Sensory Attenuation in Sport and Rehabilitation: Perspective from Research in Parkinson’s Disease
Abstract
1. Introduction
2. Sensory Attenuation and Task-Relevance
2.1. Recent and Relevant Findings
2.2. Reconsidering Sensory Attenuation Theory
2.3. Scope of the Dominant Theories
2.4. Factors Affecting Sensory Attenuation
2.5. Task-Relevance in Sensory Attenuation
3. Neural Substrate
3.1. Reconciling Discrepancies in Studies Investigating the Neural Substrates of Sensory Attenuation
3.2. The BG through a Sensory Lens: Task-Relevant Signaling
3.3. Sensation to Action
3.4. Sensorimotor Integration
4. Establishing Principles for Rehabilitation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Gaenslen, A.; Swid, I.; Liepelt-Scarfone, I.; Godau, J.; Berg, D. The Patients’ Perception of Prodromal Symptoms Before the Initial Diagnosis of Parkinson’s Disease. Mov. Disord. 2011, 26, 653–658. [Google Scholar] [CrossRef]
- Jost, W.H.; Bausch, J. Patients’ Perspective on Current Treatment Options for Parkinson’s Disease. Basal Ganglia 2017, 9, 7–11. [Google Scholar] [CrossRef]
- Sangarapillai, K.; Norman, B.M.; Almeida, Q.J. Analyzing the Effects of PDSAFExTM on the Motor Symptoms of Parkinson’s Disease: A Retrospective Study. NeuroRehabilitation 2020, 46, 589–593. [Google Scholar] [CrossRef]
- Bratsos, S.; Karponis, D.; Saleh, S.N. Efficacy and Safety of Deep Brain Stimulation in the Treatment of Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- di Biase, L.; Fasano, A. Low-Frequency Deep Brain Stimulation for Parkinson’s Disease: Great Expectation or False Hope? Mov. Disord. 2016, 31, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing Consensus among Movement Disorder Specialists on Clinical Indicators for Identification and Management of Advanced Parkinson’s Disease: A Multi-Country Delphi-Panel Approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Sage, M.D.; Almeida, Q.J. A Positive Influence of Vision on Motor Symptoms during Sensory Attention Focused Exercise for Parkinson’s Disease. Mov. Disord. 2010, 25, 64–69. [Google Scholar] [CrossRef]
- Sage, M.D.; Almeida, Q.J. Symptom and Gait Changes after Sensory Attention Focused Exercise vs Aerobic Training in Parkinson’s Disease. Mov. Disord. 2009, 24, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Ebersbach, G.; Ebersbach, A.; Edler, D.; Kaufhold, O.; Kusch, M.; Kupsch, A.; Wissel, J. Comparing Exercise in Parkinson’s Disease--the Berlin LSVT®BIG Study. Mov. Disord. 2010, 25, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Chawla, G.; Hoppe, M.; Browner, N.; Lewek, M.D. Individuals with Parkinson’s Disease Retain Spatiotemporal Gait Control with Music and Metronome Cues. Motor Control 2020, 25, 33–43. [Google Scholar] [CrossRef]
- del Olmo, M.F.; Cudeiro, J. Temporal Variability of Gait in Parkinson Disease: Effectsof a Rehabilitation Programme Based on Rhythmic Sound Cues. Parkinsonism Relat. Disord. 2005, 11, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to Modulate Walking Cadence Remains Intact in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Stride Length Regulation in Parkinson’s Disease. Normalization Strategies and Underlying Mechanisms. Brain J. Neurol. 1996, 119 Pt 2, 551–568. [Google Scholar] [CrossRef]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and Gait Improvement among People With Parkinson’s Disease: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Caligiore, D.; Mustile, M.; Spalletta, G.; Baldassarre, G. Action Observation and Motor Imagery for Rehabilitation in Parkinson’s Disease: A Systematic Review and an Integrative Hypothesis. Neurosci. Biobehav. Rev. 2017, 72, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Corcos, D.M.; Robichaud, J.A.; David, F.J.; Leurgans, S.E.; Vaillancourt, D.E.; Poon, C.; Rafferty, M.R.; Kohrt, W.M.; Comella, C.L. A Two-Year Randomized Controlled Trial of Progressive Resistance Exercise for Parkinson’s Disease. Mov. Disord. 2013, 28, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Ridgel, A.L.; Ault, D.L. High-Cadence Cycling Promotes Sustained Improvement in Bradykinesia, Rigidity, and Mobility in Individuals with Mild-Moderate Parkinson’s Disease. Park. Dis. 2019, 2019, 4076862. [Google Scholar] [CrossRef] [PubMed]
