Dynamic Functional Network Connectivity Changes Associated with fMRI Neurofeedback of Right Premotor Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging Parameters
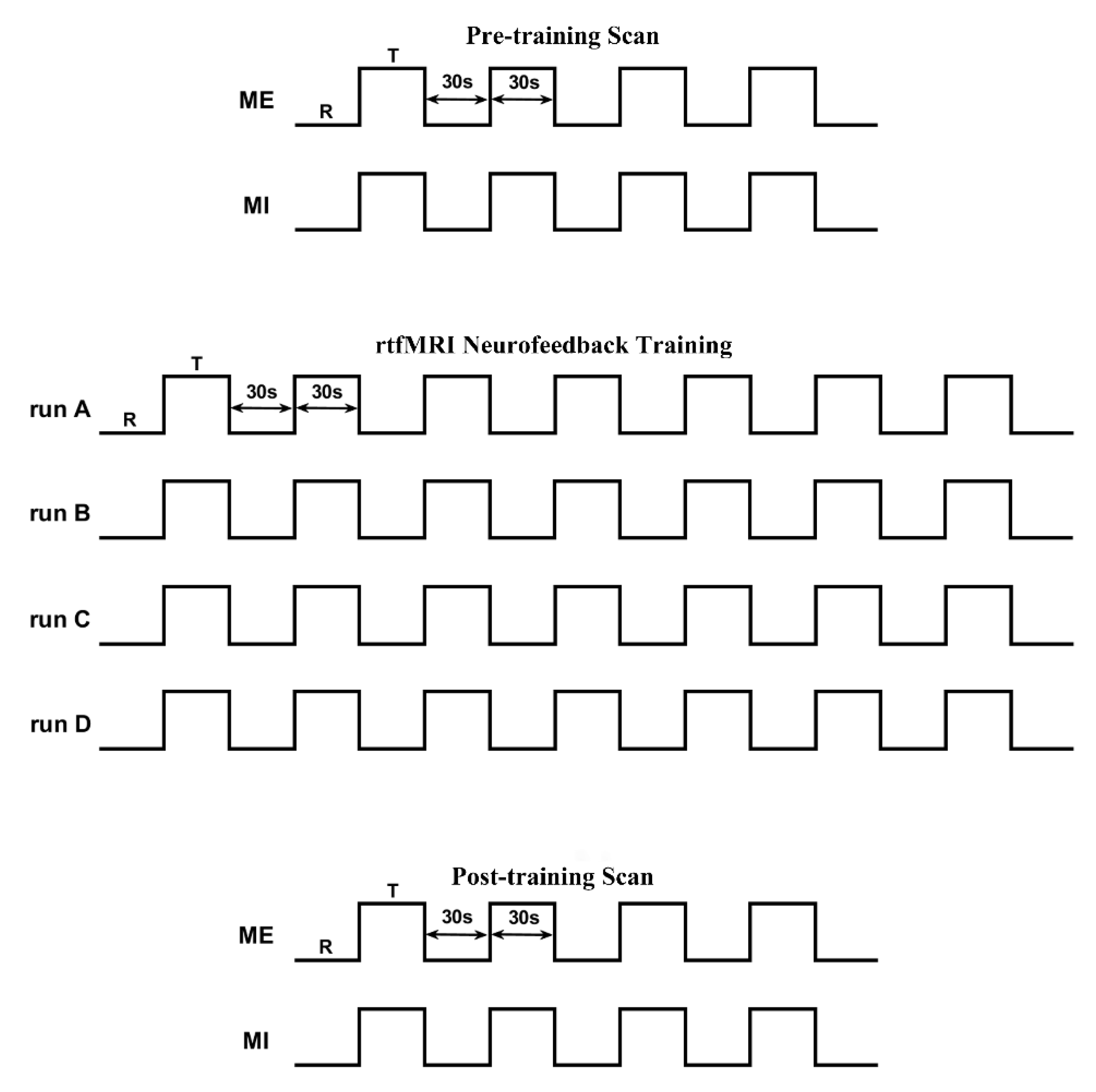
2.3. Experimental Procedure
2.3.1. Pre-Scan Practice
2.3.2. Pre- and Post-Training Scan
2.3.3. Neurofeedback Training
2.3.4. Questionnaire Interview
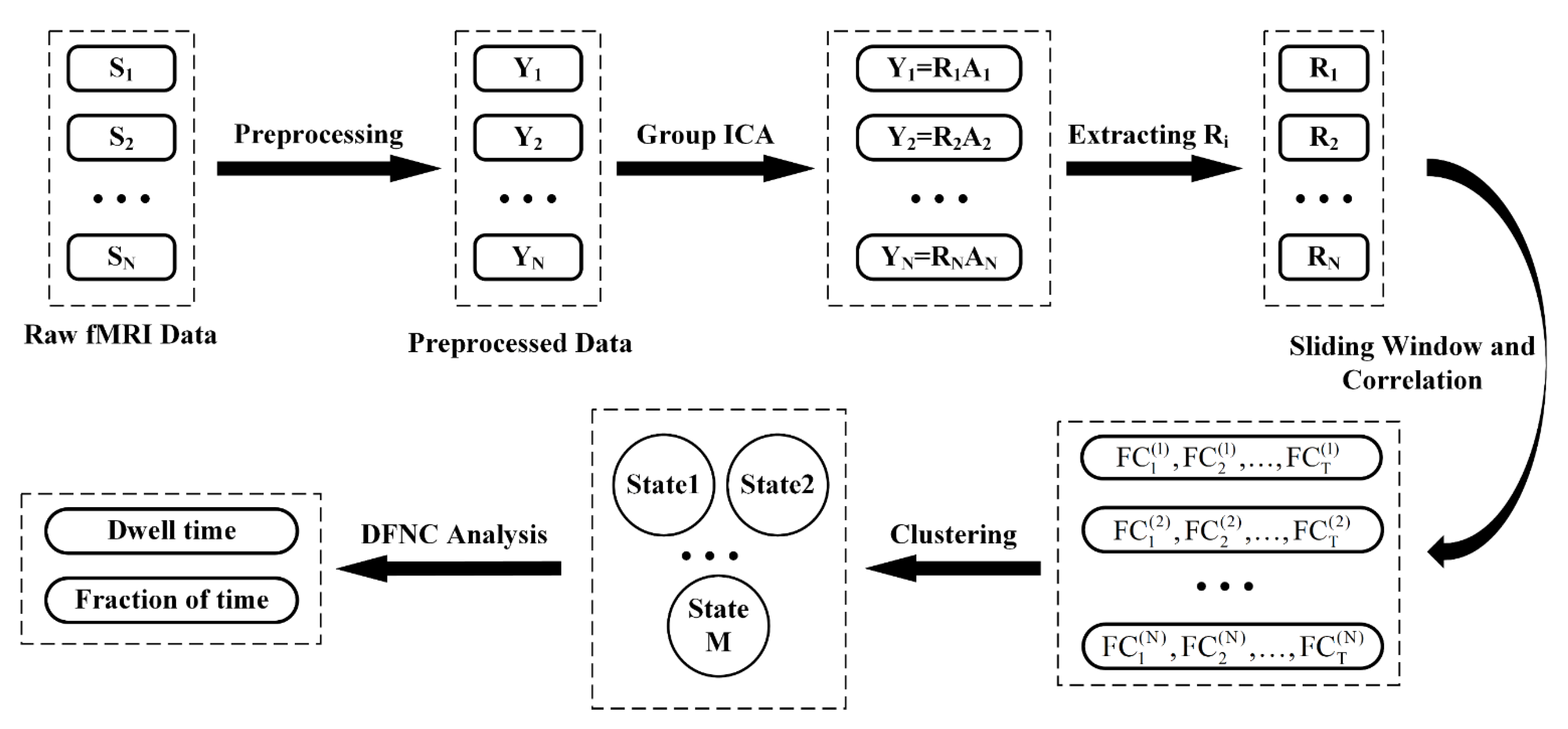
2.4. Offline Data Analysis
2.4.1. Preprocessing
2.4.2. Group ICA
2.4.3. DFNC Analysis
2.4.4. Clustering Analysis
2.4.5. Temporal Property Analysis of DFNC
2.4.6. Subnetwork Connectivity Analysis of the Target FC States
2.4.7. Relationship between the Behavioral Performance and DFNC
2.4.8. Relationship between the Neurofeedback Training Effect and DFNC/Motor Behavior
2.4.9. Network-Based Statistic (NBS) Analysis
3. Results
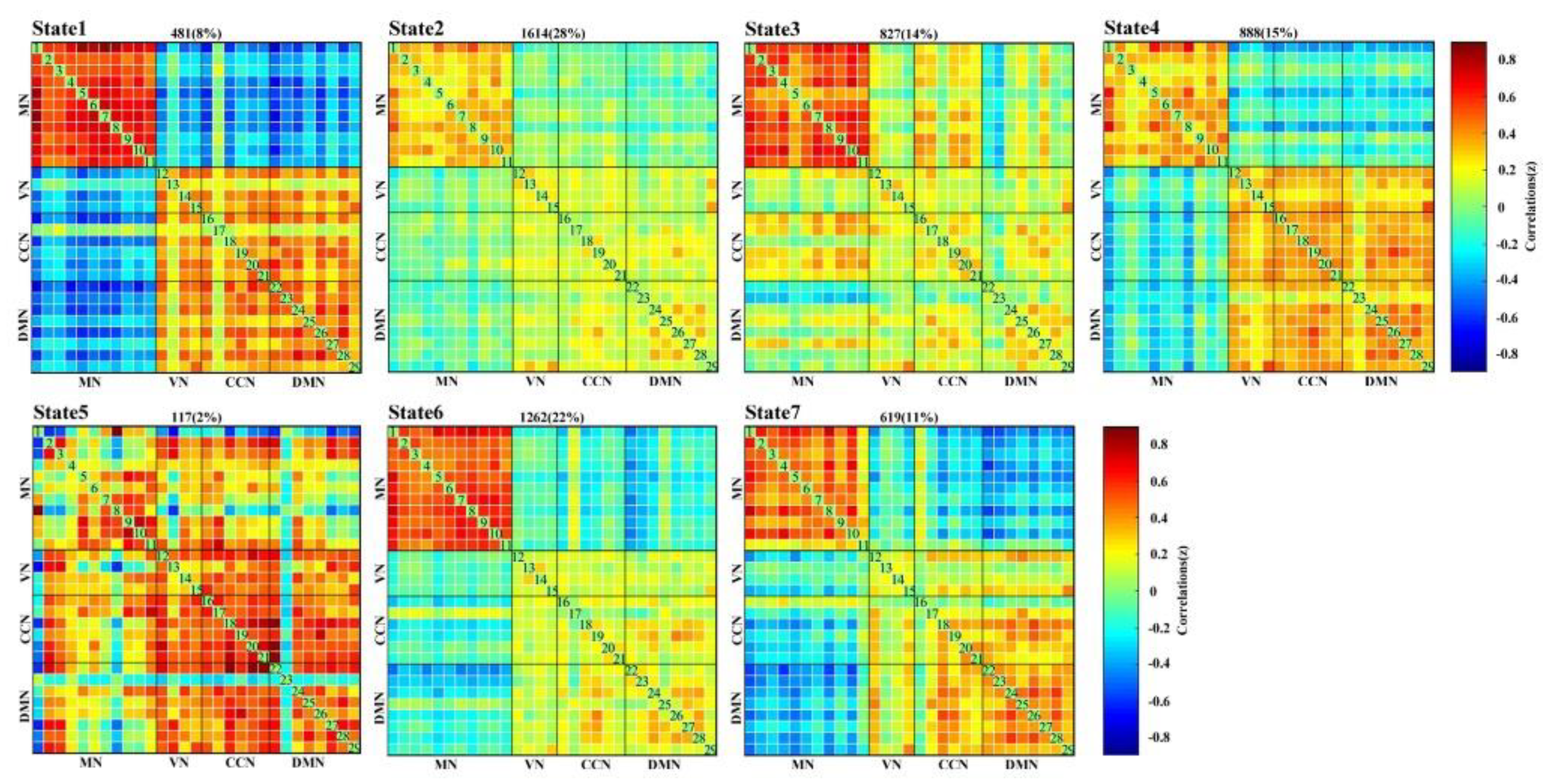
3.1. Temporal Property Analysis of DFNC
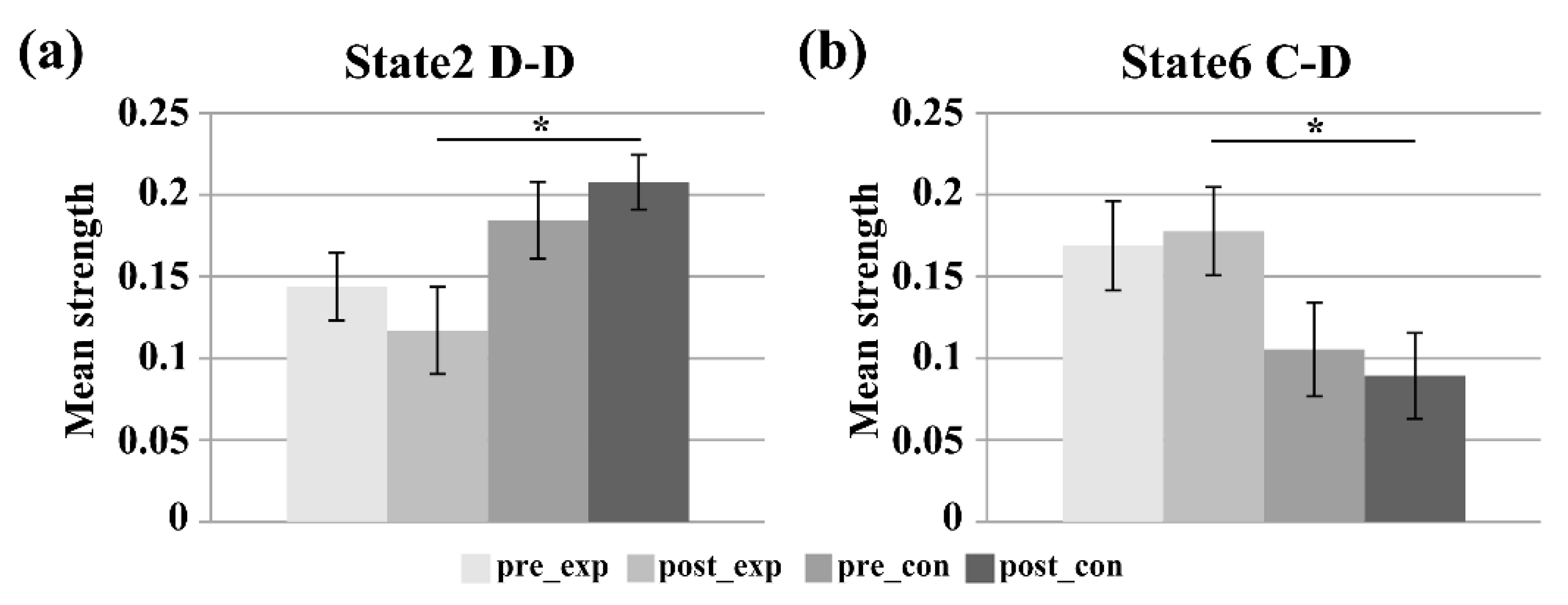
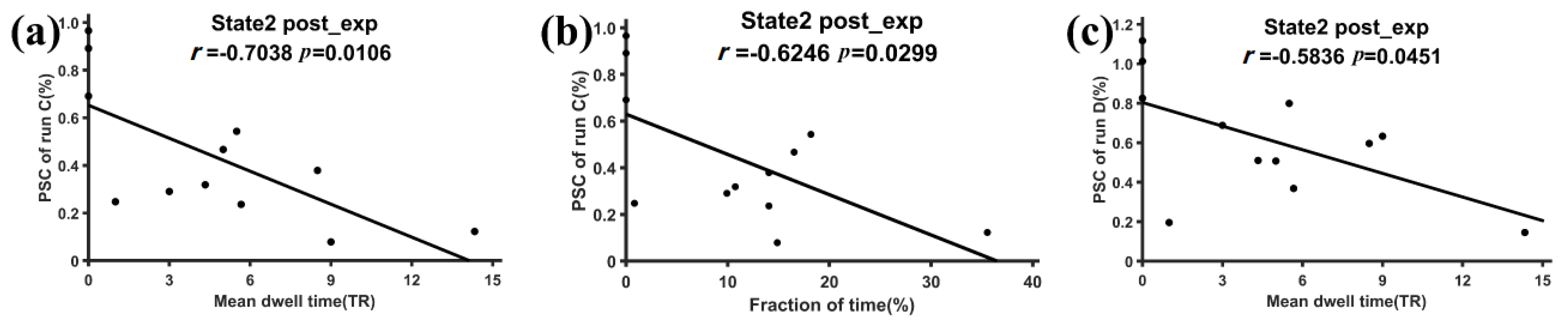
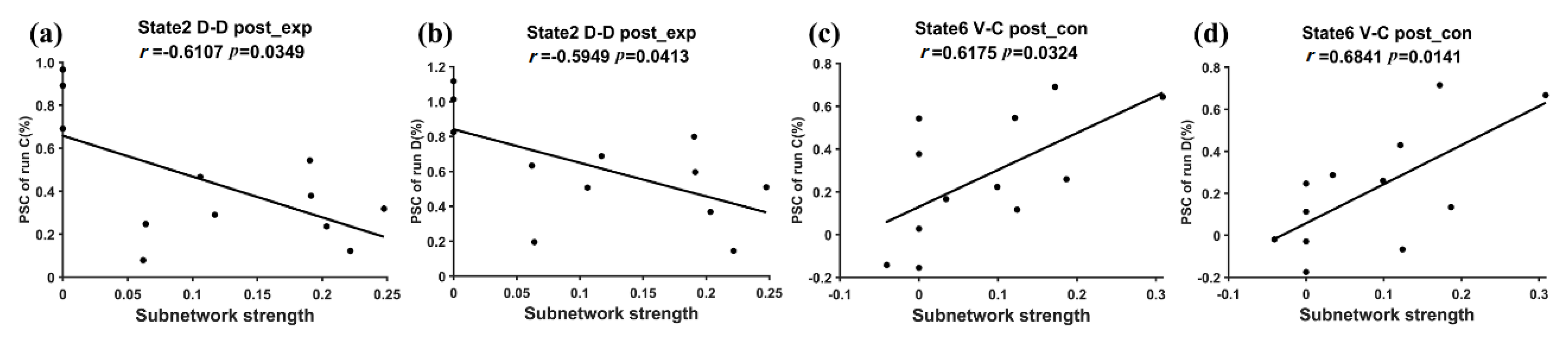
3.2. Subnetwork Connectivity Analysis of the Target FC States
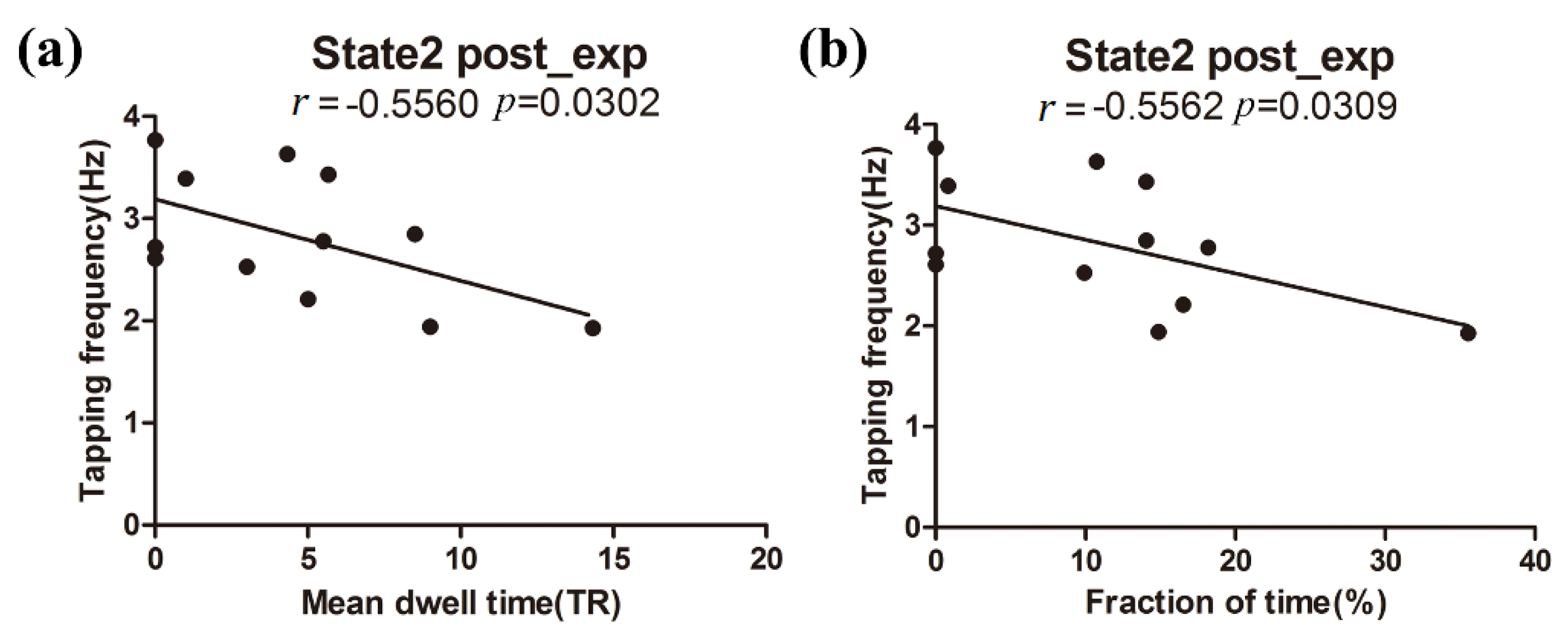
3.3. Relationship between Behavioral Performance and DFNC
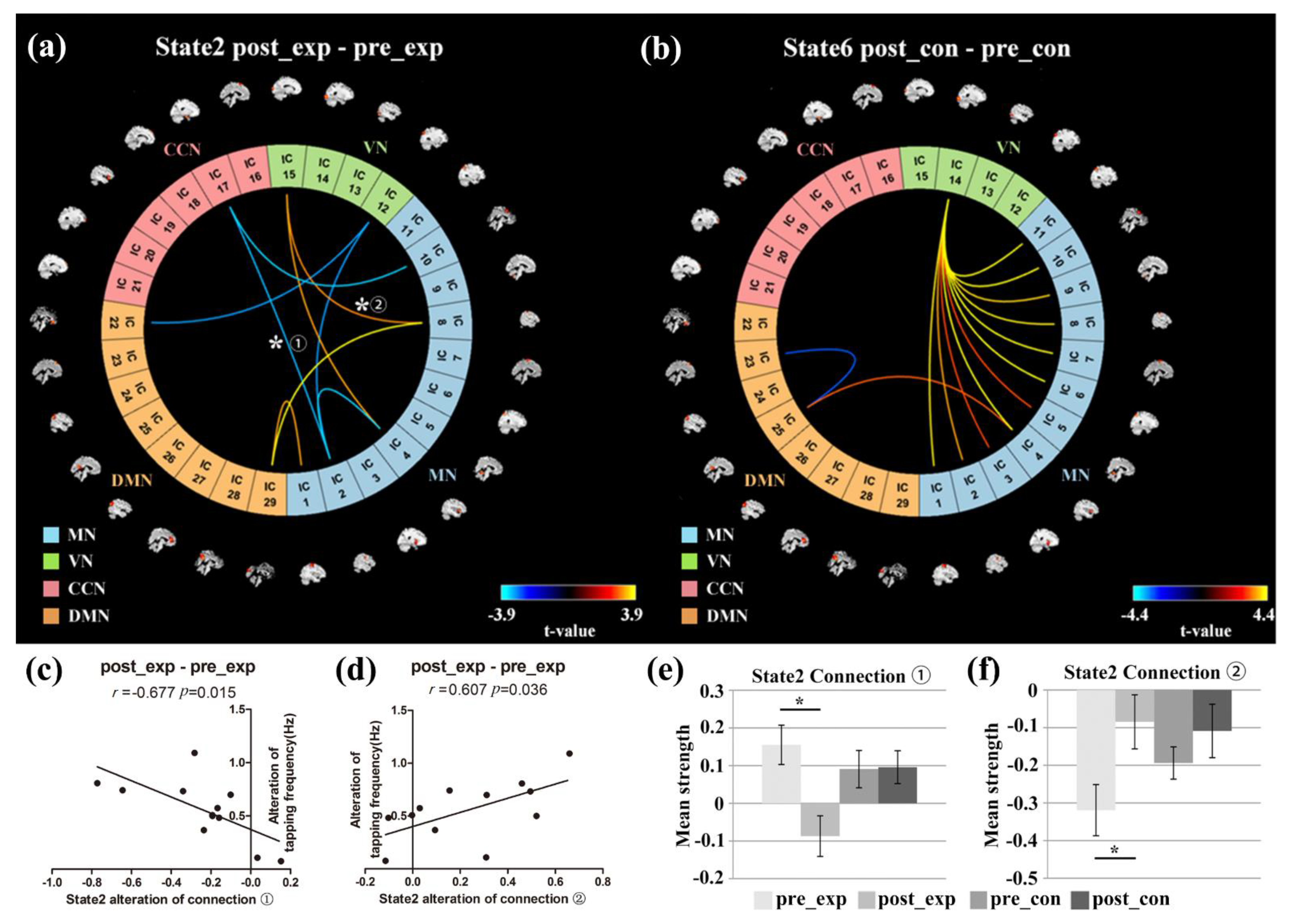
3.4. Relationship between the Neurofeedback Training Effect and DFNC/Behavioral Performance
3.5. NBS Analysis
4. Discussion
4.1. DFNC Changes after the Neurofeedback Training
4.2. Subnetwork Connectivity Changes in the Target FC States after the Neurofeedback Training
4.3. The Relation between DFNC and Motor Performance/Training Effect after the Neurofeedback Training
4.4. Network Connectivity Changes of the Target FC States
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caria, A.; Sitaram, R.; Birbaumer, N. Real-time fMRI: A tool for local brain regulation. Neuroscientist 2011, 18, 487–501. [Google Scholar] [CrossRef]
- Sitaram, R.; Caria, A.; Veit, R.; Gaber, T.; Rota, G.; Kuebler, A.; Birbaumer, N. fMRI Brain-Computer Interface: A Tool for Neuroscientific Research and Treatment. Comput. Intell. Neurosci. 2007, 2007, 025487. [Google Scholar] [CrossRef] [PubMed]
- Hui, M.; Zhang, H.; Ge, R.; Yao, L.; Long, Z. Modulation of functional network with real-time fMRI feedback training of right premotor cortex activity. Neuropsychologia 2014, 62, 111–123. [Google Scholar] [CrossRef]
