Behavioral Outcomes and Neural Network Modeling of a Novel, Putative, Recategorization Sound Therapy
Abstract
1. Introduction
2. Materials and Methods
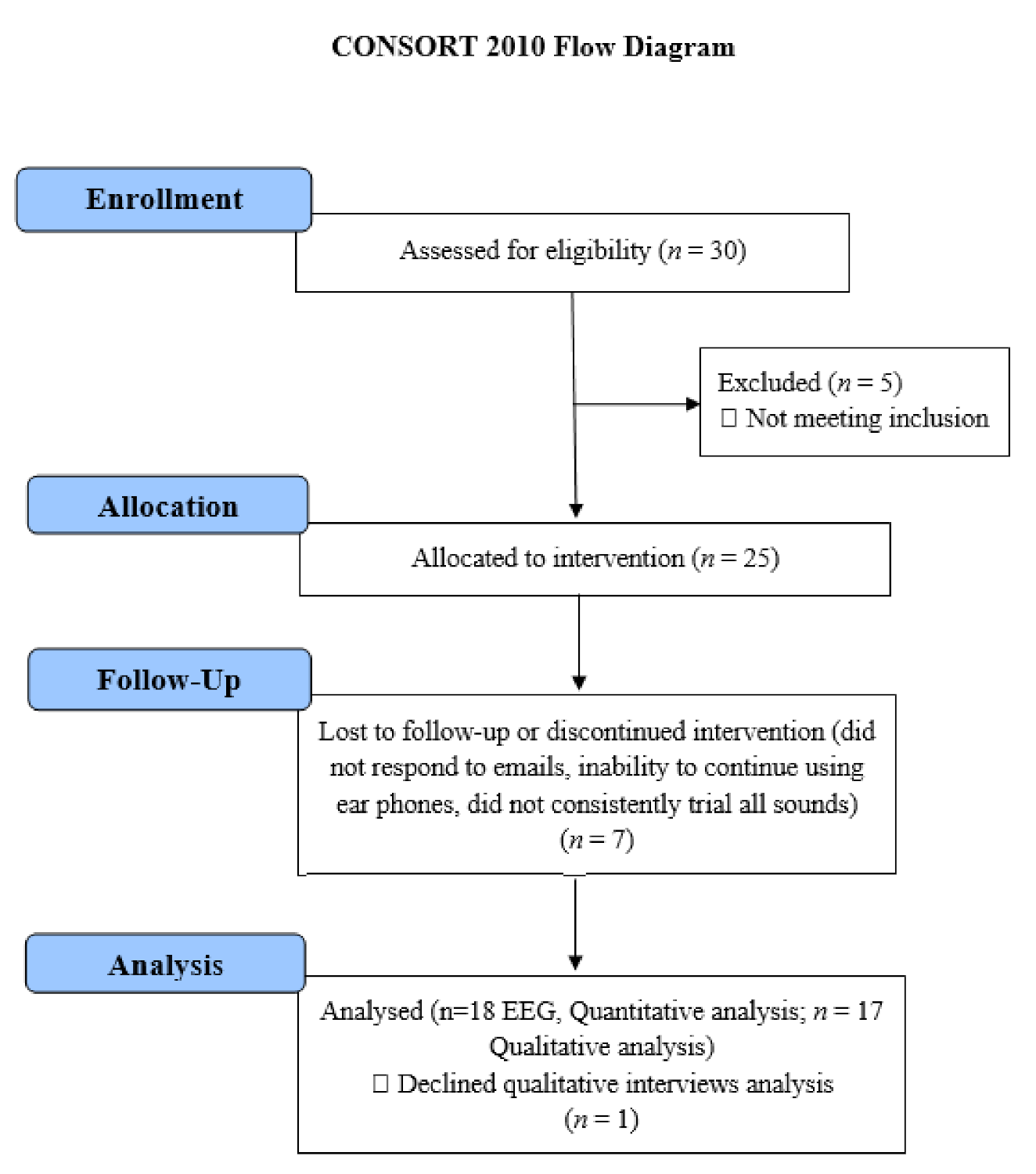
2.1. Participant Eligibility and Recruitment
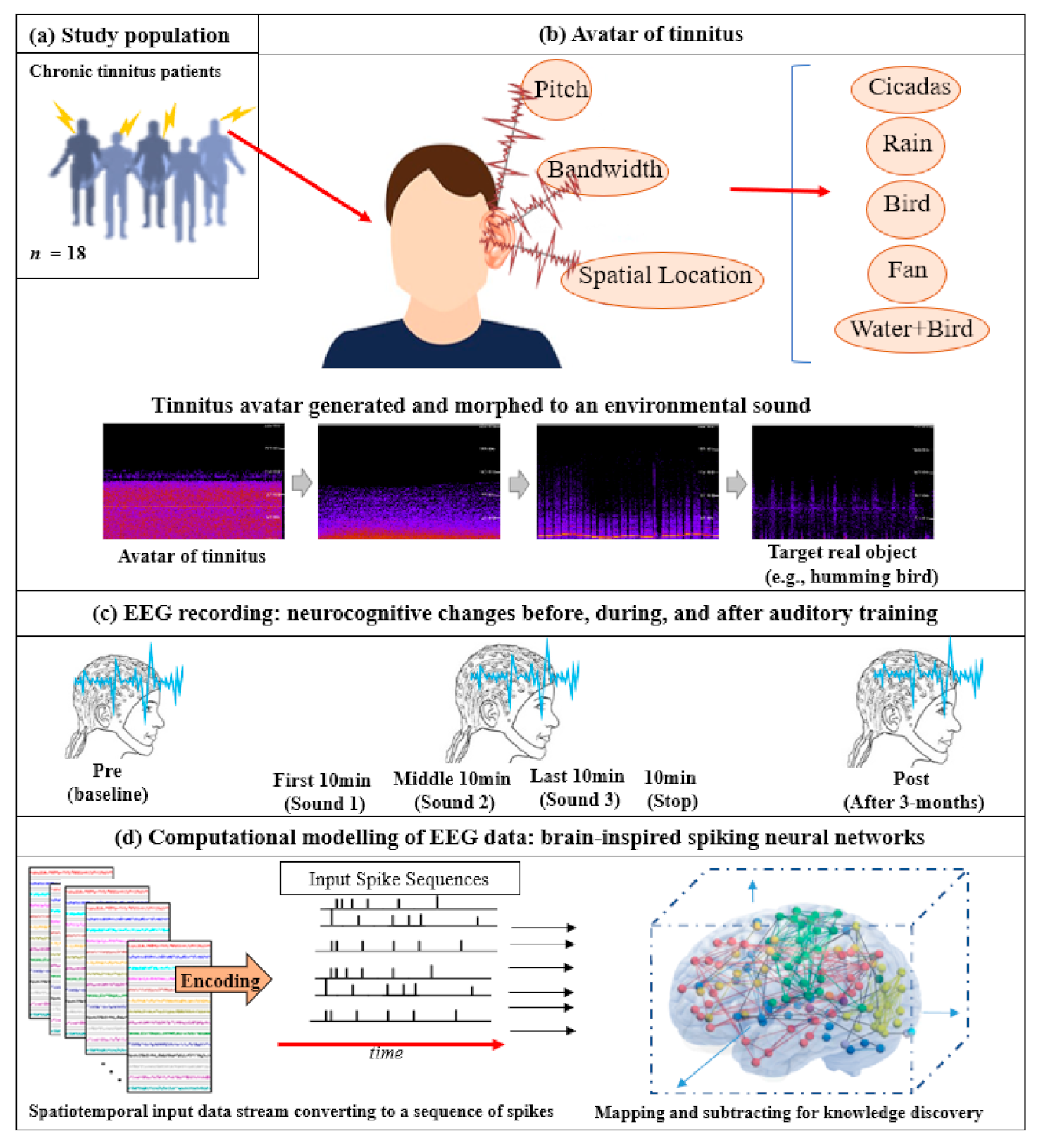
2.2. Part 1. Development of Tinnitus Avatar, Real Auditory Objects Library, and Acute Electroencephalography (EEG) Stepped Morphing Measurements
2.2.1. Tinnitus Avatar
2.2.2. Real Auditory Objects Library
2.2.3. Acute EEG Stepped Morphing
2.3. Part 2. Three-Month Feasibility Trial
Chronic Sound Stepped Morphing
2.4. Outcome Measures: Statistical Analysis and Computational Modeling of Data
2.4.1. Questionnaires
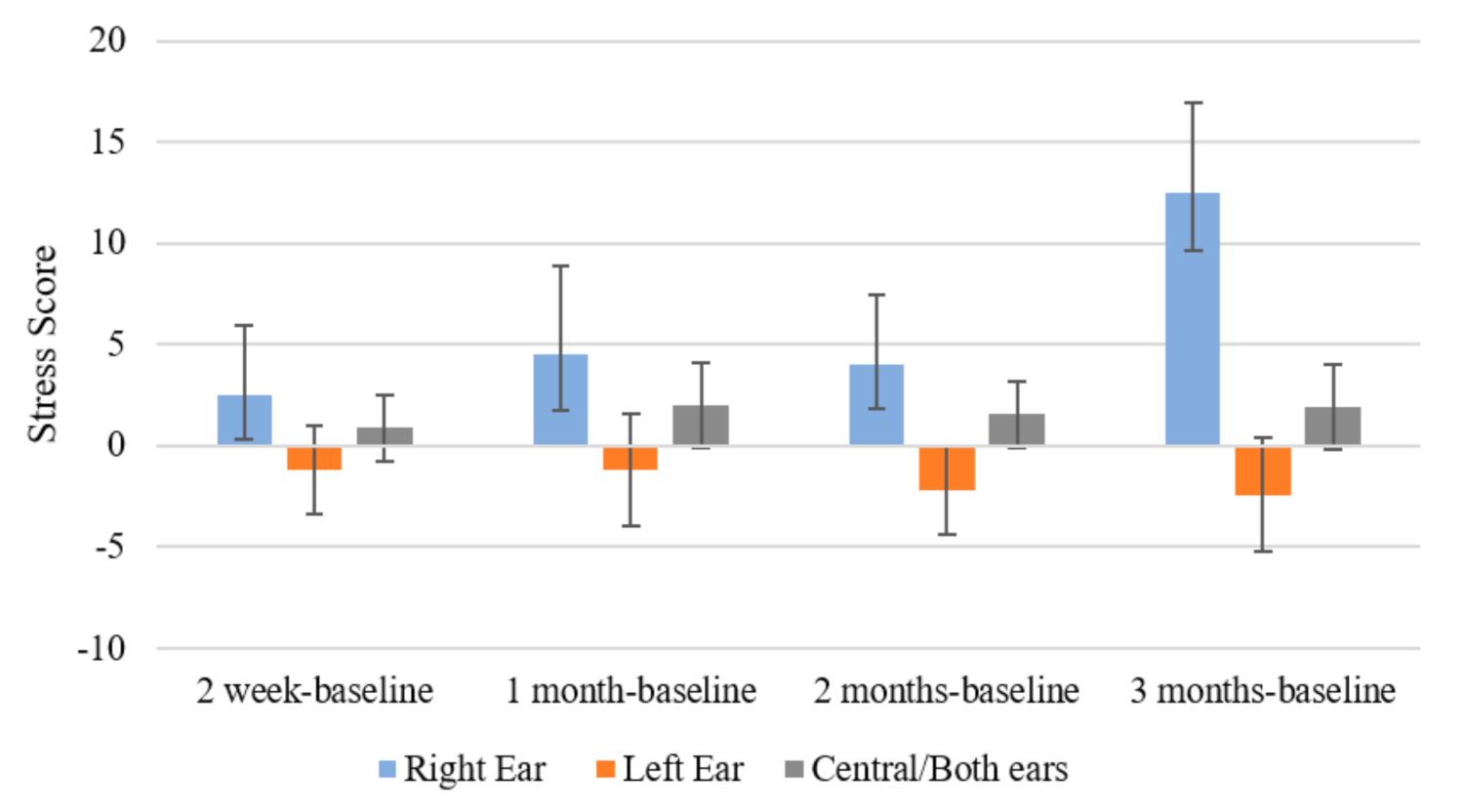
2.4.2. Psychoacoustic Tinnitus Characteristics
2.4.3. Qualitative Interviews
2.4.4. Electroencephalography (EEG)
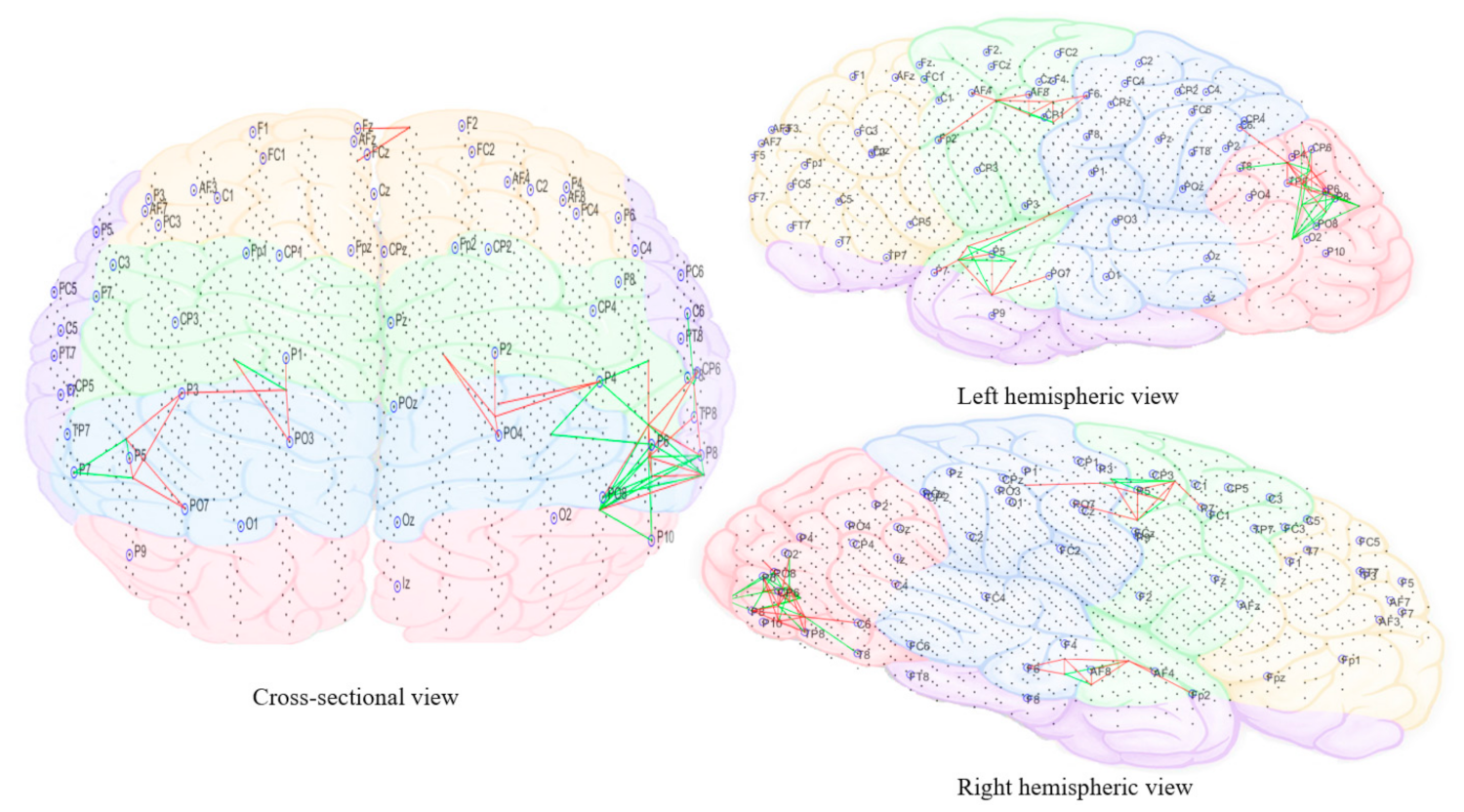
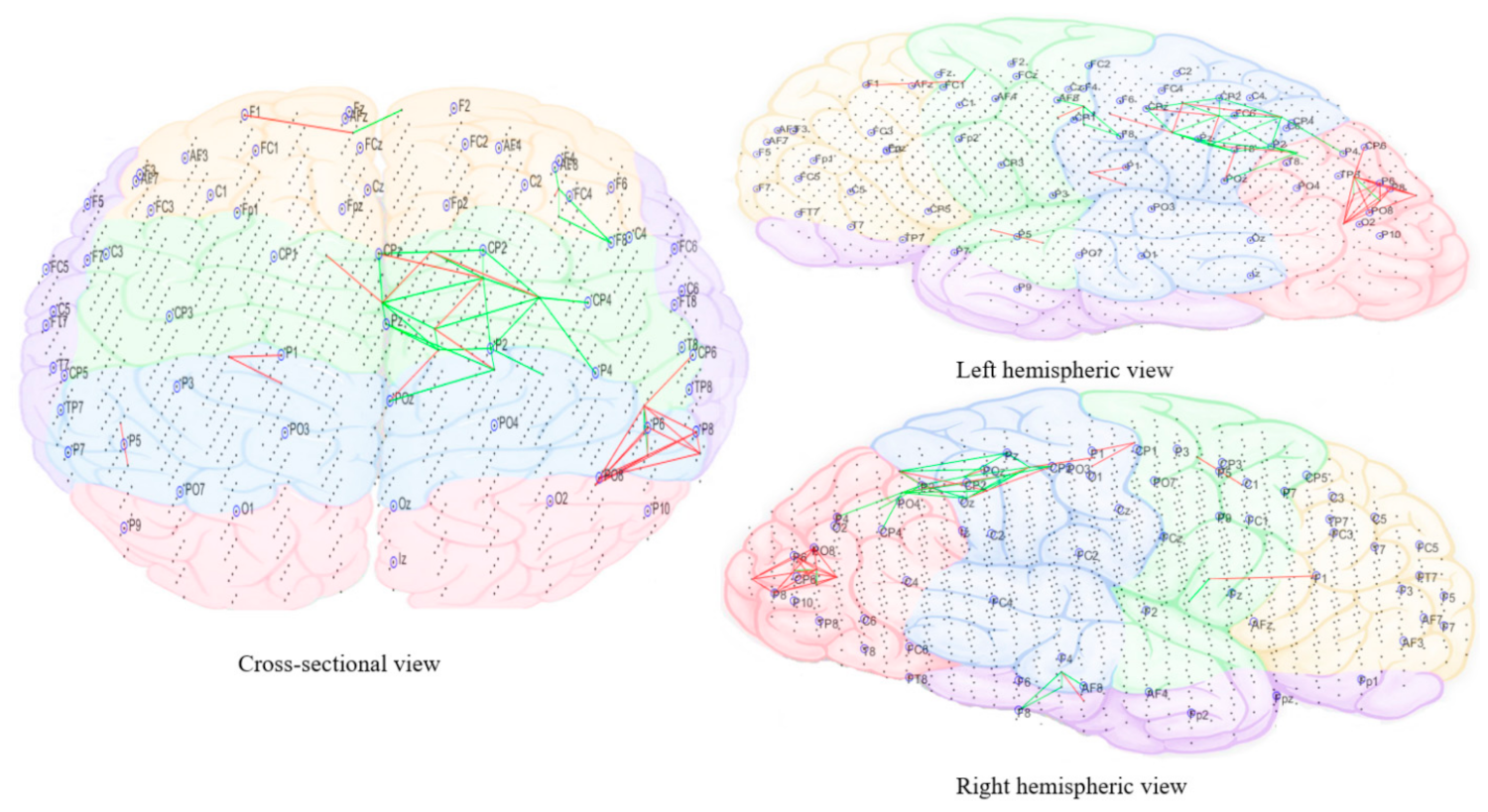
2.4.5. Electroencephalography (EEG) Modeling and Analysis Using Brain-Inspired Spiking Neural Networks Architecture
- Data encoding: In the first phase of data modeling, the real-value EEG time series was encoded to trains of spikes using an appropriate encoding method [55]. As shown by Petró et al., the Step Forward [55] algorithm best captured the characteristics of the original signals. The SF algorithm employed a fixed threshold and a moving baseline, which was