- Ridgel, A.L.; Vitek, J.L.; Alberts, J.L. Forced, Not Voluntary, Exercise Improves Motor Function in Parkinson’s Disease Patients. Neurorehabil. Neural Repair 2009, 23, 600–608. [Google Scholar] [CrossRef]
- Ridgel, A.L.; Phillips, R.S.; Walter, B.L.; Discenzo, F.M.; Loparo, K.A. Dynamic High-Cadence Cycling Improves Motor Symptoms in Parkinson’s Disease. Front. Neurol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Miner, D.G.; Aron, A.; DiSalvo, E. Therapeutic Effects of Forced Exercise Cycling in Individuals with Parkinson’s Disease. J. Neurol. Sci. 2020, 410, 116677. [Google Scholar] [CrossRef]
- Bek, J.; Arakaki, A.I.; Lawrence, A.; Sullivan, M.; Ganapathy, G.; Poliakoff, E. Dance and Parkinson’s: A Review and Exploration of the Role of Cognitive Representations of Action. Neurosci. Biobehav. Rev. 2020, 109, 16–28. [Google Scholar] [CrossRef]
- Foster, E.R.; Golden, L.; Duncan, R.P.; Earhart, G.M. Community-Based Argentine Tango Dance Program Is Associated with Increased Activity Participation among Individuals with Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 240–249. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H.J.; Brittain, J.-S.; Spitzer, B.; Hanslmayr, S.; Jenkinson, N. Memory Deficits in Parkinson’s Disease Are Associated with Reduced Beta Power Modulation. Brain Commun. 2019, 1, fcz040. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.; Jain, S. Non-Motor Symptoms of Parkinson Disease: Update on the Diagnosis and Treatment. Neurologist 2004, 10, 185–194. [Google Scholar]
- Kaji, R. Basal Ganglia as a Sensory Gating Devise for Motor Control. J. Med. Investig. JMI 2001, 48, 142–146. [Google Scholar]
- Lidsky, T.I.; Manetto, C.; Schneider, J.S. A Consideration of Sensory Factors Involved in Motor Functions of the Basal Ganglia. Brain Res. Rev. 1985, 9, 133–146. [Google Scholar] [CrossRef]
- Nakajima, M.; Schmitt, L.I.; Halassa, M.M. Prefrontal Cortex Regulates Sensory Filtering through a Basal Ganglia-to-Thalamus Pathway. Neuron 2019, 103, 445–458.e10. [Google Scholar] [CrossRef] [PubMed]
- Robbe, D. To Move or to Sense? Incorporating Somatosensory Representation into Striatal Functions. Curr. Opin. Neurobiol. 2018, 52, 123–130. [Google Scholar] [CrossRef]
- Gale, J.T.; Amirnovin, R.; Williams, Z.M.; Flaherty, A.W.; Eskandar, E.N. From Symphony to Cacophony: Pathophysiology of the Human Basal Ganglia in Parkinson Disease. Neurosci. Biobehav. Rev. 2008, 32, 378–387. [Google Scholar] [CrossRef]
- Utter, A.A.; Basso, M.A. The Basal Ganglia: An Overview of Circuits and Function. Neurosci. Biobehav. Rev. 2008, 32, 333–342. [Google Scholar] [CrossRef]
- Shergill, S.S.; Bays, P.M.; Frith, C.D.; Wolpert, D.M. Two Eyes for an Eye: The Neuroscience of Force Escalation. Science 2003, 301, 187. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.D.; Taylor, J.L.; Gandevia, S.C. Overestimation of Force during Matching of Externally Generated Forces. J. Physiol. 2011, 589, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wolpe, N.; Zhang, J.; Nombela, C.; Ingram, J.N.; Wolpert, D.M.; Cam, C.A.N.; Rowe, J.B. Sensory Attenuation in Parkinson’s Disease Is Related to Disease Severity and Dopamine Dose. Sci. Rep. 2018, 8, 15643. [Google Scholar] [CrossRef] [PubMed]
