Olfactory Perception in Relation to the Physicochemical Odor Space
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of a Physicochemical Odor Space
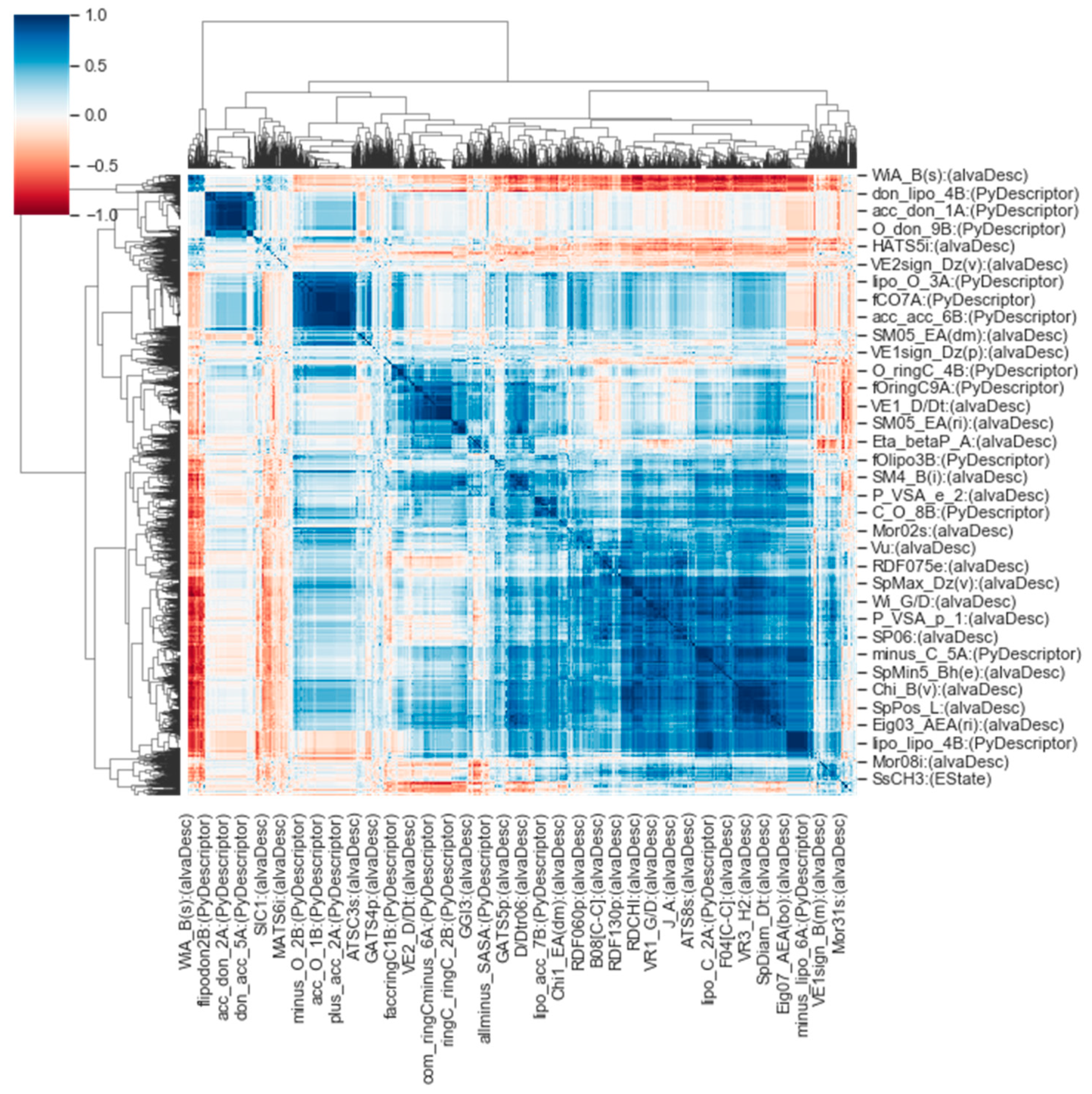
2.1.1. Descriptor Calculation and Preprocessing
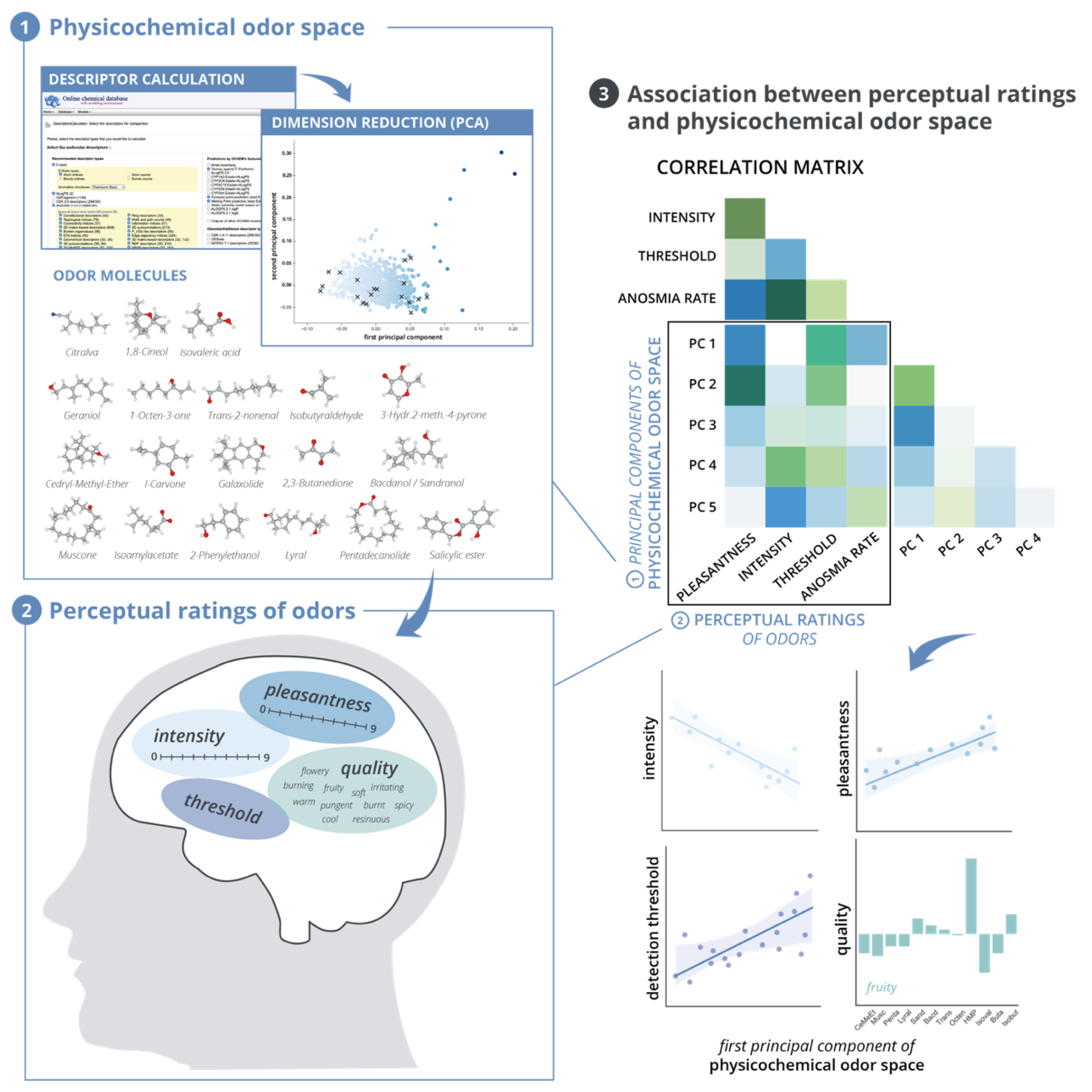
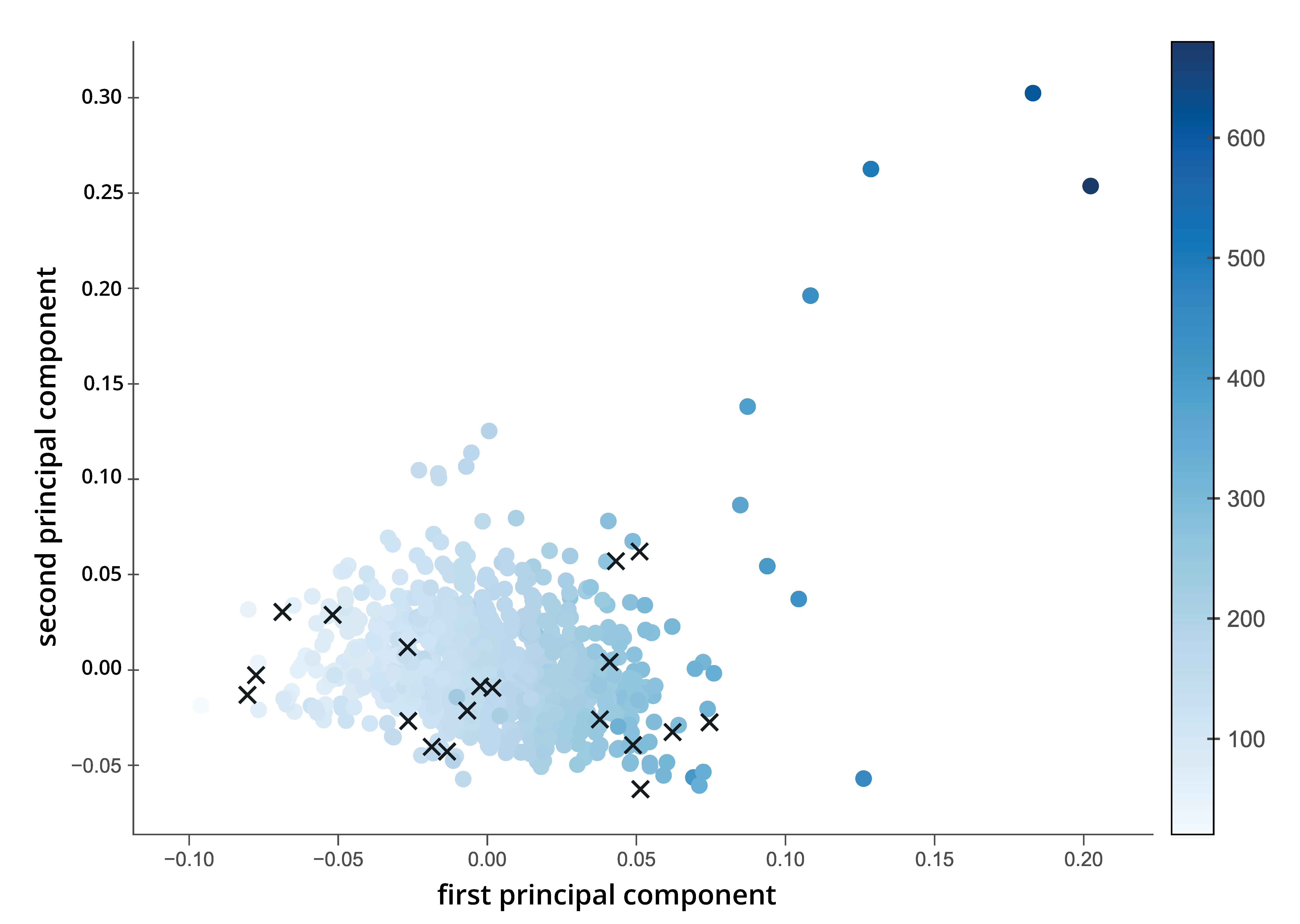
2.1.2. Dimension Reduction
2.2. Materials and Measures
2.3. Statistical Methods
3. Results
3.1. Physicochemical Odor Space
3.1.1. Factor Scores and Loadings
3.1.2. Explained Variance
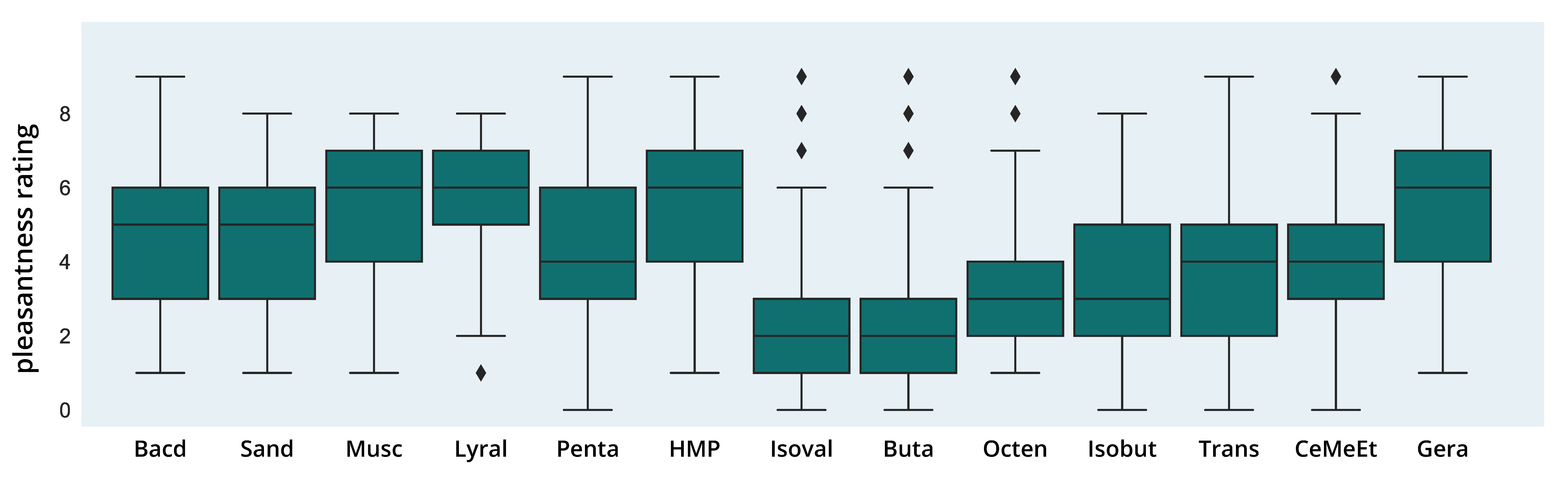
3.2. Perceptual Ratings of Odors
3.2.1. Pleasantness Ratings
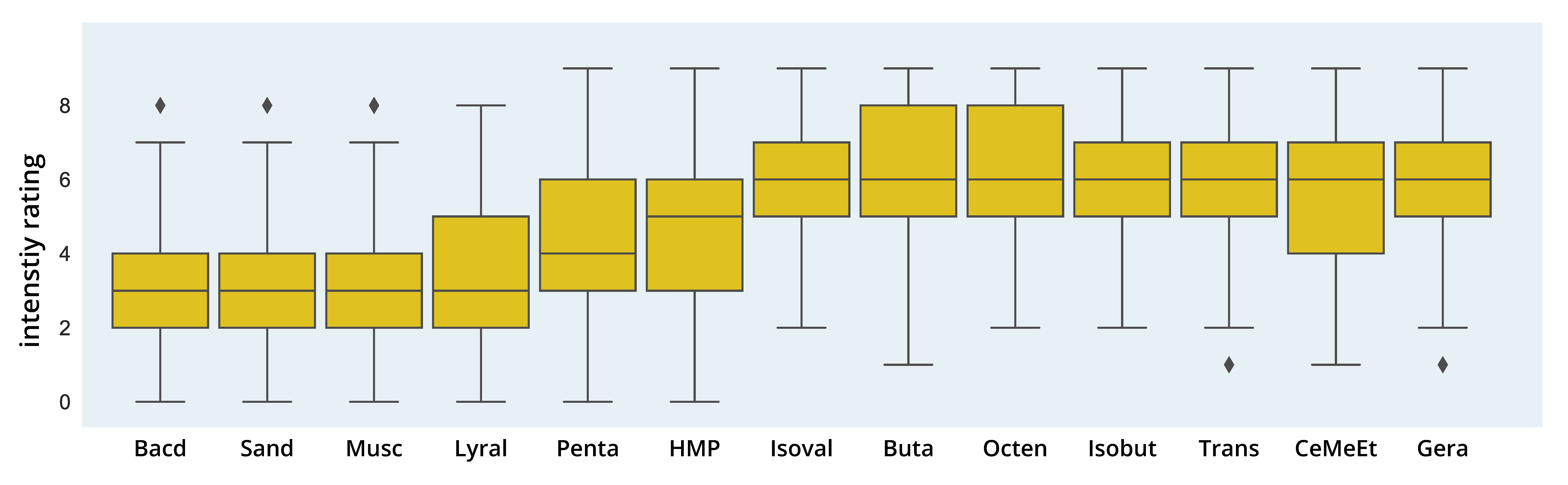
3.2.2. Intensity Ratings
3.2.3. Detection Threshold
3.2.4. Distribution of Pleasantness and Intensity Ratings
3.2.5. Interaction of Pleasantness and Intensity Ratings
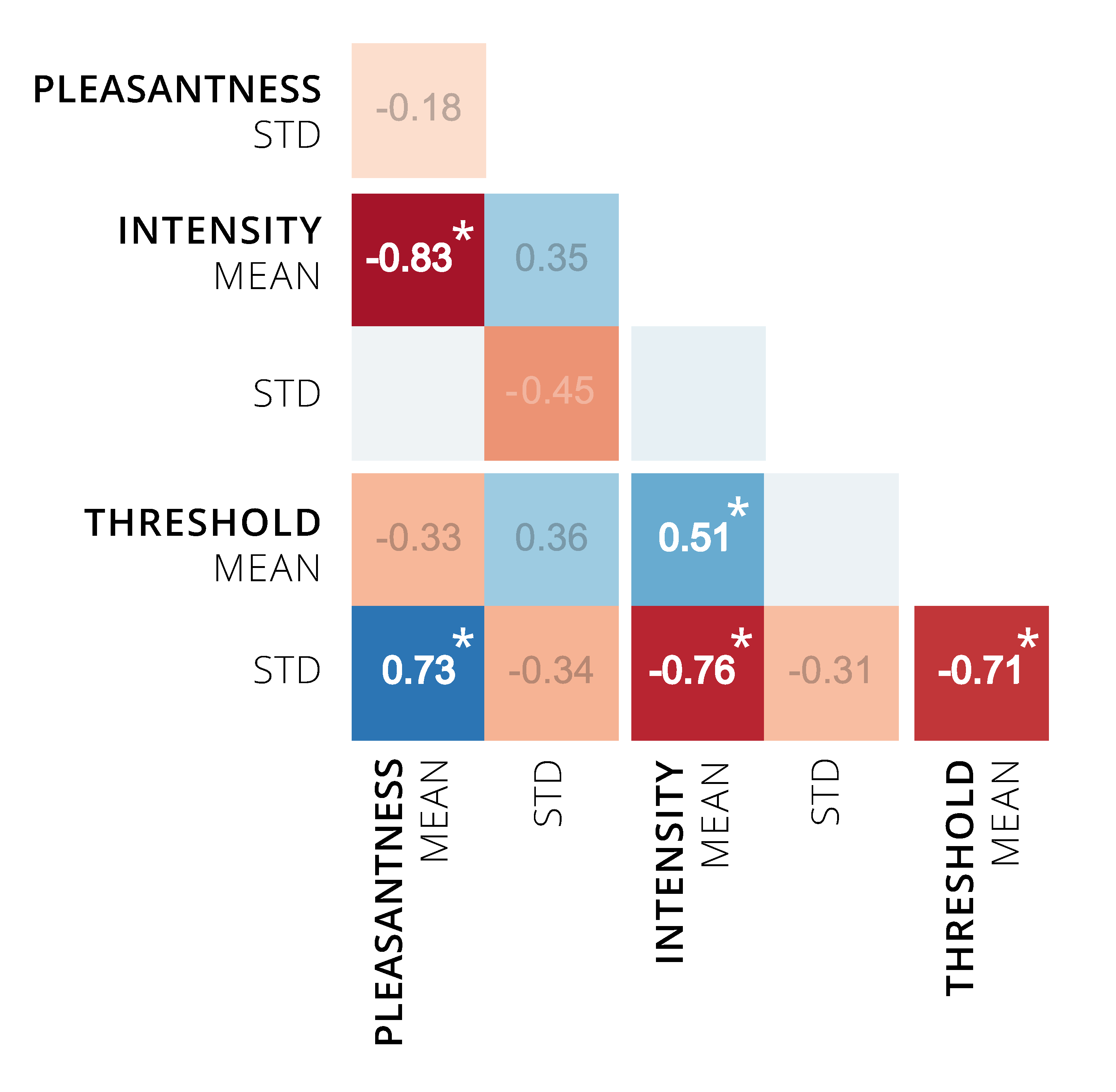
3.2.6. Correlations among Perceptual Ratings
3.2.7. Correlations of Perceptual Ratings with Vapor Pressure
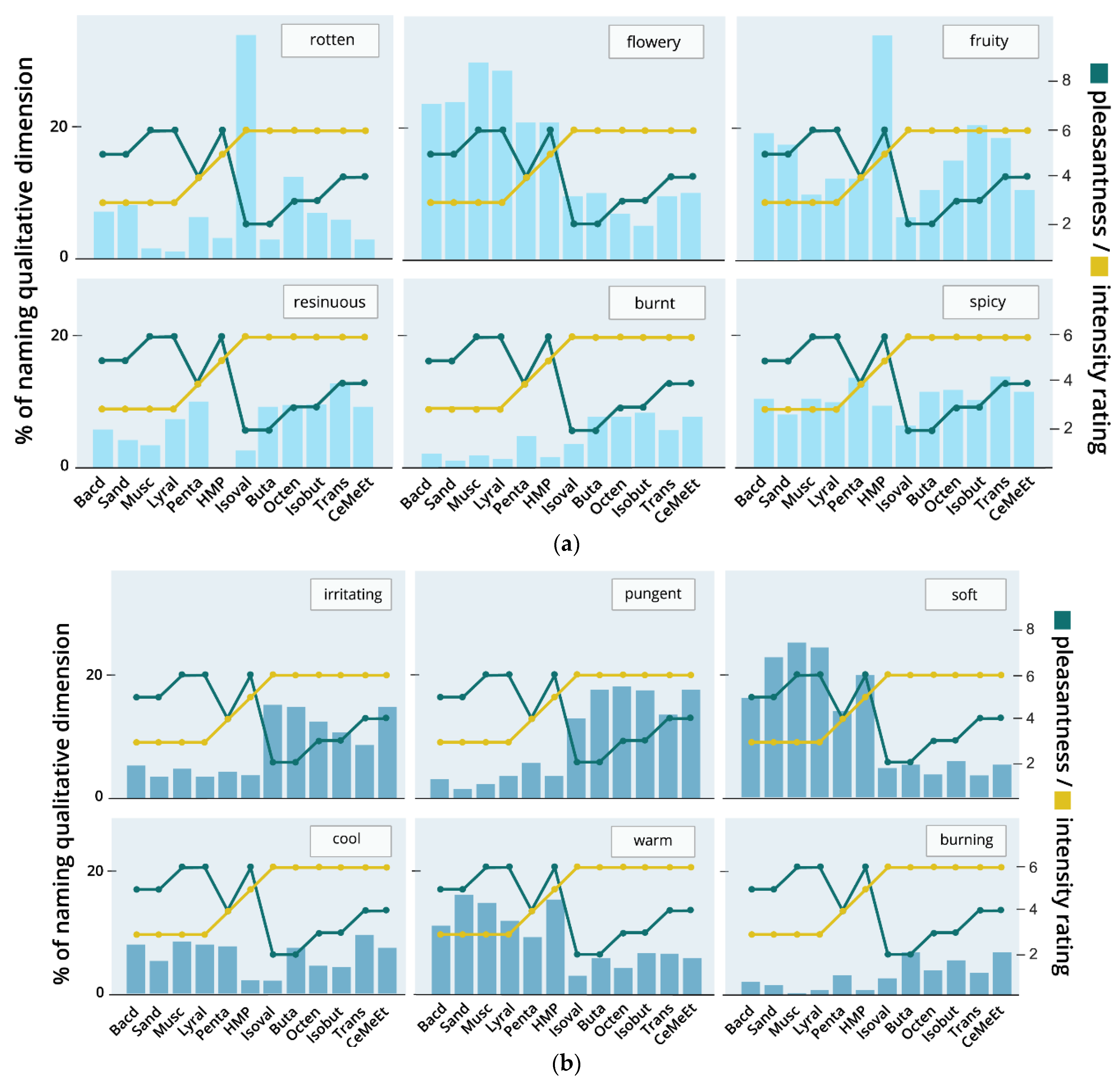
3.2.8. Associations with Qualitative Ratings
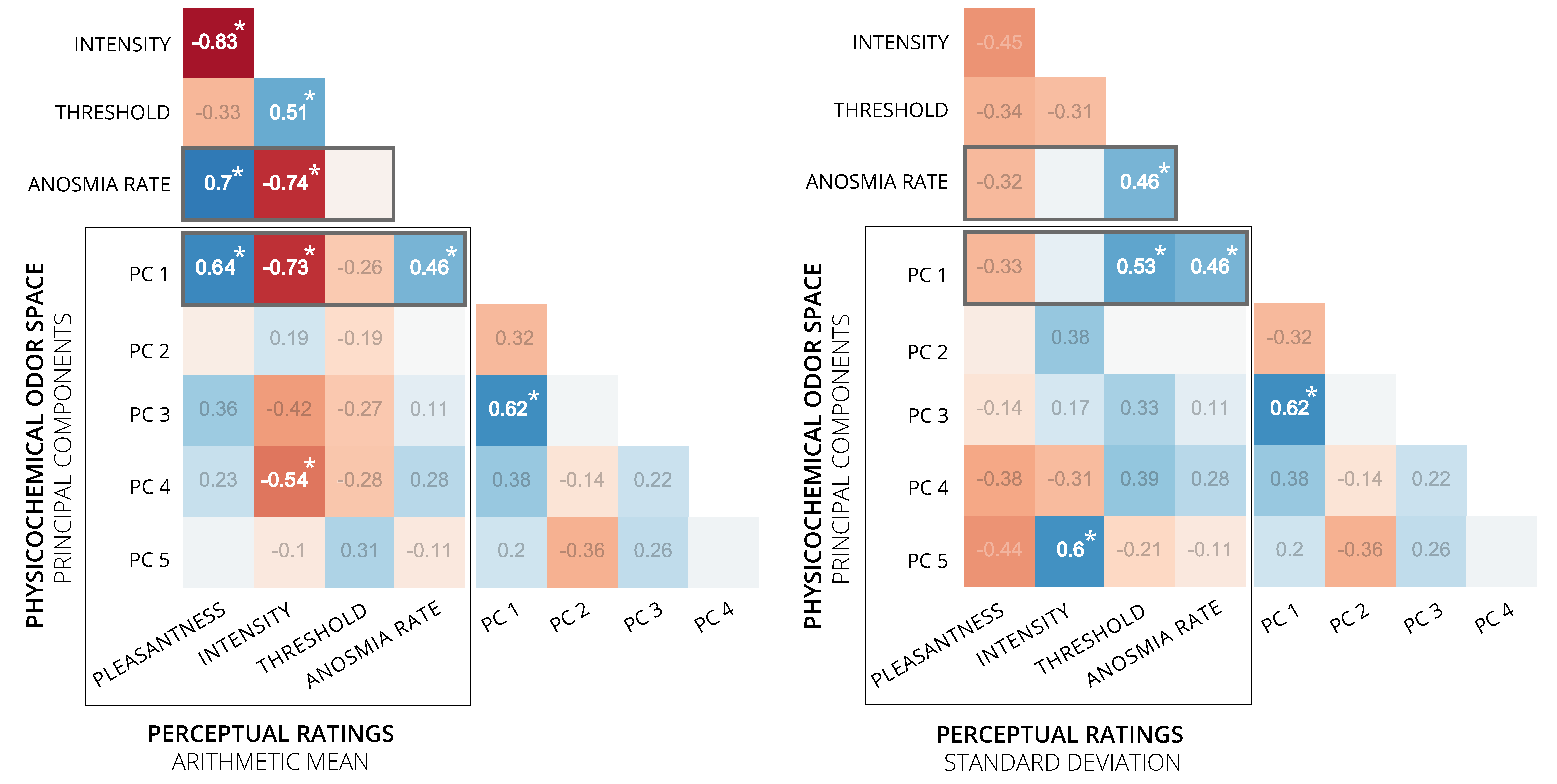
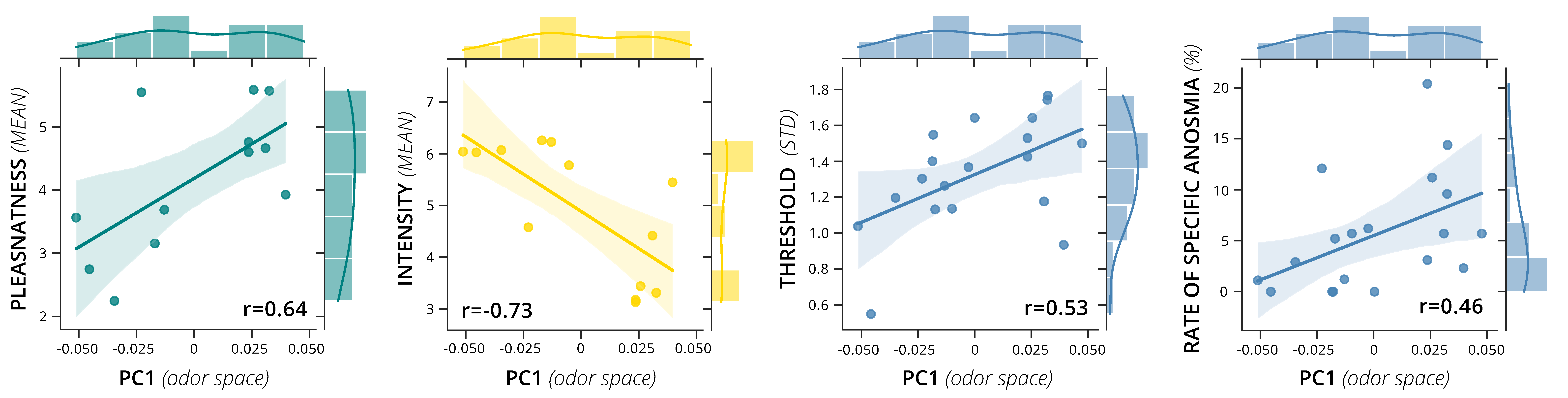
3.3. Associations between Perceptual Ratings and Physicochemical Odor Space
4. Discussion
4.1. Discussion of Results
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Genva, M.; Kemene, T.K.; Deleu, M.; Lins, L.; Fauconnier, M.-L. Is It Possible to Predict the Odor of a Molecule on the Basis of its Structure? Int. J. Mol. Sci. 2019, 20, 3018. [Google Scholar] [CrossRef]
- Mainland, J.D. Olfaction. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 2, pp. 1–46. [Google Scholar]
