Rehabilitation of Falls in Parkinson’s Disease: Self-Perception vs. Objective Measures of Fall Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
2.3. Intervention
2.4. Data Analysis
3. Results
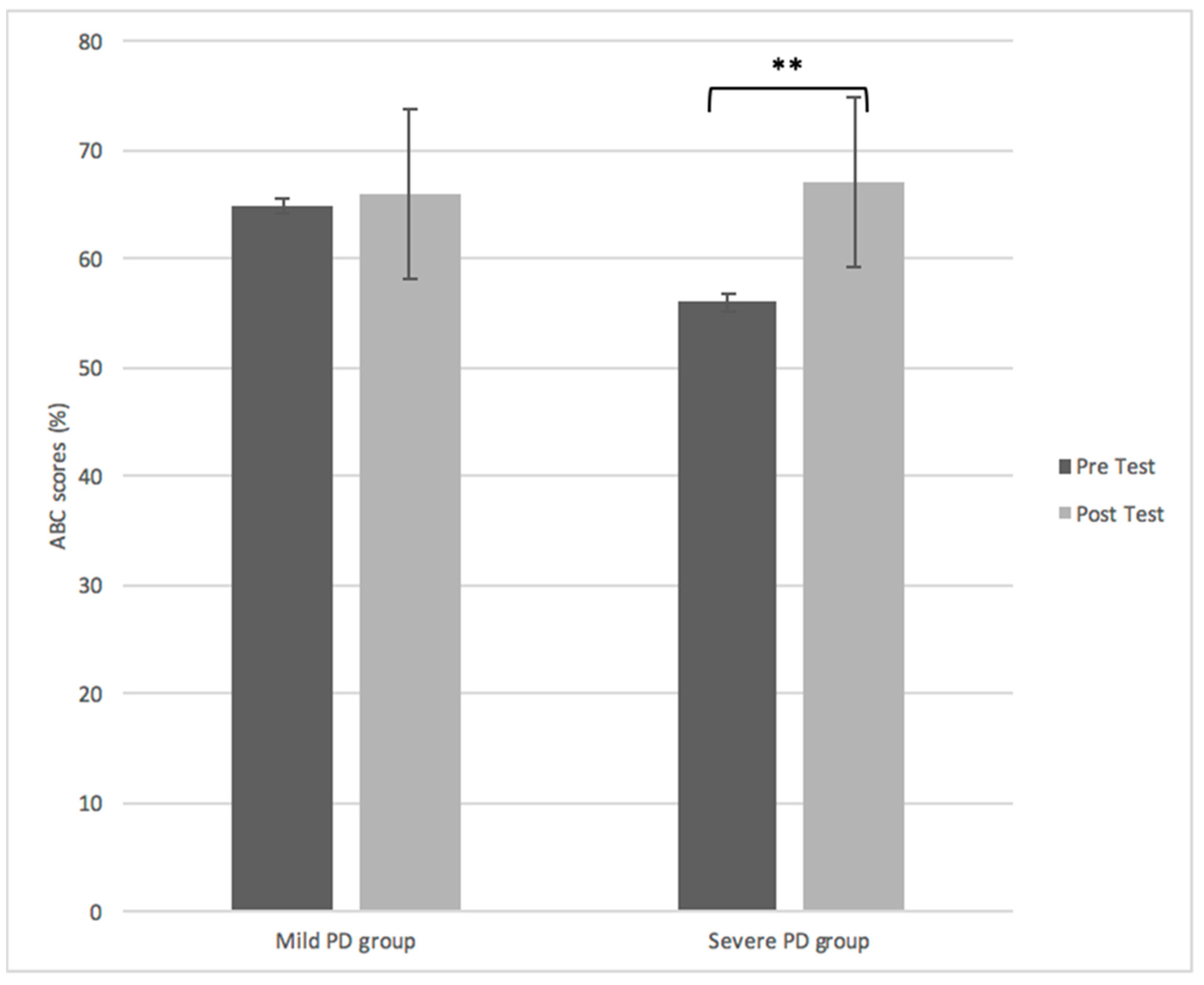
Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, B.H.; Bilclough, J.A.; Bowron, A.; Walker, R.W. Incidence and Prediction of Falls in Parkinson’s Disease: A Prospective Multidisciplinary Study. J. Neurol. Neurosurg. Psychiatry 2002, 72, 721–725. [Google Scholar] [CrossRef]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Parkinsons. Dis. 2013, 906274. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait Dynamics in Parkinson’s Disease: Common and Distinct Behavior Among Stride Length. Gait Variability, and Fractal-Like Scaling. Chaos 2009, 19, 026113. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R.; Krishnamurthi, N.; Lieberman, A.; Dhanani, S.; Pan, D. Why Levodopa May Increase Falls in Parkinson’s Disease. Neurology 2013, 80, 6–91. [Google Scholar]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease Carolin. Mov. Disord. 2016, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.; Baker, K.; Nieuwboer, A.; Burn, D. Targeting Dopa-Sensitive and Dopa-Resistant Gait Dysfunction in Parkinson’s Disease: Selective Responses to Internal and External Cues. Mov. Disord. 2011, 26, 430–435. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait Dynamics in Parkinson’s Disease: Relationship to Parkinsonian Features. Falls and Response to Levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Volpe, D.; Spolaor, F.; Sawacha, Z.; Guiotto, A.; Pavan, D.; Bakdounes, L.; Urbani, V.; Frazzitta, G.; Iansek, R. Muscular Activation Changes in Lower Limbs after Underwater Gait Training in Parkinson’s Disease: A Surface EMG Pilot Study. Gait Posture 2020, 80, 185–191. [Google Scholar] [CrossRef]
- Peppe, A.; Paravati, S.; Baldassarre, M.G.; Bakdounes, L.; Spolaor, F.; Guiotto, A.; Pavan, D.; Sawacha, Z.; Bottino, S.; Clerici, D.; et al. Proprioceptive Focal Stimulation (Equistasi®) May Improve the Quality of Gait in Middle-Moderate Parkinson’s Disease Patients. Double-Blind. Double-Dummy, Randomized, Crossover, Italian Multicentric Study. Front. Neurol. 2019, 18, 10–998. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Hou, G.; Han, P.; Jiang, L.; Guo, Q. The Impact of Tai Chi on Motor Function. Balance, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Evid Based Complement. Alternat. Med. 2021, 11, 6637612. [Google Scholar] [CrossRef]
- Jung, S.H.; Hasegawa, N.; Mancini, M.; King, L.A.; Carlson-Kuhta, P.; Smulders, K.; Peterson, D.S.; Barlow, N.; Harker, G.; Morris, R.; et al. Effects of the Agility Boot Camp with Cognitive Challenge (ABC-C) Exercise Program for Parkinson’s Disease. NPJ Parkinsons. Dis. 2020, 2, 3. [Google Scholar] [CrossRef]