- Macerollo, A.; Chen, J.-C.; Korlipara, P.; Foltynie, T.; Rothwell, J.; Edwards, M.J.; Kilner, J.M. Dopaminergic Treatment Modulates Sensory Attenuation at the Onset of the Movement in Parkinson’s Disease: A Test of a New Framework for Bradykinesia. Mov. Disord. 2016, 31, 143–146. [Google Scholar] [CrossRef]
- Horváth, J. Action-Related Auditory ERP Attenuation: Paradigms and Hypotheses. Brain Res. 2015, 1626, 54–65. [Google Scholar] [CrossRef]
- Mifsud, N.G.; Beesley, T.; Watson, T.L.; Elijah, R.B.; Sharp, T.S.; Whitford, T.J. Attenuation of Visual Evoked Responses to Hand and Saccade-Initiated Flashes. Cognition 2018, 179, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Wolpert, D.M.; Frith, C.D. Central Cancellation of Self-Produced Tickle Sensation. Nat. Neurosci. 1998, 1, 635–640. [Google Scholar] [CrossRef]
- Kilteni, K.; Houborg, C.; Ehrsson, H.H. Rapid Learning and Unlearning of Predicted Sensory Delays in Self-Generated Touch. eLife 2019, 8. [Google Scholar] [CrossRef]
- Heins, N.; Pomp, J.; Kluger, D.S.; Trempler, I.; Zentgraf, K.; Raab, M.; Schubotz, R.I. Incidental or Intentional? Different Brain Responses to One’s Own Action Sounds in Hurdling vs. Tap Dancing. Front. Neurosci. 2020, 14, 483. [Google Scholar] [CrossRef]
- Sato, A. Action Observation Modulates Auditory Perception of the Consequence of Others’ Actions. Conscious. Cogn. 2008, 17, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Macerollo, A.; Limousin, P.; Korlipara, P.; Foltynie, T.; Edwards, M.J.; Kilner, J. Dopaminergic Modulation of Sensory Attenuation in Parkinson’s Disease: Is There an Underlying Modulation of Beta Power? Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Wolpe, N.; Ingram, J.N.; Tsvetanov, K.A.; Geerligs, L.; Kievit, R.A.; Henson, R.N.; Wolpert, D.M.; Rowe, J.B. Ageing Increases Reliance on Sensorimotor Prediction through Structural and Functional Differences in Frontostriatal Circuits. Nat. Commun. 2016, 7, 13034. [Google Scholar] [CrossRef]
- Ogata, K.; Okamoto, T.; Yamasaki, T.; Shigeto, H.; Tobimatsu, S. Pre-Movement Gating of Somatosensory-Evoked Potentials by Self-Initiated Movements: The Effects of Ageing and Its Implication. Clin. Neurophysiol. 2009, 120, 1143–1148. [Google Scholar] [CrossRef]
- Simões-Franklin, C.; Whitaker, T.A.; Newell, F.N. Active and Passive Touch Differentially Activate Somatosensory Cortex in Texture Perception. Hum. Brain Mapp. 2011, 32, 1067–1080. [Google Scholar] [CrossRef]
- Railo, H.; Nokelainen, N.; Savolainen, S.; Kaasinen, V. Deficits in Monitoring Self-Produced Speech in Parkinson’s Disease. Clin. Neurophysiol. 2020, 131, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.W.; Wolpert, D.M. Computational Mechanisms of Sensorimotor Control. Neuron 2011, 72, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Adams, R.A.; Parees, I.; Edwards, M.; Friston, K. Active Inference, Sensory Attenuation and Illusions. Cogn. Process. 2013, 14, 411–427. [Google Scholar] [CrossRef]
- Körding, K.P.; Wolpert, D.M. Bayesian Integration in Sensorimotor Learning. Nature 2004, 427, 244–247. [Google Scholar] [CrossRef]
- Körding, K.P.; Wolpert, D.M. Bayesian Decision Theory in Sensorimotor Control. Trends Cogn. Sci. 2006, 10, 319–326. [Google Scholar] [CrossRef]
- Wolpert, D.; Flanagan, J.R. Motor Learning. Curr. Biol. 2010, 20, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Engeler, P.; Ehrsson, H.H. Efference Copy Is Necessary for the Attenuation of Self-Generated Touch. iScience 2020, 23, 100843. [Google Scholar] [CrossRef] [PubMed]
- Shergill, S.S.; White, T.P.; Joyce, D.W.; Bays, P.M.; Wolpert, D.M.; Frith, C.D. Functional Magnetic Resonance Imaging of Impaired Sensory Prediction in Schizophrenia. JAMA Psychiatry 2014, 71, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Clark, A. Surfing Uncertainty: Prediction, Action, and the Embodied Mind; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-021702-0. [Google Scholar]