- Nicholson, A.A.; Rabellino, D.; Densmore, M.; Frewen, P.A.; Paret, C.; Kluetsch, R.; Schmahl, C.; Théberge, J.; Neufeld, R.W.; McKinnon, M.C.; et al. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017, 38, 541–560. [Google Scholar] [CrossRef] [PubMed]
- Niziolek, C.A.; Guenther, F.H. Vowel Category Boundaries Enhance Cortical and Behavioral Responses to Speech Feedback Alterations. J. Neurosci. 2013, 33, 12090–12098. [Google Scholar] [CrossRef] [Green Version]
- Rance, M.; Ruttorf, M.; Nees, F.; Schad, L.R.; Flor, H. Real time fMRI feedback of the anterior cingulate and posterior insular cortex in the processing of pain. Hum. Brain Mapp. 2014, 35, 5784–5798. [Google Scholar] [CrossRef]
- Thibault, R.T.; MacPherson, A.; Lifshitz, M.; Roth, R.R.; Raz, A. Neurofeedback with fMRI: A critical systematic review. NeuroImage 2018, 172, 786–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Sasaki, Y.; Shibata, K.; Kawato, M. Advances in fMRI Real-Time Neurofeedback. Trends Cogn. Sci. 2017, 21, 997–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, S.; Adamovich, S.V.; Tunik, E. Mirrored Feedback in Chronic Stroke: Recruitment and Effective Connectivity of Ipsilesional Sensorimotor Networks. Neurorehabilit. Neural Repair 2014, 28, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Cordes, J.S.; Mathiak, K.A.; Dyck, M.; Alawi, E.M.; Gaber, T.J.; Zepf, F.D.; Klasen, M.; Zvyagintsev, M.; Gur, R.C.; Mathiak, K. Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Front. Behav. Neurosci. 2015, 9, 169. [Google Scholar] [CrossRef]
- Subramanian, L.; Hindle, J.V.; Johnston, S.; Roberts, M.V.; Husain, M.; Goebel, R.; Linden, D. Real-Time Functional Magnetic Resonance Imaging Neurofeedback for Treatment of Parkinson’s Disease. J. Neurosci. 2011, 31, 16309–16317. [Google Scholar] [CrossRef]