- Keller, A.; Vosshall, L.B. Olfactory perception of chemically diverse molecules. BMC Neurosci. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Morton, T.H.; Potter, H.; Corkin, S. Selective Olfactory Deficits in case H.M. Brain 1983, 106, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Jones-Gotman, M.; Zatorre, R.J. Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia 1988, 26, 387–400. [Google Scholar] [CrossRef]
- Croy, I.; Olgun, S.; Mueller, L.; Schmidt, A.; Muench, M.; Hummel, C.; Gisselmann, G.; Hatt, H.; Hummel, T. Peripheral adaptive filtering in human olfaction? Three studies on prevalence and effects of olfactory training in specific anosmia in more than 1600 participants. Cortex 2015, 73, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, H.R.; Dravnieks, A.; Klarman, L.A. Odor intensity and pleasantness for a diverse set of odorants. Percept. Psychophys. 1976, 19, 122–128. [Google Scholar] [CrossRef]
- Khan, R.M.; Luk, C.-H.; Flinker, A.; Aggarwal, A.; Lapid, H.; Haddad, R.; Sobel, N. Predicting Odor Pleasantness from Odorant Structure: Pleasantness as a Reflection of the Physical World. J. Neurosci. 2007, 27, 10015–10023. [Google Scholar] [CrossRef] [PubMed]
- Kermen, F.; Chakirian, A.; Sezille, C.; Joussain, P.; Le Goff, G.; Ziessel, A.; Chastrette, M.; Mandairon, N.; Didier, A.; Rouby, C.; et al. ERRATUM: Molecular complexity determines the number of olfactory notes and the pleasantness of smells. Sci. Rep. 2013, 3, 206. [Google Scholar] [CrossRef]
- Zarzo, M. Hedonic Judgments of Chemical Compounds Are Correlated with Molecular Size. Sensors 2011, 11, 3667–3686. [Google Scholar] [CrossRef]
- Licon, C.C.; Manesse, C.; Dantec, M.; Fournel, A.; Bensafi, M. Pleasantness and trigeminal sensations as salient dimensions in organizing the semantic and physiological spaces of odors. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Zarzo, M. Psychologic dimensions in the perception of everyday odors: Pleasantness and edibility. J. Sens. Stud. 2008, 23, 354–376. [Google Scholar] [CrossRef]
