S. Motion-Perception Deficits and Reading Impairment. Psychol. Sci. 2006, 17, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Cestnick, L.; Coltheart, M. The relationship between language-processing and visual-processing deficits in developmental dyslexia. Cognition 1999, 71, 231–255. [Google Scholar] [CrossRef]
- Facoetti, A.; Corradi, N.; Ruffino, M.; Gori, S.; Zorzi, M. Visual spatial attention and speech segmentation are both impaired in preschoolers at familial risk for developmental dyslexia. Dyslexia 2010, 16, 226–239. [Google Scholar] [CrossRef]
- Facoetti, A.; Ruffino, M.; Peru, A.; Paganoni, P.; Chelazzi, L. Sluggish engagement and disengagement of non-spatial attention in dyslexic children. Cortex 2008, 44, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Zorzi, M.; Cestnick, L.; Lorusso, M.L.; Molteni, M.; Paganoni, P.; Umiltà, C.; Mascetti, G.G. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cogn. Neuropsychol. 2006, 23, 841–855. [Google Scholar] [CrossRef]
- Ruffino, M.; Trussardi, A.N.; Gori, S.; Finzi, A.; Giovagnoli, S.; Menghini, D.; Benassi, M.; Molteni, M.; Bolzani, R.; Vicari, S.; et al. Attentional engagement deficits in dyslexic children. Neuropsychology 2010, 48, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Mascheretti, S.; Bertoni, S.; Trezzi, V.; Andreola, C.; Gori, S.; Facoetti, A. Sluggish dorsally-driven inhibition of return during orthographic processing in adults with dyslexia. Brain Lang. 2018, 179, 1–10. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, J.; Ding, Y.; Wang, Z. Dyslexic children are sluggish in disengaging spatial attention. Dyslexia 2019, 25, 158–172. [Google Scholar] [CrossRef]
- Krause, M.B. Pay Attention!: Sluggish Multisensory Attentional Shifting as a Core Deficit in Developmental Dyslexia. Dyslexia 2015, 21, 285–303. [Google Scholar] [CrossRef]
- Varoquaux, G. Cross-validation failure: Small sample sizes lead to large error bars. NeuroImage 2018, 180, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Conlon, E.G.; Lilleskaret, G.; Wright, C.M.; Power, G.F. The influence of contrast on coherent motion processing in dyslexia. Neuropsychology 2012, 50, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Mascheretti, S.; Gori, S.; Trezzi, V.; Ruffino, M.; Facoetti, A.; Marino, C. Visual motion and rapid auditory processing are solid endophenotypes of developmental dyslexia. Genes Brain Behav. 2018, 17, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A. Genes, Brains, and Behavior: Imaging Genetics for Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 81–92. [Google Scholar] [CrossRef]
- Franceschini, S.; Trevisan, P.; Ronconi, L.; Bertoni, S.; Colmar, S.; Double, K.; Facoetti, A.; Gori, S. Action video games improve reading abilities and visual-to-auditory attentional shifting in English-speaking children with dyslexia. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
| Children with DD (n = 22) | Typical Readers (n = 22) | Χ2 | df | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (Male/Female) | 16/6 | 15/7 | 0.11 | 1 | 0.741 | |||||||||
| Handedness | Right-handed | 18 | 17 | 0.31 | 2 | 0.856 | ||||||||
| Left-handed | 3 | 3 | ||||||||||||
| Ambidextrous | 1 | 2 | ||||||||||||
| Min | Max | Mean (SD) | Skewness | Kurtosis | Min | Max | Mean (SD) | Skewness | Kurtosis | t-Test | df | p | ||
| Age | 10.92 | 16.5 | 14.10 (1.48) | −0.40 | −0.22 | 11 | 16.25 | 13.18 (1.65) | 0.47 | −1.31 | −1.94 | 42 | 0.059 | |