- Subramanian, L.; Morris, M.B.; Brosnan, M.; Turner, D.L.; Morris, H.R.; Linden, D.E.J. Functional Magnetic Resonance Imaging Neurofeedback-guided Motor Imagery Training and Motor Training for Parkinson’s Disease: Randomized Trial. Front. Behav. Neurosci. 2016, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marins, T.F.; Rodrigues, E.C.; Engel, A.; Hoefle, S.; Basílio, R.; Lent, R.; Moll, J.; Tovar-Moll, F. Enhancing Motor Network Activity Using Real-Time Functional MRI Neurofeedback of Left Premotor Cortex. Front. Behav. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, P.; Sitaram, R.; Rana, M.; Montalba, C.; Tejos, C.; Ruiz, S. How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum. Brain Mapp. 2016, 37, 3153–3171. [Google Scholar] [CrossRef] [PubMed]
- Decharms, R.; Christoff, K.; Glover, G.H.; Pauly, J.M.; Whitfield, S.; Gabrieli, J.D. Learned regulation of spatially localized brain activation using real-time fMRI. NeuroImage 2004, 21, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.D.; Horovitz, S.G.; Venkataraman, G.; Hallett, M. Self-modulation of primary motor cortex activity with motor and motor imagery tasks using real-time fMRI-based neurofeedback. NeuroImage 2012, 59, 917–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.; Hayasaka, S.; Kawato, M.; Imamizu, H. Connectivity Neurofeedback Training Can Differentially Change Functional Connectivity and Cognitive Performance. Cereb. Cortex 2017, 27, 4960–4970. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Xu, L.; Long, Z.; Yao, L.; Wu, X. Functional connectivity alteration after real-time fMRI motor imagery training through self-regulation of activities of the right premotor cortex. BMC Neurosci. 2015, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Horovitz, S.G.; Berman, B.D.; Hallett, M. Real time BOLD functional MRI neuro-feedback affects functional connectivity. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology 2010, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4270–4273. [Google Scholar] [CrossRef] [Green Version]
- Papoutsi, M.; Weiskopf, N.; Langbehn, D.; Reilmann, R.; Rees, G.; Tabrizi, S.J. Stimulating neural plasticity with real-time fMRI neurofeedback in Huntington’s disease: A proof of concept study. Hum. Brain Mapp. 2017, 39, 1339–1353. [Google Scholar] [CrossRef] [Green Version]
- Megumi, F.; Yamashita, A.; Kawato, M.; Imamizu, H. Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front. Hum. Neurosci. 2015, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Preti, M.G.; Bolton, T.A.; Van De Ville, D. The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage 2017, 160, 41–54. [Google Scholar] [CrossRef]