- Köster, E.P. The Specific Characteristics of the Sense of Smell. Olfaction Taste Cogn. 2002, 27–44. [Google Scholar] [CrossRef]
- Boelens, H. Structure—activity relationships in chemoreception by human olfaction. Trends Pharmacol. Sci. 1983, 4, 421–426. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Voleti, V.; Zou, D.-J.; Hillman, E.M.C.; Firestein, S. Widespread receptor-driven modulation in peripheral olfactory coding. Science 2020, 368, eaaz5390. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Mainland, J.D.; Ihara, Y.; Yu, C.W.; Wolfinger, R.; et al. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 355, 820–826. [Google Scholar] [CrossRef]
- Snitz, K.; Yablonka, A.; Weiss, T.; Frumin, I.; Khan, R.M.; Sobel, N. Predicting Odor Perceptual Similarity from Odor Structure. PLoS Comput. Biol. 2013, 9, e1003184. [Google Scholar] [CrossRef] [PubMed]
- Ravia, A.; Snitz, K.; Honigstein, D.; Finkel, M.; Zirler, R.; Perl, O.; Secundo, L.; Laudamiel, C.; Harel, D.; Sobel, N. A measure of smell enables the creation of olfactory metamers. Nature 2020, 588, 118–123. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Ranjta, S.; Varadwaj, P.K. SMILES to Smell: Decoding the Structure–Odor Relationship of Chemical Compounds Using the Deep Neural Network Approach. J. Chem. Inf. Model. 2021, 61, 676–688. [Google Scholar] [CrossRef]
- Mayhew, E.J.; Arayata, C.J.; Gerkin, R.C.; Lee, B.K.; Magill, J.M.; Snyder, L.L.; Little, K.A.; Yu, C.W.; Mainland, J.D. Drawing the Borders of Olfactory Space. bioRxiv 2020. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Chemicals (Aroma Chemicals); Allured Publishing Company: Carol Stream, IL, USA, 1994. [Google Scholar]