- Saradjian, A.H. Sensory Modulation of Movement, Posture and Locomotion. Neurophysiol. Clin. Clin. Neurophysiol. 2015, 45, 255–267. [Google Scholar] [CrossRef]
- Blakemore, S.-J.; Wolpert, D.; Frith, C. Why Can’t You Tickle Yourself? NeuroReport 2000, 11, R11–R16. [Google Scholar] [CrossRef]
- Chapman, C.E.; Bushnell, M.C.; Miron, D.; Duncan, G.H.; Lund, J.P. Sensory Perception during Movement in Man. Exp. Brain Res. 1987, 68, 516–524. [Google Scholar] [CrossRef]
- Hohwy, J. The Self-Evidencing Brain. Noûs 2016, 50, 259–285. [Google Scholar] [CrossRef]
- Richter, D.; de Lange, F.P. Statistical Learning Attenuates Visual Activity Only for Attended Stimuli. eLife 2019, 8. [Google Scholar] [CrossRef]
- Dogge, M.; Hofman, D.; Custers, R.; Aarts, H. Exploring the Role of Motor and Non-Motor Predictive Mechanisms in Sensory Attenuation: Perceptual and Neurophysiological Findings. Neuropsychologia 2019, 124, 216–225. [Google Scholar] [CrossRef]
- Kaiser, J.; Schütz-Bosbach, S. Sensory Attenuation of Self-Produced Signals Does Not Rely on Self-Specific Motor Predictions. Eur. J. Neurosci. 2018, 47, 1303–1310. [Google Scholar] [CrossRef]
- Gandolla, M.; Ferrante, S.; Molteni, F.; Guanziroli, E.; Frattini, T.; Martegani, A.; Ferrigno, G.; Friston, K.; Pedrocchi, A.; Ward, N.S. Re-Thinking the Role of Motor Cortex: Context-Sensitive Motor Outputs? NeuroImage 2014, 91, 366–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Léonard, G.; Mercier, C.; Tremblay, L.E. Effect of Repetitive Afferent Electrical Stimulation of the Lower Limb on Corticomotor Excitability and Implications for Rehabilitation. J. Clin. Neurosci. 2013, 20, 435–439. [Google Scholar] [CrossRef]
- Roy, F.D.; Gorassini, M.A. Peripheral Sensory Activation of Cortical Circuits in the Leg Motor Cortex of Man. J. Physiol. 2008, 586, 4091–4105. [Google Scholar] [CrossRef] [PubMed]
- Saradjian, A.H.; Tremblay, L.; Perrier, J.; Blouin, J.; Mouchnino, L. Cortical Facilitation of Proprioceptive Inputs Related to Gravitational Balance Constraints during Step Preparation. J. Neurophysiol. 2013, 110, 397–407. [Google Scholar] [CrossRef]
- Reznik, D.; Mukamel, R. Motor Output, Neural States and Auditory Perception. Neurosci. Biobehav. Rev. 2019, 96, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, J.; Lopes, P.; Keck, J.; Baudisch, P.; Friston, K.; Blankenburg, F. Action-Dependent Processing of Touch in the Human Parietal Operculum and Posterior Insula. Cereb. Cortex 2020, 30, 607–617. [Google Scholar] [CrossRef]
- Yon, D.; Gilbert, S.J.; de Lange, F.P.; Press, C. Action Sharpens Sensory Representations of Expected Outcomes. Nat. Commun. 2018, 9, 4288. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Shipp, S.; Friston, K.J. Predictions Not Commands: Active Inference in the Motor System. Brain Struct. Funct. 2013, 218, 611–643. [Google Scholar] [CrossRef]
- Holland, P.J.; Sibindi, T.M.; Ginzburg, M.; Das, S.; Arkesteijn, K.; Frens, M.A.; Donchin, O. A Neuroanatomically Grounded Optimal Control Model of the Compensatory Eye Movement System in Mice. Front. Syst. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Giblin, D.R. Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann. N. Y. Acad. Sci. 1964, 112, 93–142. [Google Scholar] [CrossRef]