- Chang, C.; Glover, G.H. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 2010, 50, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Handwerker, D.A.; Roopchansingh, V.; Gonzalez-Castillo, J.; Bandettini, P.A. Periodic changes in fMRI connectivity. NeuroImage 2012, 63, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking Whole-Brain Connectivity Dynamics in the Resting State. Cereb. Cortex 2014, 24, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Bolt, T.S.; Ezie, C.C.; Uddin, L.Q.; Heller, A.S. Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan. J. Neurosci. 2017, 37, 5539–5548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marusak, H.A.; Calhoun, V.D.; Brown, S.; Crespo, L.M.; Sala-Hamrick, K.; Gotlib, I.H.; Thomason, M.E. Dynamic functional connectivity of neurocognitive networks in children. Hum. Brain Mapp. 2017, 38, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Q.; Wang, C.; Yu, J. Changes in dynamic functional connections with aging. NeuroImage 2018, 172, 31–39. [Google Scholar] [CrossRef]
- Shen, H.; Li, Z.; Qin, J.; Liu, Q.; Wang, L.; Zeng, L.-L.; Li, H.; Hu, D. Changes in functional connectivity dynamics associated with vigilance network in taxi drivers. NeuroImage 2016, 124, 367–378. [Google Scholar] [CrossRef]
- Espinoza, F.A.; Liu, J.; Ciarochi, J.; Turner, J.A.; Vergara, V.M.; Caprihan, A.; Misiura, M.; Johnson, H.J.; Long, J.D.; Bockholt, J.H.; et al. Dynamic functional network connectivity in Huntington’s disease and its associations with motor and cognitive measures. Hum. Brain Mapp. 2018, 40, 1955–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Calhoun, V.D.; Phlypo, R.; Adalı, T. Dynamic changes of spatial functional network connectivity in healthy individuals and schizophrenia patients using independent vector analysis. NeuroImage 2014, 90, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Fong, A.H.C.; Yoo, K.; Rosenberg, M.D.; Zhang, S.; Li, C.-S.R.; Scheinost, D.; Constable, R.T.; Chun, M.M. Dynamic functional connectivity during task performance and rest predicts individual differences in attention across studies. NeuroImage 2019, 188, 14–25. [Google Scholar] [CrossRef]