- Dravnieks, A. Atlas of Odor Character Profiles; American Society for Testing and Materials: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Dravnieks, A.; Masurat, T.; Lamm, R.A. Hedonics of Odors and Odor Descriptors. J. Air Pollut. Control. Assoc. 1984, 34, 752–755. [Google Scholar] [CrossRef]
- Zarzo, M.; Stanton, D.T. Understanding the underlying dimensions in perfumers’ odor perception space as a basis for developing meaningful odor maps. Attention, Perception, Psychophys. 2009, 71, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Hautus, M.J.; Urale, P.W.B. Personality and Perceptions of Common Odors. Chemosens. Percept. 2017, 10, 23–30. [Google Scholar] [CrossRef]
- Wilson, D.A.; Stevenson, R.J. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003, 26, 243–247. [Google Scholar] [CrossRef]
- Kaeppler, K.; Mueller, F. Odor Classification: A Review of Factors Influencing Perception-Based Odor Arrangements. Chem. Senses 2013, 38, 189–209. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Boakes, R.A. A mnemonic theory of odor perception. Psychol. Rev. 2003, 110, 340–364. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, J.; Rinck, F.; Bourgeat, F.; Schaal, B.; Rouby, C.; Bensafi, M.; Hummel, T. The effect of early experience on odor perception in humans: Psychological and physiological correlates. Behav. Brain Res. 2010, 208, 458–465. [Google Scholar] [CrossRef]
- Haller, R.; Rummel, C.; Henneberg, S.; Pollmer, U.; Köster, E. The influence of early experience with vanillin on food preference later in life. Chem. Senses 1999, 24, 465–467. [Google Scholar] [CrossRef]
- Szöcs, E.; Stirling, T.; Scott, E.R.; Scharmüller, A.; Schäfer, R.B. webchem: An R Package to Retrieve Chemical Information from the Web. J. Stat. Softw. 2020, 93, 1–17. [Google Scholar] [CrossRef]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online chemical modeling environment (OCHEM): Web platform for data storage, model development and publishing of chemical information. J. Comput. Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Garcia, H.; Filzmoser, P. Multivariate Statistical Analysis Using the R Package Chemometrics; Vienna University of Technology: Vienna, Austria, 2017. [Google Scholar]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010. [Google Scholar]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Bentley, R. The nose as a stereochemist. Enantiomers and odor. Chem. Rev. 2006, 106, 4099–4112. [Google Scholar] [CrossRef] [PubMed]
- Manescu, S.; Frasnelli, J.; Lepore, F.; Djordjevic, J. Now You Like Me, Now You Don’t: Impact of Labels on Odor Perception. Chem. Senses 2013, 39, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Koeppel, C.J.; Ruser, P.; Kitzler, H.; Hummel, T.; Croy, I. Interoceptive accuracy and its impact on neuronal responses to olfactory stimulation in the insular cortex. Hum. Brain Mapp. 2020, 41, 2898–2908. [Google Scholar] [CrossRef]
- Croy, I.; Springborn, M.; Lötsch, J.; Johnston, A.N.B.; Hummel, T. Agreeable Smellers and Sensitive Neurotics—Correlations among Personality Traits and Sensory Thresholds. PLoS ONE 2011, 6, e18701. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Flores-Sarnat, L. Olfactory Development, Part 2: Neuroanatomic Maturation and Dysgeneses. J. Child Neurol. 2017, 32, 579–593. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Flores-Sarnat, L.; Wei, X.-C. Olfactory Development, Part 1: Function, From Fetal Perception to Adult Wine-Tasting. J. Child Neurol. 2017, 32, 566–578. [Google Scholar] [CrossRef]
- Masand, V.H.; Rastija, V. PyDescriptor: A new PyMOL plugin for calculating thousands of easily understandable molecular descriptors. Chemom. Intell. Lab. Syst. 2017, 169, 12–18. [Google Scholar] [CrossRef]