adjusted after each spike was created. Using this technique, if the upward change in a signal’s amplitude from the baseline was more than the threshold at a certain time, a positive spike was produced. Conversely, negative spikes were created if the amplitude diminutions from the baseline below the defined threshold. When none of these cases occurred, no spikes were generated. The generated spike trains embody changed in the STBD that exceeded a threshold SFthr. Since this threshold was dependent on the signal amplitude, it was optimized before the encoding procedure to more accurately capture the signal changes. Figure 2d demonstrates an instance of encoded EEG signals into positive and negative spike trains generated from the raw EEG data.
- Mapping: Figure 2d further illustrates that a model was then pre-structured to represent the functional and structural information of the brain processes measured by spatiotemporal data. The STBD data samples were mapped spatially into 3D artificial neural space where the spatial information of brain areas was topologically preserved concerning the (x, y, z) coordinates as positioned in the Talairach brain atlas [56]. In the SNN model, after defining a biologically plausible 3D SNN, data were initialized with a Small-World Connectivity rule (SWC) [57] that defined a probability by which a neuron i can be linked to a neuron j with respect to their internal distance, the greater the distance between i and j, the smaller the connection probability. The generated initial connections were adapted during the unsupervised learning process, which takes into account the temporal dynamics of input data [58].
- Learning: The SNN model used an unsupervised learning algorithm, called Spike Time-Dependent Plasticity (STDP), which allows the model to learn the spatiotemporal relationships in the input spikes [50]. This learning process modifies the neural connection strength with respect to the timing of pre to postsynaptic neurons. The connections between neurons were updated dynamically at each time point of the input data (e.g., at a millisecond scale), resulting in deep trajectories of connectivity learnt in the 3D SNN structure. Throughout the STDP learning process, if every neuron’s potential passed an activation threshold in time t, then it produced an output spike. The spike was then transferred to other neurons linked to it. This neuron likewise kept receiving spikes over time and, after passing a threshold, fired. In this way, spikes were propagated inside the SNN model during the STDP learning and the ‘hidden’ spatiotemporal relationships between the data variables were captured in the shape of neural connectivity and the weights of these connections. The connection weights can then be visualized [59,60,61].