- Luna, N.M.S.; Brech, G.C.; Canonica, A.; Ernandes, R.C.; Bocalini, D.S.; Greve, J.M.D.; Alonso, A.C. Effects of Treadmill Training on Gait of Elders with Parkinson’s Disease: A Literature Review. Einstein 2020, 7, eRW5233. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, A.P.J.; Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Dos Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait Parameters of Parkinson’s Disease Compared with Healthy Controls: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 12, 752. [Google Scholar] [CrossRef]
- Seymour, K.C.; Pickering, R.; Rochester, L.; Roberts, H.C.; Ballinger, C.; Hulbert, S.; Ashburn, A. Multicentre, randomised Controlled Trial of PDSAFE, A Physiotherapist-Delivered Fall Prevention Programme for People with Parkinson’s. J. Neurol. Neurosurg. Psychiatry 2019, 90, 774–782. [Google Scholar] [CrossRef]
- Conradsson, D.; Löfgren, N.; Nero, H.; Hagströmer, M.; Ståhle, A.; Lökk, J.; Franzén, E. The Effects of Highly Challenging Balance Training in Elderly with Parkinson’s Disease: A Randomized Controlled Trial. Neurorehabil. Neural. Repair. 2015, 29, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Menz, H.B.; Mcginley, J.L.; Watts, J.J.; Huxham, F.E.; Murphy, A.; Danoudis, M.E.; Iansek, R. A Randomized Controlled Trial to Reduce Falls in People with Parkinson’s Disease. Neurorehabil. Neural. Repair. 2015, 29, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Protas, E.J.; Mitchell, K.; Williams, A.; Qureshy, H.; Caroline, K.; Lai, E.C. Gait and Step Training to Reduce Falls in Parkinson’s Disease. NeuroRehabilitation 2005, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; Toole, T.; Maitland, C.G.; Rider, R.A. The Effects of Balance Training and High-Intensity Resistance Training on Persons with Idiopathic Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2003, 84, 1109–1117. [Google Scholar] [CrossRef]
- Morris, M.E.; Martin, C.; McGinley, J.L.; Huxham, F.E.; Menz, H.B.; Taylor, N.F.; Danoudis, M.; Watts, J.J.; Soh, S.; Evans, A.H. Protocol for a Home-Based Integrated Physical Therapy Program to Reduce Falls and Improve Mobility in People with Parkinson’s Disease. BMC Neurol. 2012, 12, 54. [Google Scholar] [CrossRef]