- Mauri, A. alvaDesc: A Tool to Calculate and Analyze Molecular Descriptors and Fingerprints. In Ecotoxicological QSARs; Springer: New York, NY, USA, 2020; pp. 801–820. [Google Scholar]


| CAS Number | Trivial Name | Abbreviation | Vapor Pressure | |
|---|---|---|---|---|
| 1 | 204-262-9 | Salicylic ester | Salicyl | 0.000078 * |
| 2 | 99-49-0 | l-Carvon | Carvon | 0.115 * |
| 3 | 431-03-8 | 2,3-Butadione | Buta | 56.82 * |
| 4 | 31906-04-4 | Lyral | Lyral | 0.0000857 ** |
| 5 | 5146-66-7 | Citralva | Citra | 0.0362 ** |
| 6 | 106-24-1 | Geraniol | Gera | 0.03 * |
| 7 | 78-84-2 | Isobutyraldehyde | Isobut | 170 * |
| 8 | 503-74-2 | Isovaleric acid | Isoval | 0.44 * |
| 9 | 628-46-6 | Isoamylacetate | Isoamy | 0.058 * |
| 10 | 118-71-8 | 3-Hydroxy-2-methyl-4-pyrone | HMP | 0.000326 * |
| 11 | 470-82-6 | 1,8-Cineol | Cineol | 1.90 * |
| 12 | 956-82-1 | Muscone | Musc | 0.000469 ** |
| 13 | 6602-64-8 | Galaxolide | Gala | 0.000889 ** |
| 14 | 28219-61-6 1 | Sandranol | Sand | 0.0000718 ** |
| 15 | 28219-61-6 1 | Bacdanol | Bacd | 0.0000718 ** |
| 16 | 18829-56-6 | Trans-2-nonenal | Trans | 0.317 ** |
| 17 | 19870-74-7 | Cedrylmethylether | CeMeEt | 0.0201 ** |
| 18 | 106-02-5 | Pentadecanolide | Penta | 0.0000517 ** |
| 19 | 60-12-8 | Phenylethylalcohol | PEA | 0.09 * |
| 20 | 3391-86-4 | 1-Octen-3-one | Octen | 0.238 ** |
| Subjects | Odorants Used for Testing | Perceptual Ratings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| thr | int | pl | qual | ||||||||
| 1–200 | Isoval | Trans | - | - | - | - | - | X | X | X | X |
| 201–400 | HMP | Penta | CeMeEt | - | - | - | - | X | X | X | X |
| 401–600 | Sand | Bacd | Buta | - | - | - | - | X | X | X | X |
| 601–800 | Lyral | Musc | - | - | - | - | - | X | X | X | X |
| 801–1000 | Gera | - | - | - | - | - | - | X | X | X | |
| 1001–1200 | PEA | Gera | Cineol | - | - | - | - | X | |||