- Pyasik, M.; Salatino, A.; Burin, D.; Berti, A.; Ricci, R.; Pia, L. Shared Neurocognitive Mechanisms of Attenuating Self-Touch and Illusory Self-Touch. Soc. Cogn. Affect. Neurosci. 2019, 14, 119–127. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Spence, C.; Passingham, R.E. That’s My Hand! Activity in Premotor Cortex Reflects Feeling of Ownership of a Limb. Science 2004, 305, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Ehrsson, H.H. Body Ownership Determines the Attenuation of Self-Generated Tactile Sensations. Proc. Natl. Acad. Sci. USA 2017, 114, 8426–8431. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. The Sense of Agency and Its Disturbances in Schizophrenia: A Reappraisal. Exp. Brain Res. 2009, 192, 527–532. [Google Scholar] [CrossRef]
- Shergill, S.S.; Samson, G.; Bays, P.M.; Frith, C.D.; Wolpert, D.M. Evidence for Sensory Prediction Deficits in Schizophrenia. Am. J. Psychiatry 2005, 162, 2384–2386. [Google Scholar] [CrossRef]
- Almeida, Q.J.; Lebold, C.A. Freezing of Gait in Parkinson’s Disease: A Perceptual Cause for a Motor Impairment? J. Neurol. Neurosurg. Psychiatry 2010, 81, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Quintyn, M.; Cross, E. Factors Affecting the Ability to Initiate Movement in Parkinson’s Disease. Phys. Occup. Ther. Geriatr. 1986, 4, 51–60. [Google Scholar] [CrossRef]
- Miall, R.C.; Stanley, J.; Todhunter, S.; Levick, C.; Lindo, S.; Miall, J.D. Performing Hand Actions Assists the Visual Discrimination of Similar Hand Postures. Neuropsychologia 2006, 44, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Song, J.-H. Action Fluency Facilitates Perceptual Discrimination. Psychol. Sci. 2019, 30, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.; Sajda, P. Musical Experts Recruit Action-Related Neural Structures in Harmonic Anomaly Detection: Evidence for Embodied Cognition in Expertise. Brain Cogn. 2013, 83, 190–202. [Google Scholar] [CrossRef][Green Version]
- Bosch, F. Anatomy of Agility: Movement Analysis in Sport; 2010Publishers: Rotterdam, The Netherlands, 2020; ISBN 978-94-90951-59-7. [Google Scholar]
- Wykowska, A.; Schubö, A. Perception and Action as Two Sides of the Same Coin. A Review of the Importance of Action-Perception Links in Humans for Social Robot Design and Research. Int. J. Soc. Robot. 2012, 4, 5–14. [Google Scholar] [CrossRef]
- Kammers, M.P.M.; de Vignemont, F.; Verhagen, L.; Dijkerman, H.C. The Rubber Hand Illusion in Action. Neuropsychologia 2009, 47, 204–211. [Google Scholar] [CrossRef]
- Wolpe, N.; Hezemans, F.H.; Rowe, J.B. Alien Limb Syndrome: A Bayesian Account of Unwanted Actions. Cortex J. Devoted Study Nerv. Syst. Behav. 2020, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Debener, S.; Spychala, N.; Bongartz, E.; Sörös, P.; Müller, H.H.O.; Philipsen, A. The Senses of Agency and Ownership: A Review. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.D.; Gurney, K.; Prescott, T.J. Is There a Brainstem Substrate for Action Selection? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1627–1639. [Google Scholar] [CrossRef][Green Version]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-Directed and Habitual Control in the Basal Ganglia: Implications for Parkinson’s Disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P. Cortical Mechanisms of Action Selection: The Affordance Competition Hypothesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1585–1599. [Google Scholar] [CrossRef]
- Cisek, P.; Pastor-Bernier, A. On the Challenges and Mechanisms of Embodied Decisions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Staines, W.R.; Graham, S.J.; Black, S.E.; McIlroy, W.E. Task-Relevant Modulation of Contralateral and Ipsilateral Primary Somatosensory Cortex and the Role of a Prefrontal-Cortical Sensory Gating System. NeuroImage 2002, 15, 190–199. [Google Scholar] [CrossRef]
- Riley, M.A.; Stoffregen, T.A.; Grocki, M.J.; Turvey, M.T. Postural Stabilization for the Control of Touching. Hum. Mov. Sci. 1999, 18, 795–817. [Google Scholar] [CrossRef]