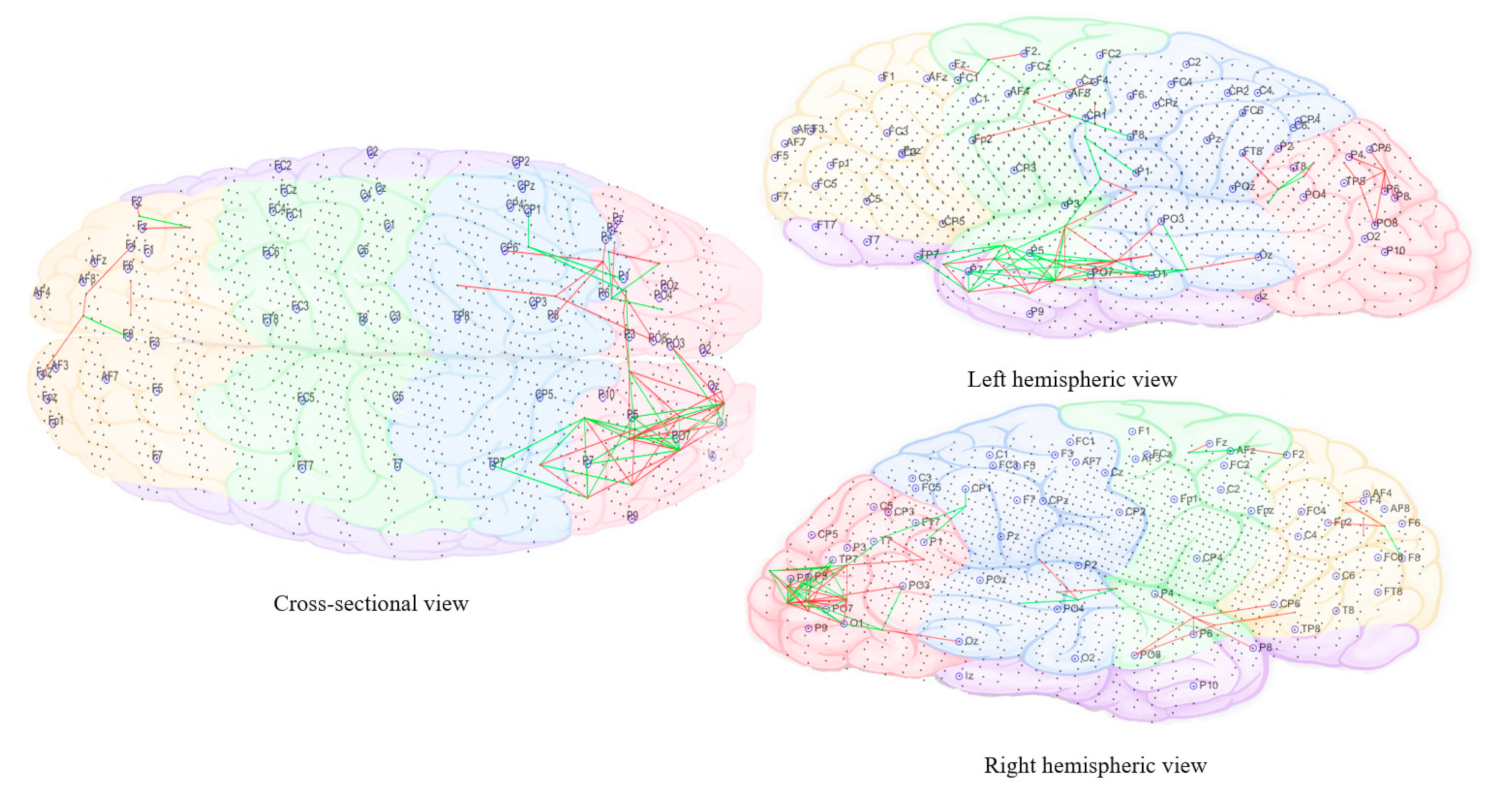
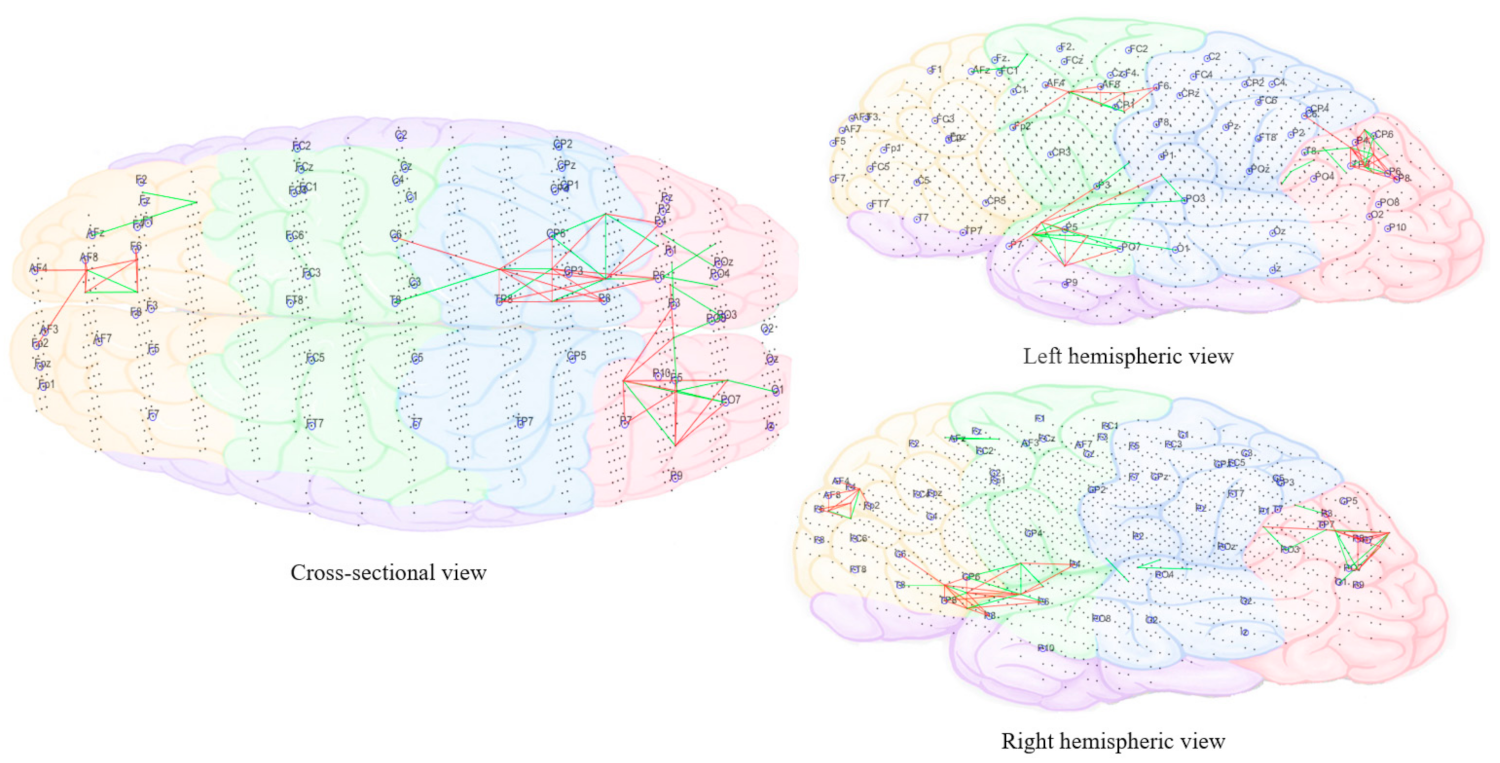
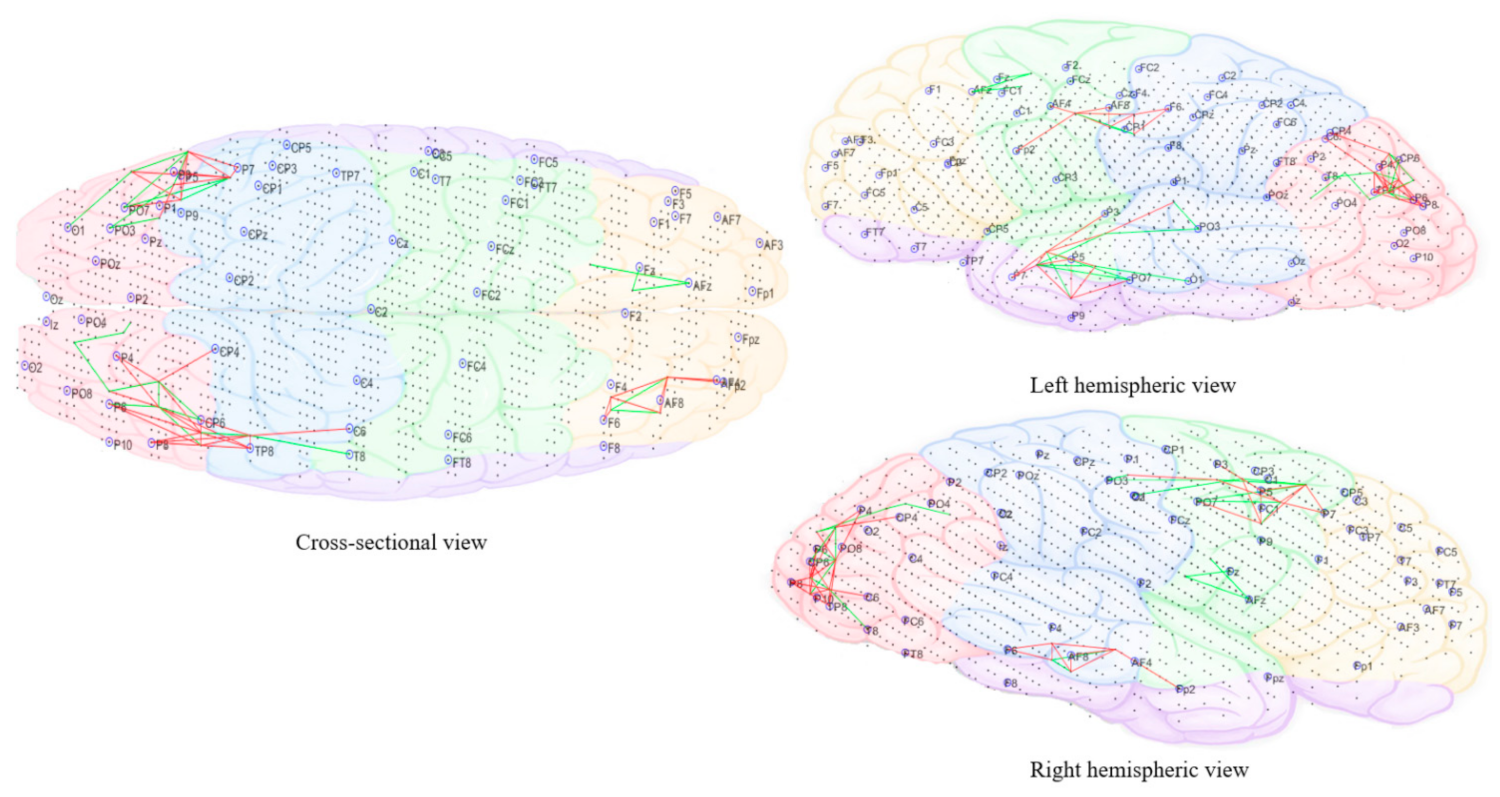
- Pattern visualization: Different models were then trained with the EEG data extracted from different brain mental states, e.g., before, during, and after sound training across participants, thus different models were produced thus they could be further compared and analyzed. The models were subtracted to understand the difference between the two states as a result of different brain activities.