| 1201–1400 | Carvon | Isoamy | Salicyl | - | - | - | - | X | |||
| 1401–1600 | Citra | Gala | - | - | - | - | - | X | |||
| 1601–1800 | Isobut | Octen | - | - | - | - | - | X | X | X | X |
| 1801–2000 | PEA | Citra | Cineol | Isoamy | Salicyl | Gala | Carvon | X | |||
| Pleasantness | Intensity | Detection Threshold | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | mean | median | std | mean | median | std | N | mean | median | std | |
| Bacd | 200 | 4.76 | 5 | 1.88 | 3.13 | 3 | 1.76 | 300 | 5.50 | 6 | 1.53 |
| Sand | 200 | 4.61 | 5 | 1.88 | 3.18 | 3 | 1.61 | 300 | 5.35 | 6 | 1.43 |
| Musc | 200 | 5.58 | 6 | 1.69 | 3.31 | 3 | 1.79 | 300 | 5.33 | 6 | 1.77 |
| Lyral | 200 | 5.59 | 6 | 1.65 | 3.44 | 3 | 1.86 | 300 | 5.44 | 6 | 1.64 |
| Penta | 200 | 4.67 | 4 | 1.87 | 4.42 | 4 | 1.89 | 300 | 6.19 | 7 | 1.18 |
| HMP | 200 | 5.55 | 6 | 1.88 | 4.58 | 5 | 1.88 | 300 | 5.88 | 6 | 1.30 |
| Isoval | 178 | 2.25 | 2 | 1.66 | 6.07 | 6 | 1.86 | 300 | 5.02 | 5 | 1.20 |
| Buta | 200 | 2.75 | 2 | 1.87 | 6.03 | 6 | 1.89 | 300 | 6.78 | 7 | 0.55 |
| Octen | 200 | 3.16 | 3 | 1.85 | 6.26 | 6 | 1.69 | 300 | 6.82 | 7 | 1.13 |
| Isobut | 200 | 3.57 | 3 | 1.97 | 6.04 | 6 | 1.72 | 300 | 7.29 | 7 | 1.04 |
| Trans | 178 | 3.69 | 4 | 2.07 | 6.22 | 6 | 1.70 | 300 | 5.32 | 5 | 1.26 |
| CeMeEt | 200 | 3.93 | 4 | 1.79 | 5.45 | 6 | 1.94 | 300 | 6.28 | 7 | 0.93 |
| Gera | 198 | 5.49 | 6 | 1.75 | 5.78 | 6 | 1.51 | 376 | 4.19 | 4 | 1.70 |
| Cineol | - | - | - | - | - | - | - | 300 | 5.20 | 5 | 1.64 |
| PEA | - | - | - | - | - | - | - | 300 | 3.94 | 4 | 1.55 |
| Carvon | - | - | - | - | - | - | - | 300 | 5.17 | 5 | 1.37 |
| Isoamy | - | - | - | - | - | - | - | 300 | 4.76 | 5 | 1.40 |
| Salicyl | - | - | - | - | - | - | - | 300 | 4.77 | 5 | 1.74 |
| Citra | - | - | - | - | - | - | - | 300 | 6.22 | 7 | 1.14 |