- Harro, C.C.; Shoemaker, M.J.; Frey, O.J.; Gamble, A.C.; Harring, K.B.; Karl, K.L.; McDonald, J.; Murray, C.; Tomassi, E.M.; Dyke, J.M.V. The Effects of Speed-Dependent Treadmill Training and Rhythmic Auditory-Cued Overground Walking on Gait Function and Fall Risk in Individuals with Idiopathic Parkinson’s Disease: A Randomized Controlled Trial. NeuroRehabilitation 2014, 34, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Fuzhong, L.; Harmer, P.; Fitzgerald, K.; Eckstrom, E.; Stock, R.; Galver, J.; Maddalozzo, G.; Batya, S.S. Tai Chi and Postural Stability in Patients with Parkinson’s Disease. New Engl. J. Med. 2012, 366, 511–519. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear. J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sangarapillai, K.; Norman, B.M.; Almeida, Q.J. Analyzing the Effects of PD SAFExTM on the Motor Symptoms of Parkinson’s Disease: A Retrospective Study. NeuroRehabilitation 2020, 46, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Sage, M.D.; Almeida, Q.J. Symptom and Gait Changes After Sensory Attention Focused Exercise vs Aerobic Training in Parkinson’s Disease. Mov. Disord. 2009, 24, 1132–1138. [Google Scholar] [CrossRef]
- Beck, E.N.; Intzandt, B.N.; Almeida, Q.J. Can Dual Task Walking Improve in Parkinson’s Disease After External Focus of Attention Exercise? A Single Blind Randomized Controlled Trial. Neurorehabil. Neural. Repair. 2018, 32, 18–23. [Google Scholar] [CrossRef]
- Lefaivre, S.C.; Almeida, Q.J. Can Sensory Attention Focused Exercise Facilitate the Utilization of Proprioception for Improved Balance Control in PD? Gait Posture 2015, 41, 630–633. [Google Scholar] [CrossRef]
- Cole, M.H.; Naughton, G.A.; Silburn, P.A. Neuromuscular Impairments are Associated with Impaired Head and Trunk Stability during Gait in Parkinson Fallers. Neurorehabil. Neural. Repair. 2017, 2017, 34–47. [Google Scholar] [CrossRef]
- Shen, X.; Mak, M.K.Y. Balance and Gait Training with Augmented Feedback Improves Balance Confidence in People with Parkinson’s Disease: A Randomized Controlled Trial. Neurorehabil. Neural. Repair. 2014, 28, 524–535. [Google Scholar] [CrossRef]
- Pelicioni, P.H.S.; Menant, J.C.; Latt, M.D.; Lord, S.R. Falls in Parkinson’s Disease Subtypes: Risk Factors. Locations and Circumstances. Int. J. Environ. Res. Public Health 2019, 16, 2216. [Google Scholar] [CrossRef] [PubMed]
- Creaby, M.W.; Cole, M.H. Gait Characteristics and Falls in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsonism Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Skorvanek, M.; Martinez-Martin, P.; Kovacs, N.; Rodriguez-Violante, M.; Corvol, J.C.; Taba, P.; Seppi, K.; Levin, O.; Scrag, A.; Foltynie, T.; et al. Differences in MDS-UPDRS Scores Based on Hoehn and Yahr Stage and Disease Duration. Mov. Disor. Clin. Pract. 2017, 4, 536–544. [Google Scholar] [CrossRef]
- Almeida, L.R.S.; Valenca, G.T.; Negreiros, N.N.; Pinto, E.B.; Oliveira-Filho, J. Comparison of Self-Report and Performance-Based Balance Measures for Predicting Recurrent Falls in People with Parkinson Disease: Cohort Study. Phys. Ther. 2016, 96, 1074–1084. [Google Scholar] [CrossRef]