- Reiser, J.E.; Wascher, E.; Arnau, S. Recording Mobile EEG in an Outdoor Environment Reveals Cognitive-Motor Interference Dependent on Movement Complexity. Sci. Rep. 2019, 9, 13086. [Google Scholar] [CrossRef] [PubMed]
- Teufel, C.; Subramaniam, N.; Dobler, V.; Perez, J.; Finnemann, J.; Mehta, P.R.; Goodyer, I.M.; Fletcher, P.C. Shift toward Prior Knowledge Confers a Perceptual Advantage in Early Psychosis and Psychosis-Prone Healthy Individuals. Proc. Natl. Acad. Sci. USA 2015, 112, 13401–13406. [Google Scholar] [CrossRef] [PubMed]
- Teufel, C.; Fletcher, P.C. Forms of Prediction in the Nervous System. Nat. Rev. Neurosci. 2020, 21, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.A.E.; Staines, W.R. Age-Related Loss in Attention-Based Modulation of Tactile Stimuli at Early Stages of Somatosensory Processing. Neuropsychologia 2012, 50, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Gulberti, A.; Hamel, W.; Buhmann, C.; Boelmans, K.; Zittel, S.; Gerloff, C.; Westphal, M.; Engel, A.K.; Schneider, T.R.; Moll, C.K.E. Subthalamic Deep Brain Stimulation Improves Auditory Sensory Gating Deficit in Parkinson’s Disease. Clin. Neurophysiol. 2015, 126, 565–574. [Google Scholar] [CrossRef]
- Conte, A.; Khan, N.; Defazio, G.; Rothwell, J.; Berardelli, A. Pathophysiology of Somatosensory Abnormalities in Parkinson Disease. Nat. Rev. Neurol. 2013, 9. [Google Scholar] [CrossRef]
- Bradley, D.; Whelan, R.; Kimmich, O.; O’Riordan, S.; Mulrooney, N.; Brady, P.; Walsh, R.; Reilly, R.B.; Hutchinson, S.; Molloy, F.; et al. Temporal Discrimination Thresholds in Adult-Onset Primary Torsion Dystonia: An Analysis by Task Type and by Dystonia Phenotype. J. Neurol. 2012, 259, 77–82. [Google Scholar] [CrossRef]
- Rao, S.M.; Mayer, A.R.; Harrington, D.L. The Evolution of Brain Activation during Temporal Processing. Nat. Neurosci. 2001, 4, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Anderson, M.E. Context-Dependent Modulation of Movement-Related Discharge in the Primate Globus Pallidus. J. Neurosci. 2005, 25, 2965–2976. [Google Scholar] [CrossRef]
- Beeler, J.A.; Kisbye Dreyer, J. Synchronicity: The Role of Midbrain Dopamine in Whole-Brain Coordination. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Bakhurin, K.I.; Goudar, V.; Shobe, J.L.; Claar, L.D.; Buonomano, D.V.; Masmanidis, S.C. Differential Encoding of Time by Prefrontal and Striatal Network Dynamics. J. Neurosci. 2017, 37, 854–870. [Google Scholar] [CrossRef]
- Jo, H.-G.; Habel, U.; Schmidt, S. Role of the Supplementary Motor Area in Auditory Sensory Attenuation. Brain Struct. Funct. 2019, 224, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Ehrsson, H.H. Functional Connectivity between the Cerebellum and Somatosensory Areas Implements the Attenuation of Self-Generated Touch. J. Neurosci. 2020, 40, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Boehme, R.; Frost Karlsson, M.; Heilig, M.; Olausson, H.; Capusan, A.J. Sharpened Self-Other Distinction in Attention Deficit Hyperactivity Disorder. NeuroImage Clin. 2020, 27, 102317. [Google Scholar] [CrossRef]
- Leube, D.T.; Knoblich, G.; Erb, M.; Schlotterbeck, P.; Kircher, T.T.J. The Neural Basis of Disturbed Efference Copy Mechanism in Patients with Schizophrenia. Cogn. Neurosci. 2010, 1, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ackerley, R.; Hassan, E.; Curran, A.; Wessberg, J.; Olausson, H.; McGlone, F. An FMRI Study on Cortical Responses during Active Self-Touch and Passive Touch from Others. Front. Behav. Neurosci. 2012, 6. [Google Scholar] [CrossRef]
- Eliades, S.J.; Wang, X. Neural Substrates of Vocalization Feedback Monitoring in Primate Auditory Cortex. Nature 2008, 453, 1102–1106. [Google Scholar] [CrossRef]