- Li, G.; Zhou, L.; Chen, Z.; Luo, N.; Niu, M.; Li, Y.; Kang, W.; Liu, J. Dynamic functional connectivity impairments in idiopathic rapid eye movement sleep behavior disorder. Park. Relat. Disord. 2020, 79, 11–17. [Google Scholar] [CrossRef]
- Kupis, L.; Romero, C.; Dirks, B.; Hoang, S.; Parladé, M.V.; Beaumont, A.L.; Cardona, S.M.; Alessandri, M.; Chang, C.; Nomi, J.S.; et al. Evoked and intrinsic brain network dynamics in children with autism spectrum disorder. NeuroImage Clin. 2020, 28, 102396. [Google Scholar] [CrossRef]
- Wu, X.; He, H.; Shi, L.; Xia, Y.; Zuang, K.; Feng, Q.; Zhang, Y.; Ren, Z.; Wei, D.; Qiu, J. Personality traits are related with dynamic functional connectivity in major depression disorder: A resting-state analysis. J. Affect. Disord. 2019, 245, 1032–1042. [Google Scholar] [CrossRef]
- Rubia, K.; Criaud, M.; Wulff, M.; Alegria, A.; Brinson, H.; Barker, G.; Stahl, D.; Giampietro, V. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. NeuroImage 2019, 188, 43–58. [Google Scholar] [CrossRef]
- Calhoun, V.; Adali, T.; Pearlson, G.; Pekar, J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Li, M.; Wang, W.; Li, R.; Zhang, Z.; Lu, G.; Chen, H. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum. Brain Mapp. 2017, 38, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, E.; Allen, E.; Belger, A.; Ford, J.; McEwen, S.; Mathalon, D.; Mueller, B.; Pearlson, G.; Potkin, S.; Preda, A.; et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014, 5, 298–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomi, J.S.; Farrant, K.; Damaraju, E.; Rachakonda, S.; Calhoun, V.D.; Uddin, L.Q. Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum. Brain Mapp. 2016, 37, 1770–1787. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, N.; Van De Ville, D. Erratum to “On spurious and real fluctuations of dynamic functional connectivity during rest”. NeuroImage 2015, 104, 464–465. [Google Scholar] [CrossRef]
- Aggarwal, C.C.; Hinneburg, A.; Keim, D.A. On the Surprising Behavior of Distance Metrics in High Dimensional Space. Lect. Notes Comput. Sci. 2001, 1973, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; Volume xxvi, p. 488. [Google Scholar]