- Foongsathaporn, C.; Panyakaew, P.; Jitkritsadakul, O.; Bhidayasiri, R. What Daily Activities Increase the Risk of Falling in Parkinson Patients? An Analysis of the Utility of the ABC-16 Scale. J. Neurol. Sci. 2016, 364, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bello-Haas, V.D.; Klassen, L.; Sheppard, M.S.; Metcalfe, A. Psychometric Properties of Activity, Self-Efficacy and Quality-Of-Life Measures in Individuals with Parkinson Disease. Physiother. Can. 2011, 63, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Raad, J.; Moore, J.; Hamby, R.L.; Rivadelo, D.; Straube, A. A Brief Review of the Activities-Specific Balance Confidence Scale in Older Adults. Arch. Phys. Med. Rehabil. 2013, 94, 1426–1427. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait Variability and Fall Risk in Community-Living Older Adults: A 1-Year Prospective Study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- McArthur, C.; Gibbs, J.C.; Patel, R.; Papaioannou, A.; Neves, P.; Killingbeck, J.; Hirdes, J.; Milligan, J.; Berg, K.; Giangregorio, L. A Scoping Review of Physical Rehabilitation in Long-Term Care: Interventions. Outcomes, Tools. Can. J. Aging 2017, 36, 435–452. [Google Scholar] [CrossRef]
- Martinez, P.; Kurtis, M. Health-Related Quality of Life as an Outcome Variable in Parkinson’s Disease. Ther. Adv. Neurol. Disord. 2012, 5, 105. [Google Scholar] [CrossRef]
- Lee, H.K.; Altmann, L.J.; McFarland, N.; Hass, C.J. The Relationship Between Balance Confidence and Control in Individuals with Parkinson’s Disease. Parkinsonism Relat. Disord. 2016, 26, 24–28. [Google Scholar] [CrossRef][Green Version]
- Muir, S.W.; Berg, K.; Chesworth, B.; Klar, N.; Speechley, M. Balance Impairment as a Risk Factor for Falls in Community-Dwelling Older Adults who are High Functioning: A Prospective Study. Phys. Ther. 2010, 90, 338–347. [Google Scholar] [CrossRef]
- Mak, M.K.Y.; Pang, M.Y.C. Balance Confidence and Functional Mobility are Independently Associated with Falls in People with Parkinson’s Disease. J. Neurol. 2009, 256, 742–749. [Google Scholar] [CrossRef]
- Tidman, M.; Skotzke, E. Effects of a Community-Based Exercise Program on Mobility. Balance, Cognition, Sleep, Activities of Daily Living, and Quality of Life in PD: A Pilot Study. Neurodogener. Dis. Manag. 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M.; Munoz, B.; West, S.K.; Rubin, G.S.; Fried, L.P. Falls and Fear of Falling: Which Comes First? A Longitudinal Prediction Model Suggests Strategies for Primary and Secondary Prevention. J. Am. Geriatr. Soc. 2002, 50, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Shah, V.V.; Harker, G.; Carlso-Kuhta, P.; Nutt, J.G.; Lapidus, J.A.; Jung, S.H.; Barlow, N.; King, L.A.; Horak, F.B.; et al. Responsiveness of Objective vs. Clinical Balance Domain Outcomes for Exercise Intervention in Parkinson’s Disease. Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Mild PD (UPDRS-III <20) | Severe PD (UPDRS-III >27) | |