- Synofzik, M.; Thier, P.; Leube, D.T.; Schlotterbeck, P.; Lindner, A. Misattributions of Agency in Schizophrenia Are Based on Imprecise Predictions about the Sensory Consequences of One’s Actions. Brain J. Neurol. 2010, 133, 262–271. [Google Scholar] [CrossRef]
- Martinu, K.; Monchi, O. Cortico-Basal Ganglia and Cortico-Cerebellar Circuits in Parkinson’s Disease: Pathophysiology or Compensation? Behav. Neurosci. 2013, 127, 222–236. [Google Scholar] [CrossRef]
- van Donkelaar, P.; Stein, J.F.; Passingham, R.E.; Miall, R.C. Temporary Inactivation in the Primate Motor Thalamus during Visually Triggered and Internally Generated Limb Movements. J. Neurophysiol. 2000, 83, 2780–2790. [Google Scholar] [CrossRef]
- Yamazaki, T.; Lennon, W. Revisiting a Theory of Cerebellar Cortex. Neurosci. Res. 2019, 148, 1–8. [Google Scholar] [CrossRef]
- Bosch, F. Strength Training and Coordination: An Integrative Approach; 2010Publishers: Rotterdam, The Netherlands, 2015; ISBN 978-94-90951-27-6. [Google Scholar]
- Ehgoetz Martens, K.A.; Ellard, C.G.; Almeida, Q.J. Does Anxiety Cause Freezing of Gait in Parkinson’s Disease? PLoS ONE 2014, 9, e106561. [Google Scholar] [CrossRef]
- Ehgoetz Martens, K.A.; Ellard, C.G.; Almeida, Q.J. Virtually-Induced Threat in Parkinson’s: Dopaminergic Interactions between Anxiety and Sensory–Perceptual Processing While Walking. Neuropsychologia 2015, 79, 322–331. [Google Scholar] [CrossRef]
- Klockgether, T.; Dichgans, J. Visual Control of Arm Movement in Parkinson’s Disease. Mov. Disord. 1994, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. Basal Ganglia Role in Behavior: Importance of Sensory Gating and Its Relevance to Psychiatry. Biol. Psychiatry 1984, 19, 1693–1710. [Google Scholar] [PubMed]
- Gilbert, C.D.; Li, W. Top-down Influences on Visual Processing. Nat. Rev. Neurosci. 2013, 14, 350–363. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Klingberg, T. Prefrontal Cortex and Basal Ganglia Control Access to Working Memory. Nat. Neurosci. 2008, 11, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. Ingestion-Related Activity of Caudate and Entopeduncular Neurons in the Cat. Exp. Neurol. 1987, 95, 216–223. [Google Scholar] [CrossRef]
- Andres, D.S.; Darbin, O. Complex Dynamics in the Basal Ganglia: Health and Disease Beyond the Motor System. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 101–114. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Cowan, N.; Vogel, E.K.; Rolan, T.; Valle-Inclán, F.; Hackley, S.A. Visual Working Memory Deficits in Patients with Parkinson’s Disease Are Due to Both Reduced Storage Capacity and Impaired Ability to Filter out Irrelevant Information. Brain J. Neurol. 2010, 133, 2677–2689. [Google Scholar] [CrossRef]
- Flynn, J.P.; Edwards, S.B.; Bandler, R.J. Changes in Sensory and Motor Systems during Centrally Elicited Attack. Behav. Sci. 1971, 16, 1–19. [Google Scholar] [CrossRef]
- Sippy, T.; Lapray, D.; Crochet, S.; Petersen, C.C.H. Cell-Type-Specific Sensorimotor Processing in Striatal Projection Neurons during Goal-Directed Behavior. Neuron 2015, 88, 298–305. [Google Scholar] [CrossRef]
- Haber, S.N.; Calzavara, R. The Cortico-Basal Ganglia Integrative Network: The Role of the Thalamus. Brain Res. Bull. 2009, 78, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Connor, N.P.; Abbs, J.H. Task-Dependent Variations in Parkinsonian Motor Impairments. Brain J. Neurol. 1991, 114 Pt 1A, 321–332. [Google Scholar]
- Vitório, R.; Lirani-Silva, E.; Pieruccini-Faria, F.; Moraes, R.; Almeida, Q. Visual Cues and Gait Improvement in Parkinson’s Disease: Which Piece of Information Is Really Important? Neuroscience 2014. [Google Scholar] [CrossRef]