3. Results
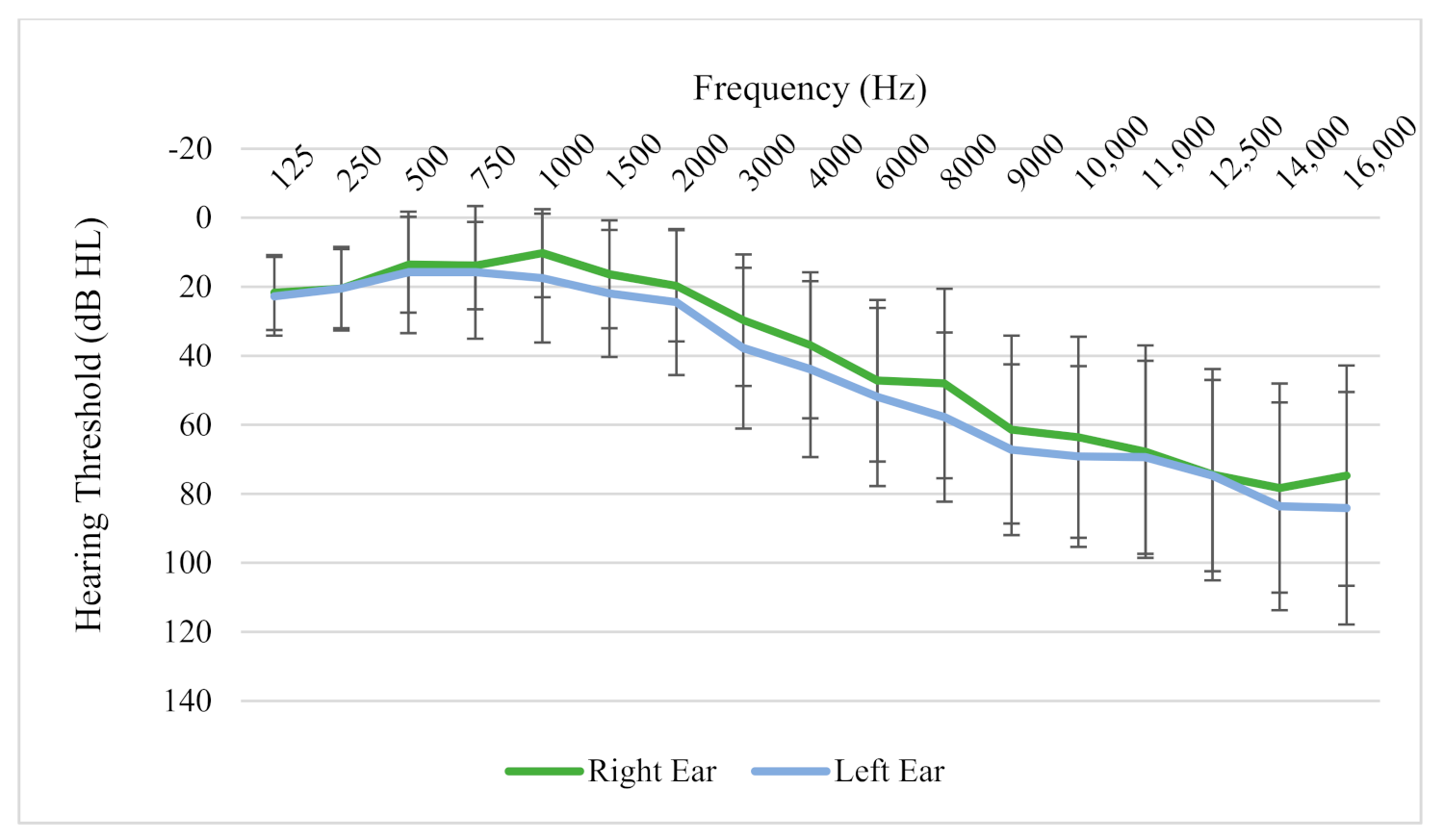
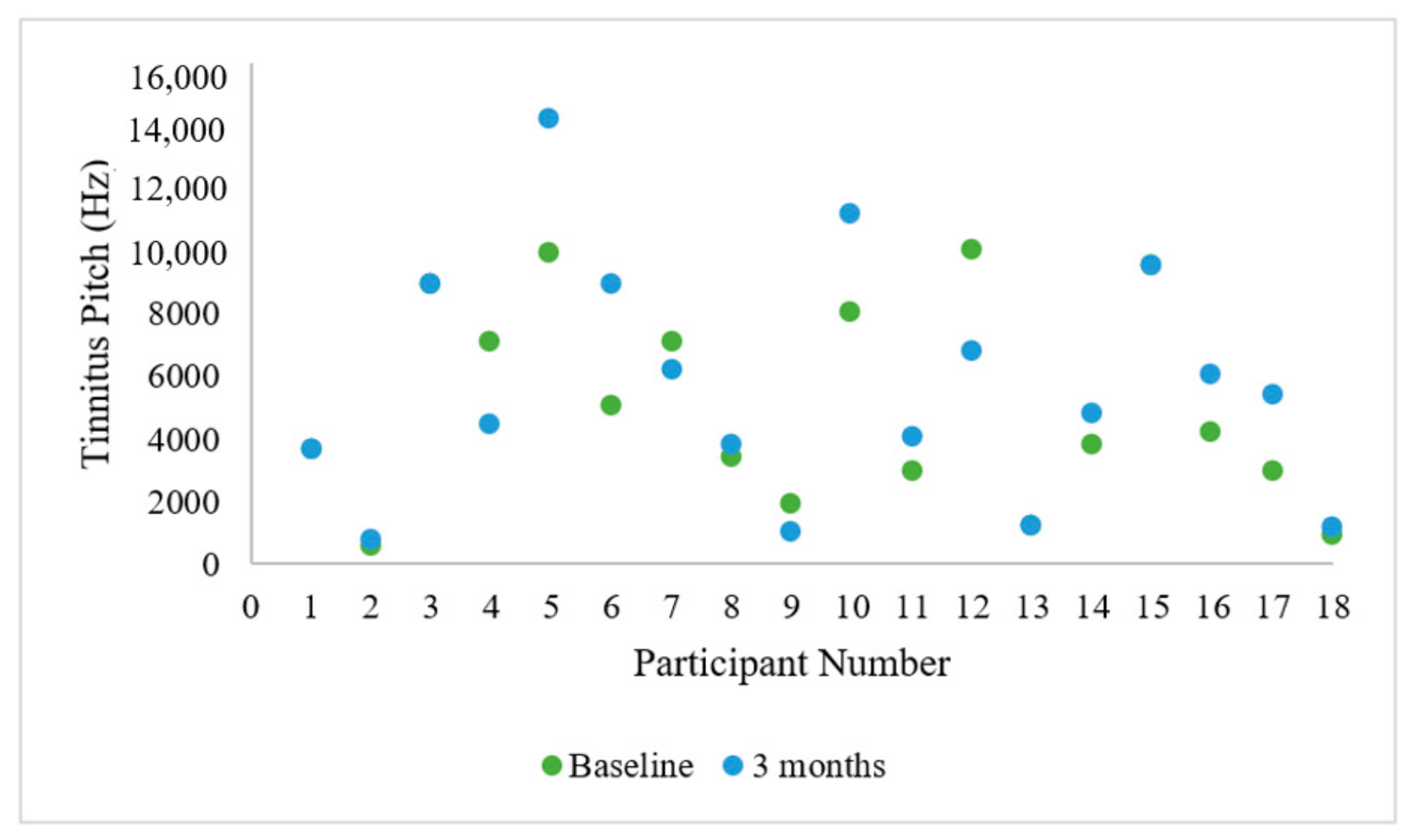
3.1. Participant Hearing and Tinnitus Characteristics
3.2. The Tinnitus Avatar
3.3. Questionnaires Outcomes
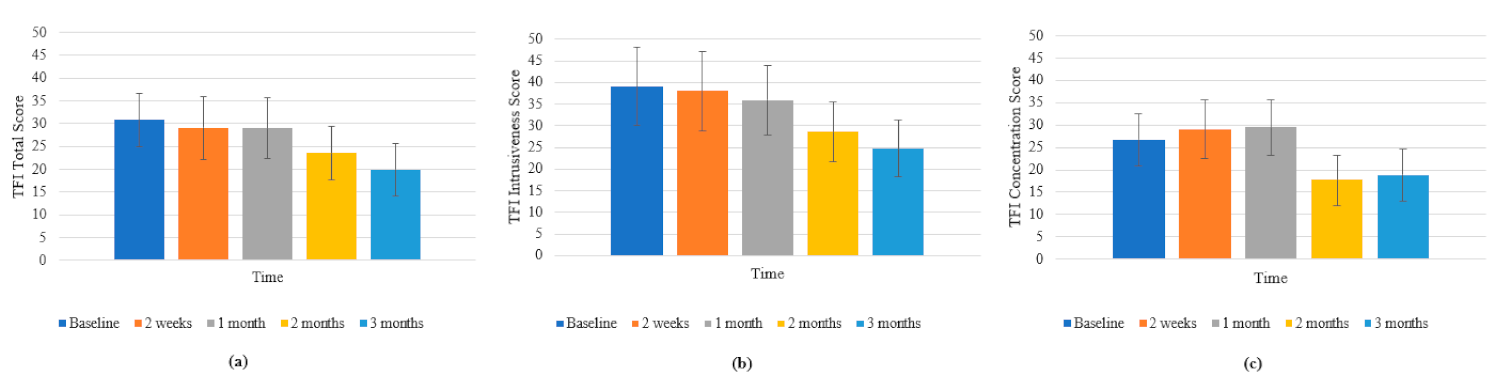
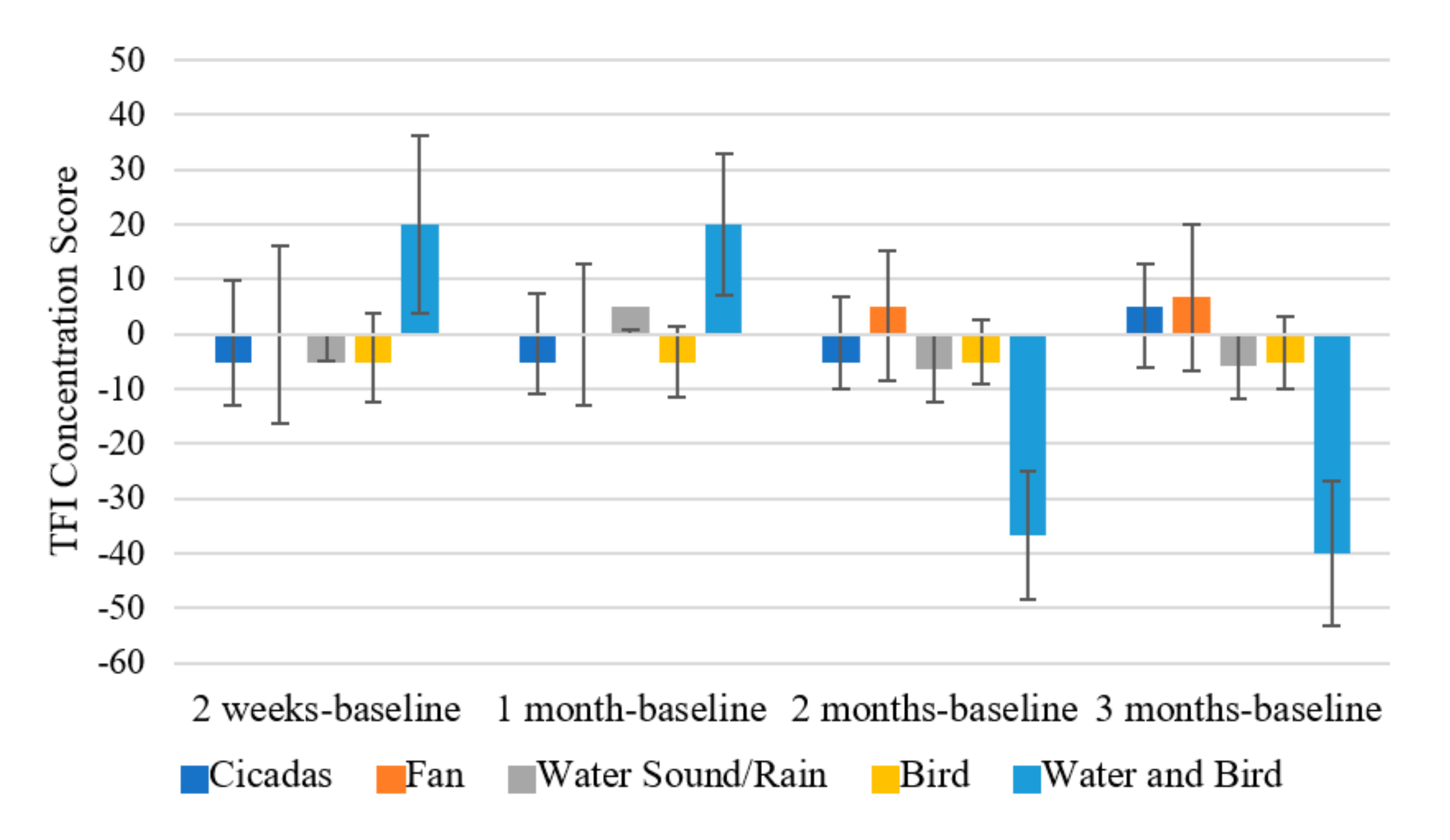
3.3.1. Tinnitus Functional Index (TFI)
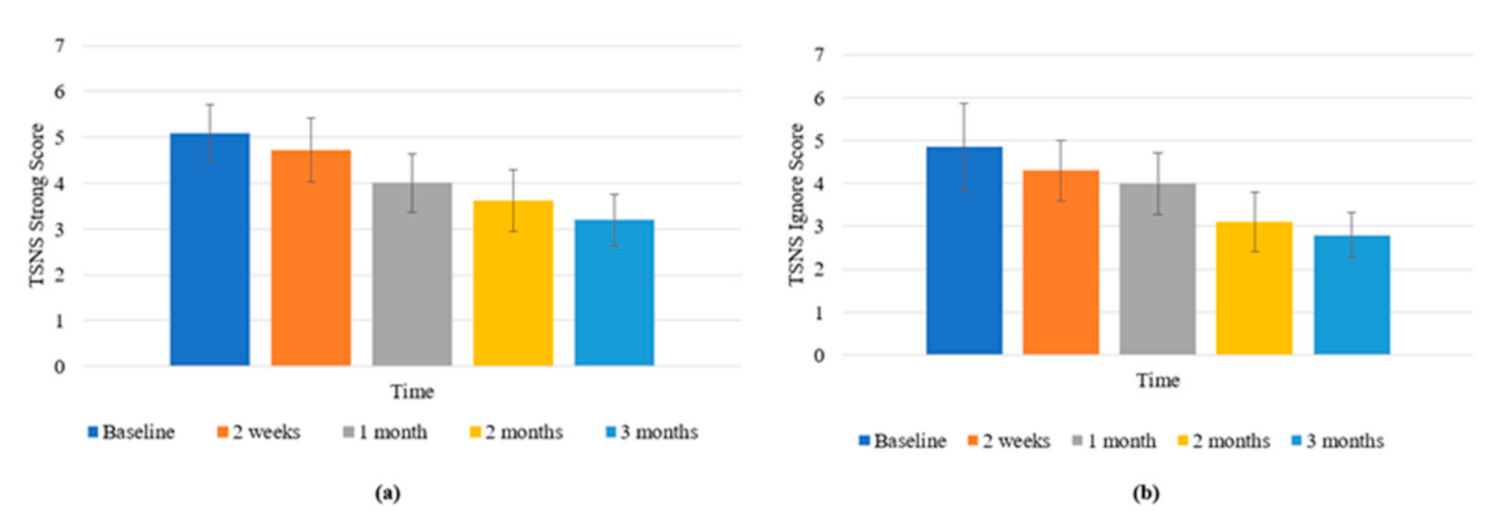
3.3.2. Tinnitus Severity Numeric Rating Scales
3.3.3. Depression, Anxiety, and Stress Scale (DASS)
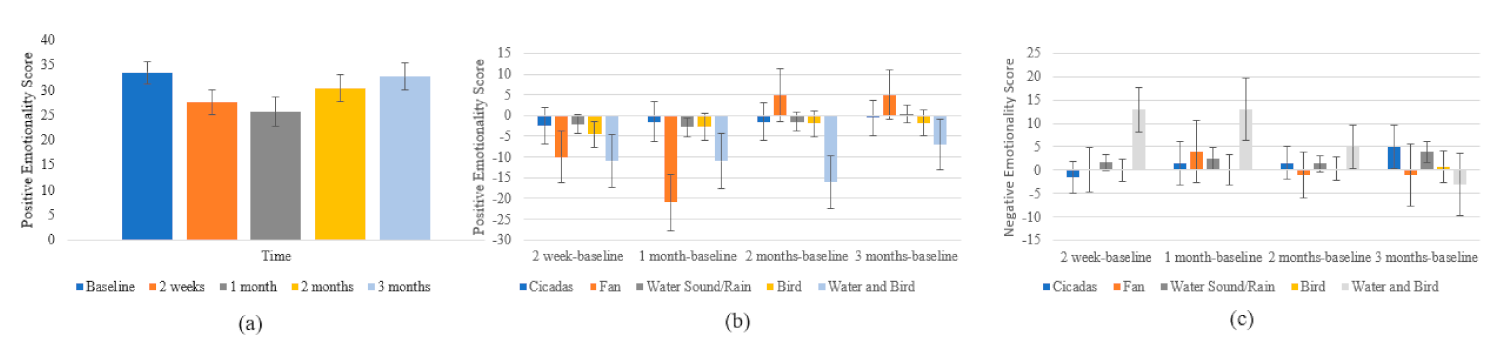
3.3.4. Positive and Negative Affect Schedule (PANAS)
3.3.5. Computational Modeling of EEG Data Using SNN Architecture
3.3.6. Qualitative Analysis (Participant Interview Excerpts Attached as Appendix A)
“It seems that the lower sound is more frequent and the higher sound only occasionally. Reverts to higher and more annoying pitch when tired or continuing to process thoughts when going to sleep”[Fan] [Participant #2]
“Less uniform sound, more variation in pitch and sounds like more than one sound”[Birds] [Participant #4]
“Overall enjoyed the sound but did not change tinnitus much—only changed from multiple component sound to single electronic scream—but can’t tell if due to life stress”[Birds] [Participant #6]
“Yes, when I missed a few days, it got worse so I have been more careful to listen every day, since then it has decreased and evened out. Strong left-sided tinnitus had more distinct ringing has now stopped, seemed to have helped with that.”[Water sound/Rain] [Participant #3]
“Yes, it is much calmer after a session. Hardly noticing the tinnitus, change in loudness from perceived 9/10 to 2/10. The location is still the same. Much lower and worked better.”[Water sound/Rain] [Participant #9]
“I was thinking today how the tinnitus has become less noticeable during the day. Enjoyed water sound more than tinnitus. Like the idea of sound covering up tinnitus. Tinnitus is not unnoticeable, just acceptable—more relaxed and readier to try ongoing management.”[Water and Bird] [Participant #10]
“I am more aware of tinnitus as paying attention to it when filling in questionnaires.” [Cicadas] [Participant #8] “Will get a residual hum in head for 5 min after finishing.”[Water sound/Rain] [Participant #15]
“Yes, I have left my job of 23 years and am 2 days into a less demanding job, to a much less stressful environment and workload.”[Water sound/Rain] [Participant #9]
“Holiday stress that causes jaw clenching and teeth grinding, not being in my own bed causing sore stiff neck.”[Water sound/Rain] [Participant #3]
“Yeah absolutely, I’ve gotten super stressed about university because tests and deadlines are coming up and I’m so behind. My sleep schedule is completely out of whack, I’m struggling to stay hydrated, I haven’t been exercising much, etc. I’ve been anxious and demotivated and depressed.”[Cicadas] [Participant #5]
“My father died and I was helping arrange his memorial, which was stressful, involved international travel, an over-full house and upset routine. Finding an hour of quiet was at times impossible.”[Birds] [Participant #6]
“For the first 6 weeks, sometimes the tinnitus wasn’t there at times. The silence was very prominent, marked. About 4 weeks ago, there was work tension and noticed an increase in sound. Also flew down to Wellington 2 weekends ago—in middle of tension period—not sure if flying might have had an effect.”[Water sound/Rain] [Participant #14]
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| How is your tinnitus at 3 months? Tinnitus is like always [Cicadas] [#1] Less intrusive mostly [Fan] [#2] Variable but less intrusive than before [Water sound/Rain] [#3] Seems a little louder at times [Birds] [#4] Not very good, it’s louder [Cicadas] [#5] Noticeable and consistent [Birds] [#6] No change [Birds] [#7] Have not noticed any significant difference [Cicadas] [#8] Much calmer [Water sound/Rain] [#9] Mostly it seems somewhat subdued; I am aware of it, it just doesn’t seem so aggressive. Unless of course I get stressed. Then my head pounds, and the tinnitus is louder [Water and Bird] [#10] Less of a problem than usual [Birds] [#11] Constant. No change [Birds] [#12] No change. Still annoying! [Water sound/Rain] [#13] Much the same [Water sound/Rain] [#14] Just the same. Have several tinnitus components: hissing, bells, buzzing, chiming, tones [Water sound/Rain] [#15] About the same [Water sound/Rain] [#17] Much as usual. I may be less aware of it [Water sound/Rain] [#18] |