- Zhang, Z.; Yuan, K.H. Practical Statistical Power Analysis Using Web Power and R; ISDSA Press: Granger, IN, USA, 2018. [Google Scholar]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage 2010, 53, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Denkova, E.; Nomi, J.S.; Uddin, L.Q.; Jha, A.P. Dynamic brain network configurations during rest and an attention task with frequent occurrence of mind wandering. Hum. Brain Mapp. 2019, 40, 4564–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auer, T.; Schweizer, R.; Frahm, J. Training Efficiency and Transfer Success in an Extended Real-Time Functional MRI Neurofeedback Training of the Somatomotor Cortex of Healthy Subjects. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. From the Cover: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Díez-Cirarda, M.; Strafella, A.P.; Kim, J.; Peña, J.; Ojeda, N.; Cabrera-Zubizarreta, A.; Ibarretxe-Bilbao, N. Dynamic functional connectivity in Parkinson’s disease patients with mild cognitive impairment and normal cognition. NeuroImage Clin. 2018, 17, 847–855. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, E.J.R.; Schactera, D.L. The Brain’s Default Network. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, A.P. The role of the prefrontal cortex in dynamic filtering. Psychobiology 2000, 28, 207–218. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Criaud, M.; Cho, S.S.; Díez-Cirarda, M.; Mihaescu, A.; Coakeley, S.; Ghadery, C.; Valli, M.; Jacobs, M.F.; Houle, S.; et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 2017, 140, 2955–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, J.; Peraza, L.R.; Firbank, M.; Thomas, A.J.; Kaiser, M.; Gallagher, P.; O’Brien, J.T.; Blamire, A.M.; Taylor, J.-P. Dynamic functional connectivity changes in dementia with Lewy bodies and Alzheimer’s disease. NeuroImage Clin. 2019, 22, 101812. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Shi, J.; Zhang, Z.; Zheng, W.; Hu, T.; Li, Y.; Yu, Y.; Zhang, Z.; Fu, Y.; Zou, Y.; et al. Altered dynamic functional connectivity in weakly-connected state in major depressive disorder. Clin. Neurophysiol. 