- Ketzef, M.; Spigolon, G.; Johansson, Y.; Bonito-Oliva, A.; Fisone, G.; Silberberg, G. Dopamine Depletion Impairs Bilateral Sensory Processing in the Striatum in a Pathway-Dependent Manner. Neuron 2017, 94, 855–865.e5. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.-S.; Sharott, A.; Brown, P. The Highs and Lows of Beta Activity in Cortico-Basal Ganglia Loops. Eur. J. Neurosci. 2014, 39, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, N.; Brown, P. New Insights into the Relationship between Dopamine, Beta Oscillations and Motor Function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef]
- Brown, P. Bad Oscillations in Parkinson’s Disease. J. Neural Transm. Suppl. 2006, 27–30. [Google Scholar] [CrossRef]
- Pollok, B.; Krause, V.; Martsch, W.; Wach, C.; Schnitzler, A.; Südmeyer, M. Motor-Cortical Oscillations in Early Stages of Parkinson’s Disease. J. Physiol. 2012, 590, 3203–3212. [Google Scholar] [CrossRef]
- Marsden, J.F.; Limousin-Dowsey, P.; Ashby, P.; Pollak, P.; Brown, P. Subthalamic Nucleus, Sensorimotor Cortex and Muscle Interrelationships in Parkinson’s Disease. Brain 2001, 124, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Pogosyan, A.; Kuhn, A.A.; Brown, P. Beta Band Stability over Time Correlates with Parkinsonian Rigidity and Bradykinesia. Exp. Neurol. 2012, 236, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsu, Y.T.; Chan, H.L.; Chiou, S.M.; Tu, P.H.; Lee, S.T.; Tsai, C.H.; Lu, C.S.; Brown, P. Complexity of Subthalamic 13-35 Hz Oscillatory Activity Directly Correlates with Clinical Impairment in Patients with Parkinson’s Disease. Exp. Neurol. 2010, 224, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Roeder, L.; Boonstra, T.W.; Kerr, G.K. Corticomuscular Control of Walking in Older People and People with Parkinson’s Disease. Sci. Rep. 2020, 10, 2980. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.-K.R.; Debener, S.; Nobre, A.C. Synchronisation of Neural Oscillations and Cross-Modal Influences. Trends Cogn. Sci. 2020, 24, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.-S.; Brown, P. Oscillations and the Basal Ganglia: Motor Control and Beyond. NeuroImage 2014, 85 Pt 2, 637–647. [Google Scholar] [CrossRef]
- Brown, L.L.; Schneider, J.S.; Lidsky, T.I. Sensory and Cognitive Functions of the Basal Ganglia. Curr. Opin. Neurobiol. 1997, 7, 157–163. [Google Scholar] [CrossRef]
- Nonnekes, J.; Ružicka, E.; Nieuwboer, A.; Hallett, M.; Fasano, A.; Bloem, B.R. Compensation Strategies for Gait Impairments in Parkinson Disease: A Review. JAMA Neurol. 2019, 76, 718–725. [Google Scholar] [CrossRef]
- Godden, D.R.; Baddeley, A.D. Context-Dependent Memory in Two Natural Environments: On Land and Underwater. Br. J. Psychol. 1975, 66, 325–331. [Google Scholar] [CrossRef]
- Song, J.-H.; Bédard, P. Paradoxical Benefits of Dual-Task Contexts for Visuomotor Memory. Psychol. Sci. 2015, 26, 148–158. [Google Scholar] [CrossRef]
- Azmi, F. Playing Basketball in Hong Kong.
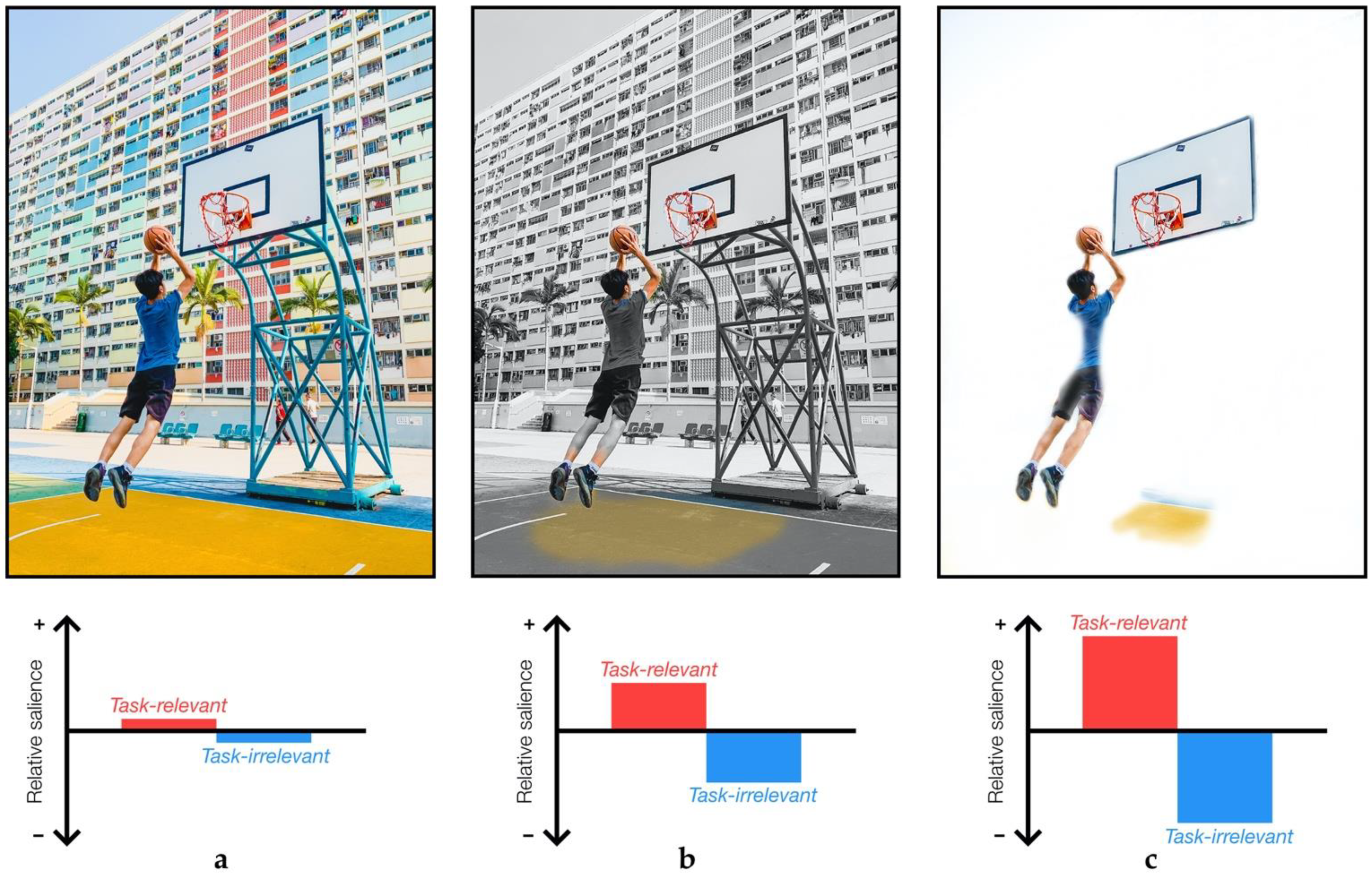
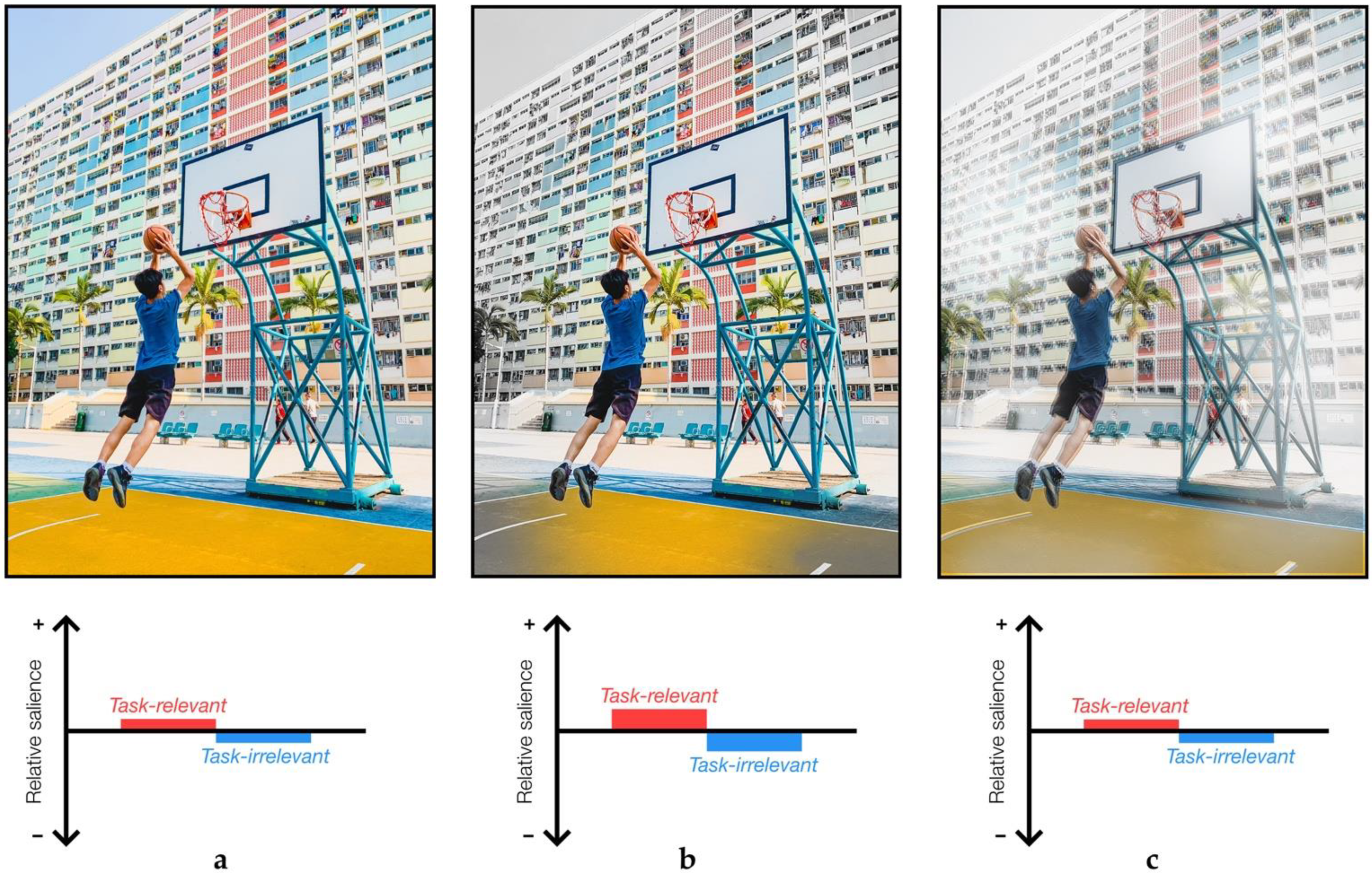
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kearney, J.; Brittain, J.-S. Sensory Attenuation in Sport and Rehabilitation: Perspective from Research in Parkinson’s Disease. Brain Sci. 2021, 11, 580. https://doi.org/10.3390/brainsci11050580
Kearney J, Brittain J-S. Sensory Attenuation in Sport and Rehabilitation: Perspective from Research in Parkinson’s Disease. Brain Sciences. 2021; 11(5):580. https://doi.org/10.3390/brainsci11050580
Chicago/Turabian StyleKearney, Joshua, and John-Stuart Brittain. 2021. "Sensory Attenuation in Sport and Rehabilitation: Perspective from Research in Parkinson’s Disease" Brain Sciences 11, no. 5: 580. https://doi.org/10.3390/brainsci11050580
APA StyleKearney, J., & Brittain, J.-S. (2021). Sensory Attenuation in Sport and Rehabilitation: Perspective from Research in Parkinson’s Disease. Brain Sciences, 11(5), 580. https://doi.org/10.3390/brainsci11050580