| IQ † | 7.0 | 13.5 | 10.59 (1.78) | −0.10 | −0.61 | 7.5 | 18.5 | 13.70 (2.82) | −0.67 | 0.23 | 4.38 | 42 | <0.001 | |
| IQ, Vocabulary | 5.0 | 16.0 | 9.73 (2.75) | 0.25 | −0.24 | 8.0 | 18.0 | 13.23 (3.09) | −0.27 | −0.95 | 3.98 | 42 | <0.001 | |
| IQ, Block Design | 8.0 | 18.0 | 11.82 (2.04) | 1.16 | 3.10 | 7.0 | 19.0 | 14.18 (3.65) | −0.47 | −0.49 | 2.65 | 42 | 0.012 | |
| TR, accuracy | −10.43 | 0.74 | −3.34 (2.42) | −1.30 | 2.52 | −0.13 | 1.42 | 0.61 (0.34) | 0.10 | 0.88 | 7.61 | 42 | <0.001 | |
| TR, speed | −4.21 | −0.09 | −2.55 (1.01) | 0.44 | −0.11 | −1.00 | 1.18 | 0.16 (0.65) | 0.02 | −1.00 | 10.55 | 42 | <0.001 | |
| SWR, accuracy | −10.00 | 0.33 | −3.41 (2.63) | −0.74 | 0.22 | −0.67 | 1.00 | 0.27 (0.56) | −0.04 | −1.16 | 6.42 | 42 | <0.001 | |
| SWR, speed | −10.12 | −0.41 | −3.66 (2.16) | −1.49 | 2.77 | −1.11 | 0.87 | 0.04 (0.57) | −0.19 | −0.96 | 7.75 | 42 | <0.001 | |
| SPWR, accuracy | −8.50 | 0.33 | −2.28 (1.91) | −1.57 | 4.33 | −0.67 | 1.33 | 0.50 (0.46) | −0.47 | 0.60 | 6.61 | 42 | <0.001 | |
| SPWR, speed | −9.42 | −0.67 | −3.38 (2.42) | −1.43 | 1.36 | −1.03 | 1.40 | 0.26 (0.64) | −0.26 | −0.65 | 6.82 | 42 | <0.001 | |
| SLFS | −2.30 | 0.65 | −1.15 (0.72) | 0.46 | 0.61 | −1.35 | 1.35 | 0.14 (0.76) | −0.69 | −0.28 | 5.72 | 41 | <0.001 | |
| SLBS | −2.20 | 1.35 | −0.71 (0.75) | 0.93 | 2.25 | −1.35 | 1.60 | −0.07 (0.89) | 0.73 | −0.41 | 2.53 | 41 | 0.015 | |
| SDFS | −2.20 | 0.00 | −1.38 (0.62) | 0.49 | −0.08 | −2.00 | 1.00 | −0.61 (0.74) | 0.18 | −0.21 | 3.68 | 41 | 0.001 | |
| SDBS | −1.35 | 0.65 | −0.56 (0.47) | 0.56 | 1.01 | −1.40 | 2.00 | 0.10 (0.96) | 0.91 | −0.20 | 2.84 | 41 | 0.007 | |
| SNWR | −7.00 | 3.53 | −1.66 (2.71) | −0.1 | −0.41 | −2.79 | 3 | 1.18 (1.40) | −1.45 | 2.26 | 4.38 | 42 | <0.001 | |
| ADHD | DSM-IV-I ‡ | 41 | 82 | 60.09 (10.73) | 0.33 | −0.32 | 39 | 59 | 46.11 (5.49) | 0.94 | 0.34 | −5.43 | 42 | <0.001 |
| DSM-IV-HI § | 38 | 71 | 49.20 (7.96) | 1.13 | 1.57 | 38 | 65 | 47.36 (7.63) | 1.099 | 0.377 | −0.76 | 40 | 0.450 | |
| SES ¶ | 20 | 90 | 58.84 (19.16) | −0.08 | −0.37 | 30 | 90 | 60.23 (19.42) | 0.19 | −1.04 | 0.56 | 39 | 0.579 | |
| Hemisphere | ROI † | ROI-Region † | ROI Weight | Contrast Weight | |||||
|---|---|---|---|---|---|---|---|---|---|
| M-vs-B | P-vs-B | CML6-vs-B | CML15-vs-B | CML40-vs-B | |||||
| 1 | Right | Lateral Area 7P | Superior Parietal Cortex | 1.000 | −0.381 | −0.277 | −0.583 | −0.525 | −0.404 |
| 2 | Left | Area PGp | Inferior Parietal Cortex | 0.704 | −0.405 | −0.298 | −0.613 | −0.491 | −0.361 |
| 3 | Left | Area V6A | Dorsal Stream Visual Cortex | 0.644 | −0.407 | −0.066 | −0.549 | −0.693 | −0.220 |
| 4 | Right | Medial Area 7A | Superior Parietal Cortex | 0.633 | −0.209 | −0.257 | −0.759 | −0.458 | −0.324 |
| 5 | Left | Ventro-Medial Visual Area 1 | Ventral Stream Visual Cortex | 0.585 | −0.112 | 0.119 | −0.524 | −0.664 | −0.507 |
| 6 | Left | Area Lateral Occipital 2 | MT+ Complex and Neighboring Visual Area | 0.539 | −0.388 | 0.157 | −0.495 | −0.483 | −0.589 |
| 7 | Right | Area IFJ posterior | Inferior Frontal Cortex | 0.521 | −0.685 | −0.477 | −0.500 | −0.230 | −0.033 |
| 8 | Right | Ventro-Medial Visual Area 1 | Ventral Stream Visual Cortex | 0.436 | −0.546 | −0.243 | −0.436 | −0.497 | −0.454 |
| 9 | Right | Area 5-L | Paracentral Lobular and Mid-Cingulate Cortex | 0.315 | −0.060 | −0.077 | −0.773 | −0.524 | −0.344 |
| 10 | Right | Area PH | MT+ complex—Ventral stream fusiform face complex | 0.290 | −0.561 | −0.573 | −0.321 | −0.424 | −0.272 |