2019, 130, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- De Lange, F.P.; Roelofs, K.; Toni, I. Motor imagery: A window into the mechanisms and alterations of the motor system. Cortex 2008, 44, 494–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, L.; Wang, S.; Xie, B.; Guo, J.; Long, Z.; Yao, L. Behavioral improvements and brain functional alterations by motor imagery training. Brain Res. 2011, 1407, 38–46. [Google Scholar] [CrossRef]
- Calvo-Merino, B.; Glaser, D.; Grèzes, J.; Passingham, R.; Haggard, P. Action Observation and Acquired Motor Skills: An fMRI Study with Expert Dancers. Cereb. Cortex 2005, 15, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Albouy, G.; Sterpenich, V.; Balteau, E.; Vandewalle, G.; Desseilles, M.; Dang-Vu, T.; Darsaud, A.; Ruby, P.; Luppi, P.-H.; Degueldre, C.; et al. Both the Hippocampus and Striatum Are Involved in Consolidation of Motor Sequence Memory. Neuron 2008, 58, 261–272. [Google Scholar] [CrossRef]

| Component Index 1 | Network 1 | Region 1 | Component Index 2 | Network 2 | Region 2 | t-Score | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | MN | Left postcentral gyrus | 29 | DMN | Cuneus | 3.227 | 0.0081 |
| 2 | MN | Bilateral precentral gyrus | 4 | MN | Bilateral inferior frontal gyrus, triangular part | −3.349 | 0.0065 |
| 2 | MN | Bilateral precentral gyrus | 12 | VN | Bilateral middle occipital gyrus, superior occipital gyrus | −3.126 | 0.0096 |
| 2 | MN | Bilateral precentral gyrus | 17 | CCN | Hippocampus | −3.276 | 0.0074 |
| 4 | MN | Bilateral inferior frontal gyrus, triangular part | 15 | VN | Bilateral middle occipital gyrus | 3.268 | 0.0075 |
| 8 | MN | Bilateral postcentral gyrus | 15 | VN | Bilateral middle occipital gyrus | 3.109 | 0.0099 |
| 8 | MN | Bilateral postcentral gyrus | 29 | DMN | Cuneus | 3.934 | 0.0023 |
| 10 | MN | Supplementary motor area | 17 | CCN | Hippocampus | −3.381 | 0.0061 |
| 12 | VN | Bilateral middle occipital gyrus, superior occipital gyrus | 22 | VN | Cingulate and paracingulate gyrus | −3.142 | 0.0094 |
| Component Index 1 | Network 1 | Region 1 | Component Index 2 | Network 2 | Region 2 | t-Score | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | MN | Left postcentral gyrus | 29 | DMN | Cuneus | 3.227 | 0.0081 |