| How is the sound? (you can comment on any features such as quality, annoyance, pleasantness of the sound, etc.) Sound is getting annoying after 30 plus minutes, quality is OK I guess. Boring as ever [Cicadas] [#1] More subdued/lower/less intrusive [Fan] [#2] It is now balanced with a very high-pitched lining that is constant [Water sound/Rain] [#3] A few more tones in it—and sound varies a little more [Birds] [#4] I kind of got used to it and maybe it’s just my imagination but it seems to help me ignore the tinnitus a little better [Cicadas] [#5] Quality is very good, although I have trouble fitting the left earphone snugly—my ears are tricky. The sound is clear, although there is sometimes interference when the cables to the ear plugs rub against clothing or hair [Birds] [#6] Not too annoying but wouldn’t want it to be any louder. The sound wasn’t different enough from original bird noise and also sounded like the clip was created artificially by modulated, different bird noises merged. Seemed repetitive [Birds] [#7] OK [Cicadas] [#8] Very pleasant. Enjoyed the sound and feels there is no need to improve anything. [Water sound/Rain] [#9] I enjoy listening to the sounds, especially when they have all mingled together. It is very relaxing. I sometimes would like the sound to last longer. I used to listen to, “forestbythesea” frequently, now I find I listen a lot less. It is, however, my first go to tune when I am stressed, as it masks the tinnitus wonderfully. File was fine, possibly bit repetitive (too much water) but finds it builds to a crescendo so needed to make it lower in volume [Water and Bird] [#10] Easy to listen to. Does not interfere with other activities [Birds] [#11] Enjoy sound track. The sounds are pleasant and nice background noise, helped to relax. [Birds] [#12] I feel really content listening to the sound. It sounds similar to the running water in a pool I was staying by on my recent holiday so conjures up happy memories [Water sound/Rain] [#13] It’s pleasant enough, not a problem [Water sound/Rain] [#14] Fine [Water sound/Rain] [#15] It’s OK and quite nice to fall asleep with [Water sound/Rain] [#17] It is OK [Water sound/Rain] [#18] |
| Do you feel like your tinnitus (e.g., characteristics of your tinnitus such as the pitch, duration, fluctuation, intensity in different environments) has changed? Please describe. Has not changed [Cicadas] [#1] It seems that the lower sound is more frequent and the higher sound only occasionally. Reverts to higher and more annoying pitch when tired or continuing to process thoughts when going to sleep [Fan] [#2] Yes—left side is matching right, which is an improvement. Volume has decreased [Water sound/Rain] [#3] Yes—less uniform sound, more variation in pitch and sounds like more than one sound. Sound comes and goes a little more [Birds] [#4] It’s gotten worse, I feel like there are new tones appearing too, especially at night. If I start focusing on it then many new tones will appear that I didn’t notice before. I think it varies day by day slightly but on the whole, it got louder and harder to ignore, and I’m noticing it much more often [Cicadas] [#5] This trial it was difficult to judge if tinnitus changed because my father passed away 2 weeks in [Birds] [#6] No [Birds] [#7] Tinnitus has become more variable after beginning trial. At the moment it seems reasonably quiet. However, during trial I became more aware of it, as having to listen to it and thinking about whether it has changed when filling in questionnaires [Cicadas] [#8] My tinnitus