|---|---|---|
| N | 22 | 22 |
| Age (years) | 68.12 (8.6) | 77.27 (6.52) |
| No. of years since diagnosis | 6.9 (5.0) | 8.65 (3.33) |
| Program adherence % | 97.81 (2.25) | 98.99 (1.60) |
| Levodopa equivalent dose (mg/d) | 596.45 (320.75) | 647.67 (282.63) |
| Motor severity (UPDRS-III) | 13.68 (6.1) | 36.66 (8.99) |
| BMI | 27.76 (10.1) | 27.59 (6.29) |
| Pre | Post | Effect | |
|---|---|---|---|
| Stride width (cm) | |||
| Mild PD | 7.41 (5.8) | 6.40 (6.1) | Time F(1, 22) = 7.17, p < 0.001 |
| Severe PD | 12.03 (8.1) | 10.17 (7.9) | |
| Stride width variability (CV) | |||
| Mild PD | 7.29 (5.6) | 5.20 (6.3) | Time F(1, 22) = 6.56, p < 0.017 |
| Severe PD | 8.33 (7.0) | 6.20 (5.9) | |
| Stride length (cm) | |||
| Mild PD | 53.12 (14.2) | 56.36 (12.3) | Time F(1, 22) = 9.37, p < 0.0001 |
| Severe PD | 48.07 (18.6) | 56.67 (15.1) | |
| Stride length variability (CV) | |||
| Mild PD | 8.93 (5.9) | 6.19 (5.7) | Time F(1, 22) = 3.87, p < 0.039 |
| Severe PD | 10.68 (5.6) | 6.15 (5.8) | |
| Stride time (s) | |||
| Mild PD | 1.62 (0.59) | 0.68 (0.46) | Time F(1, 22) = 9.22, p < 0.0001 |
| Severe PD | 2.74 (0.60) | 0.88 (0.59) | |
| Stride time variability (CV) | |||
| Mild PD | 5.89 (6.3) | 5.06 (4.29) | Time F(1, 22) = 3.48, p < 0.006 |
| Severe PD | 6.72 (8.3) | 5.12 (3.19) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangarapillai, K.; Norman, B.M.; Almeida, Q.J. Rehabilitation of Falls in Parkinson’s Disease: Self-Perception vs. Objective Measures of Fall Risk. Brain Sci. 2021, 11, 320. https://doi.org/10.3390/brainsci11030320
Sangarapillai K, Norman BM, Almeida QJ. Rehabilitation of Falls in Parkinson’s Disease: Self-Perception vs. Objective Measures of Fall Risk. Brain Sciences. 2021; 11(3):320. https://doi.org/10.3390/brainsci11030320
Chicago/Turabian StyleSangarapillai, Kishoree, Benjamin M. Norman, and Quincy J. Almeida. 2021. "Rehabilitation of Falls in Parkinson’s Disease: Self-Perception vs. Objective Measures of Fall Risk" Brain Sciences 11, no. 3: 320. https://doi.org/10.3390/brainsci11030320
APA StyleSangarapillai, K., Norman, B. M., & Almeida, Q. J. (2021). Rehabilitation of Falls in Parkinson’s Disease: Self-Perception vs. Objective Measures of Fall Risk. Brain Sciences, 11(3), 320. https://doi.org/10.3390/brainsci11030320