| 2 | MN | Bilateral precentral gyrus | 4 | MN | Bilateral inferior frontal gyrus, triangular part | −3.349 | 0.0065 |
| 2 | MN | Bilateral precentral gyrus | 12 | VN | Bilateral middle occipital gyrus, superior occipital gyrus | −3.126 | 0.0096 |
| 2 | MN | Bilateral precentral gyrus | 17 | CCN | Hippocampus | −3.276 | 0.0074 |
| 4 | MN | Bilateral inferior frontal gyrus, triangular part | 15 | VN | Bilateral middle occipital gyrus | 3.268 | 0.0075 |
| 8 | MN | Bilateral postcentral gyrus | 15 | VN | Bilateral middle occipital gyrus | 3.109 | 0.0099 |
| 8 | MN | Bilateral postcentral gyrus | 29 | DMN | Cuneus | 3.934 | 0.0023 |
| 10 | MN | Supplementary motor area | 17 | CCN | Hippocampus | −3.381 | 0.0061 |
| 12 | VN | Bilateral middle occipital gyrus, superior occipital gyrus | 22 | VN | Cingulate and paracingulate gyrus | −3.142 | 0.0094 |
| 1 | MN | Left postcentral gyrus | 29 | DMN | Cuneus | 3.227 | 0.0081 |
| 2 | MN | Bilateral precentral gyrus | 4 | MN | Bilateral inferior frontal gyrus, triangular part | −3.349 | 0.0065 |
| 2 | MN | Bilateral precentral gyrus | 12 | VN | Bilateral middle occipital gyrus, superior occipital gyrus | −3.126 | 0.0096 |
| 2 | MN | Bilateral precentral gyrus | 17 | CCN | Hippocampus | −3.276 | 0.0074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Z.; Guo, Z.; Guo, Z.; Zhang, H.; Yao, L. Dynamic Functional Network Connectivity Changes Associated with fMRI Neurofeedback of Right Premotor Cortex. Brain Sci. 2021, 11, 582. https://doi.org/10.3390/brainsci11050582
Long Z, Guo Z, Guo Z, Zhang H, Yao L. Dynamic Functional Network Connectivity Changes Associated with fMRI Neurofeedback of Right Premotor Cortex. Brain Sciences. 2021; 11(5):582. https://doi.org/10.3390/brainsci11050582
Chicago/Turabian StyleLong, Zhiying, Zhaoxi Guo, Zhitao Guo, Hang Zhang, and Li Yao. 2021. "Dynamic Functional Network Connectivity Changes Associated with fMRI Neurofeedback of Right Premotor Cortex" Brain Sciences 11, no. 5: 582. https://doi.org/10.3390/brainsci11050582
APA StyleLong, Z., Guo, Z., Guo, Z., Zhang, H., & Yao, L. (2021). Dynamic Functional Network Connectivity Changes Associated with fMRI Neurofeedback of Right Premotor Cortex. Brain Sciences, 11(5), 582. https://doi.org/10.3390/brainsci11050582