is quieter since going on the 2nd version. Yes, less noticeable a lot of the time [Water sound/Rain] [#9] Perhaps the intensity of the tinnitus is slightly less. I haven’t noticed any change to the tinnitus in a stressful situation. I am acutely aware of the tinnitus, and know I need to calm myself asap [Water and Bird] [#10] I think the intensity is reduced [Birds] [#11] No change. Thought early on that tinnitus was louder after listening session but may be just greater awareness. Once I stopped “listening” to tinnitus seemed to go back to the usual [Birds] [#12] The volume in my right ear seems to have reduced in the last few weeks (probably settled down after long plane journey). The left ear is probably still louder than before I travelled but does have short periods of a lower (more usual) volume. I’m a lot more aware of it at night time. [Water sound/Rain] [#13] Occasionally it seems to be silent for a little while [Water sound/Rain] [#14] No [Water sound/Rain] [#15] Not really, it’s about the same loudness and more in my left ear as before [Water sound/Rain] [#17] No change. May be slightly less conscious of it [Water sound/Rain] [#18] |
| Do you feel like the sound has been interacting with your tinnitus since the last appointment? Please describe. Would say no [Cicadas] [#1] I think so. Tinnitus has only been noticeable perhaps when I am more stressed or tired?? [Fan] [#2] Yes, when I missed a few days it got worse so I have been more careful to listen every day, since then it has decreased and evened out. Strong left sided tinnitus had more distinct ringing has now stopped, seemed to have helped with that. [Water sound/Rain] [#3] I certainly here bird sounds outside a bit more—or at least I pay more attention to them [Birds] [#4] If I listen to the old [version] mp3, it really helps to ignore the tinnitus while it’s playing, and even if I try to notice my tinnitus ringing, it’s hard to actually hear it. The new one is ok too [Cicadas] [#5] No. Overall enjoyed the sound but did not change tinnitus much—only changed from multiple component sound to single electronic scream—but can’t tell if due to life stress [Birds] [#6] Yes—tinnitus is less noticeable while listening. The first set of sounds had a certain amount of effectiveness, the tinnitus was fading in to environmental sound. The later sounds I found tinnitus faded out only because of concentrating on the sound, not due to ‘auditory’ interaction with tinnitus [Birds] [#7] I am more aware of tinnitus as paying attention to it when filling in questionnaires [Cicadas] [#8] Yes, it is much calmer after a session. Hardly noticing the tinnitus, change in loudness from perceived 9/10 to 2/10. The location is still the same. Much lower and worked better. [Water sound/Rain] [#9] I was thinking today how the tinnitus has become less noticeable during the day. Enjoyed water sound more than tinnitus. Like the idea of sound covering up tinnitus. Tinnitus is not unnoticeable, just acceptable—more relaxed and ready to try ongoing management [Water and Bird] [#10] I feel that the MP3 sound is having a beneficial effect [Birds] [#11] No change. Tinnitus hasn’t changed, and even when birds chirping on sound file the tinnitus still sits in the background [Birds] [#12] I find that when I am getting frustrated with my tinnitus, I choose to go and listen to the sounds to distract myself from it. I sometimes wonder if the sounds are causing the ongoing increased volume but it may just be co-incidental with the travel. I do find that I seem to be going to sleep much faster than I normally would when listening to the sounds. When sound is playing, tinnitus is not as bothersome/audibility is reduced. When tinnitus is very loud, want to listen to it. [Water sound/Rain] [#13] They just seem to be alongside each other and it’s ok [Water sound/Rain] [#14] Will get a residual hum in head for 5 min after finishing [Water sound/Rain] [#15] I think there is a slight improvement, in the sense of a connection between the water sound and the tinnitus [Water sound/Rain] [#17] Maybe. Slightly less conscious of my tinnitus [Water sound/Rain] [#18] |