| 11 | Right | Ventro-Medial Visual Area 2 | Ventral Stream Visual Cortex | 0.222 | −0.655 | −0.369 | −0.396 | −0.409 | −0.333 |
| Hemisphere | ROI † | ROI-Region † | M-vs-B | P-vs-B | CML6-vs-B | CML15-vs-B | CML40-vs-B | |
|---|---|---|---|---|---|---|---|---|
| 1 | Right | Lateral Area 7P | Superior Parietal Cortex | 2.309 (0.026) | 1.713 (0.094) | 3.662 (0.001) | 2.993 (0.005) | 2.009 (0.051) |
| 2 | Left | Area PGp | Inferior Parietal Cortex | 2.023 (0.049) | 1.770 (0.084) | 2.985 (0.005) | 2.503 (0.016) | 1.579 (0.122) |
| 3 | Left | Area V6A | Dorsal Stream Visual Cortex | 1.324 (0.193) | 0.262 (0.795) | 2.230 (0.031) | 2.342 (0.024) | 0.866 (0.391) |
| 4 | Right | Medial Area 7A | Superior Parietal Cortex | 1.131 (0.264) | 1.327 (0.192) | 4.497 (<0.001) | 2.413 (0.020) | 1.916 (0.062) |
| 5 | Left | Ventro-Medial Visual Area 1 | Ventral Stream Visual Cortex | 0.839 (0.406) | −0.751 (0.457) | 2.481 (0.017) | 2.906 (0.006) | 2.289 (0.027) |
| 6 | Left | Area Lateral Occipital 2 | MT+ Complex and Neighboring Visual Area | 1.805 (0.078) | −0.615 (0.542) | 2.151 (0.037) | 2.026 (0.049) | 2.727 (0.009) |
| 7 | Right | Area IFJ posterior | Inferior Frontal Cortex | 3.948 (<0.001) | 2.468 (0.018) | 2.698 (0.010) | 1.430 (0.160) | 0.194 (0.847) |
| 8 | Right | Ventro-Medial Visual Area 1 | Ventral Stream Visual Cortex | 3.014 (0.004) | 1.288 (0.205) | 1.845 (0.072) | 1.905 (0.064) | 1.810 (0.077) |
| 9 | Right | Area 5-L | Paracentral Lobular and Mid-Cingulate Cortex | 0.312 (0.756) | 0.316 (0.754) | 4.441 (<0.001) | 2.509 (0.016) | 1.642 (0.108) |
| 10 | Right | Area PH | MT+ complex—Ventral stream fusiform face complex | 3.538 (0.001) | 3.078 (0.004) | 1.553 (0.128) | 2.110 (0.041) | 1.401 (0.169) |
| 11 | Right | Ventro-Medial Visual Area 2 | Ventral Stream Visual Cortex | 2.628 (0.012) | 1.437 (0.158) | 1.467 (0.150) | 1.789 (0.081) | 1.148 (0.257) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascheretti, S.; Peruzzo, D.; Andreola, C.; Villa, M.; Ciceri, T.; Trezzi, V.; Marino, C.; Arrigoni, F. Selecting the Most Relevant Brain Regions to Classify Children with Developmental Dyslexia and Typical Readers by Using Complex Magnocellular Stimuli and Multiple Kernel Learning. Brain Sci. 2021, 11, 722. https://doi.org/10.3390/brainsci11060722
Mascheretti S, Peruzzo D, Andreola C, Villa M, Ciceri T, Trezzi V, Marino C, Arrigoni F. Selecting the Most Relevant Brain Regions to Classify Children with Developmental Dyslexia and Typical Readers by Using Complex Magnocellular Stimuli and Multiple Kernel Learning. Brain Sciences. 2021; 11(6):722. https://doi.org/10.3390/brainsci11060722
Chicago/Turabian StyleMascheretti, Sara, Denis Peruzzo, Chiara Andreola, Martina Villa, Tommaso Ciceri, Vittoria Trezzi, Cecilia Marino, and Filippo Arrigoni. 2021. "Selecting the Most Relevant Brain Regions to Classify Children with Developmental Dyslexia and Typical Readers by Using Complex Magnocellular Stimuli and Multiple Kernel Learning" Brain Sciences 11, no. 6: 722. https://doi.org/10.3390/brainsci11060722
APA StyleMascheretti, S., Peruzzo, D., Andreola, C., Villa, M., Ciceri, T., Trezzi, V., Marino, C., & Arrigoni, F. (2021). Selecting the Most Relevant Brain Regions to Classify Children with Developmental Dyslexia and Typical Readers by Using Complex Magnocellular Stimuli and Multiple Kernel Learning. Brain Sciences, 11(6), 722. https://doi.org/10.3390/brainsci11060722