| Gala | - | - | - | - | - | - | - | 300 | 5.40 | 6 | 1.50 |
| Bacd | Sand | Musc | Lyral | Penta | HMP | Isoval | Buta | Octen | Isobut | Trans | CeMeEt | Gera | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +/− 1.5 IQR | [1, 9] | [1, 8] | [1, 8] | [2, 8] | [0, 9] | [1, 9] | [0, 6] | [0, 6] | [1, 7] | [0, 8] | [0, 9] | [0, 8] | [1, 9] |
| IQR | 3–6 | 3–6 | 4–7 | 5–7 | 3–6 | 4–7 | 1–3 | 1–3 | 2–4 | 2–5 | 2–5 | 3–5 | 4–7 |
| median | 5 | 5 | 6 | 6 | 4 | 6 | 2 | 2 | 3 | 3 | 4 | 4 | 6 |
| Bacd | Sand | Musc | Lyral | Penta | HMP | Isoval | Buta | Octen | Isobut | Trans | CeMeEt | Gera | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +/− 1.5 IQR | [0, 7] | [0, 7] | [0, 7] | [0, 8] | [0, 9] | [0, 9] | [2, 9] | [1, 9] | [2, 9] | [2, 9] | [2, 9] | [1, 9] | [2, 9] |
| IQR | 2–4 | 2–4 | 2–4 | 2–5 | 3–6 | 3–6 | 5–7 | 5–8 | 5–8 | 5–7 | 5–7 | 4–7 | 5–7 |
| median | 3 | 3 | 3 | 3 | 4 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierling, A.L.; Croy, I.; Hummel, T.; Cuniberti, G.; Croy, A. Olfactory Perception in Relation to the Physicochemical Odor Space. Brain Sci. 2021, 11, 563. https://doi.org/10.3390/brainsci11050563
Bierling AL, Croy I, Hummel T, Cuniberti G, Croy A. Olfactory Perception in Relation to the Physicochemical Odor Space. Brain Sciences. 2021; 11(5):563. https://doi.org/10.3390/brainsci11050563
Chicago/Turabian StyleBierling, Antonie Louise, Ilona Croy, Thomas Hummel, Gianaurelio Cuniberti, and Alexander Croy. 2021. "Olfactory Perception in Relation to the Physicochemical Odor Space" Brain Sciences 11, no. 5: 563. https://doi.org/10.3390/brainsci11050563
APA StyleBierling, A. L., Croy, I., Hummel, T., Cuniberti, G., & Croy, A. (2021). Olfactory Perception in Relation to the Physicochemical Odor Space. Brain Sciences, 11(5), 563. https://doi.org/10.3390/brainsci11050563