| What environments have you been using the sound in? Quiet environments, e.g., computer work, reading book/reading on the computer, quiet housework, cooking [Cicadas] [#1] Mostly reading. A couple of times when doing mindless household chores [Fan] [#2] In bed at night (before I go to sleep or as I go), when I go for a walk, or sitting at my desk [Water sound/Rain] [#3] Quiet office at work [Birds] [#4] At my desk while studying usually. Sometimes it’s hard to find a quiet hour because I live with my girlfriend and she always wants to talk to me about something [Cicadas] [#5] At home with no radio or television, sometimes reading or on a computer. Occasional conversation with a family member [Birds] [#6] Working at desk, reading [Birds] [#7] At home [Cicadas] [#8] Mainly at home alone cooking or reading or doing crossword [Water sound/Rain] [#9] Generally, I listen before going to sleep, then I replay the sounds as I go to sleep. I feel I have to listen to the sounds continuously to get the full benefit, not stop and start. Sometimes I have uninterrupted time to listen during the day. If I wake in the early morning, and the tinnitus is annoying, I will play the sounds to relax me [Water and Bird] [#10] Usually at home when reading. Occasionally while TV is on [Birds] [#11] Used them mainly in quiet environments, sometimes in early morning or in mid-late afternoon, when reading [Birds] [#12] I mostly listen to them at home during the day when going about normal activities—housework, admin, working from home on computer. A few times I have listened when out walking and I have also got into the habit of listening to them when I go to bed at night. Usually do other things while listening to it, walking children to/from school, house work, reading book (don’t really pay attention when reading). Prefer the birds/more environmental sounds—rain sound is more background [Water sound/Rain] [#13] Mostly when I am meditating [Water sound/Rain] [#14] In the lounge and mostly in the bedroom before I go to sleep [Water sound/Rain] [#15] Mainly at night [Water sound/Rain] [#17] At my desk when working on my computer [Water sound/Rain] [#18] |
| Are there any major changes in your life circumstances or incidents since the last appointment or email follow-up, which you believe may have had an influence on your tinnitus? Only change was school holidays as well as my ones [holidays] (3 weeks) [Cicadas] [#1] No [Fan] [#2] Holiday stress that causes jaw clenching and teeth grinding, not being in my own bed causing sore stiff neck [Water sound/Rain] [#3] No [Birds] [#4] Yeah absolutely, I’ve gotten super stressed about university because tests and deadlines are coming up and I’m so behind. My sleep schedule is completely out of whack, I’m struggling to stay hydrated, I haven’t been exercising much, etc. I’ve been anxious and demotivated and depressed [Cicadas] [#5] My father died and I was helping arrange his memorial, which was stressful, involved international travel, an over-full house and upset routine. Finding an hour of quiet was at times impossible [Birds] [#6] No [Birds] [#7] No [Cicadas] [#8] Yes, I have left my job of 23 years and am 2 days into a less demanding job, to a much less stressful environment and workload [Water sound/Rain] [#9] No [Water and Bird] [#10] I don’t believe so. I have started regular Gym activity during this trial [Birds] [#11] No incidents or circumstances [Birds] [#12] No [Water sound/Rain] [#13] For the first 6 weeks, sometimes the tinnitus wasn’t there at times. The silence was very prominent, marked. About 4 weeks ago, there was work tension and noticed an increase in sound. Also flew down to Wellington 2 weekends ago—in middle of tension period—not sure if flying might have had an effect [Water sound/Rain] [#14] No [Water sound/Rain] [#15] No [Water sound/Rain] [#17] No changes [Water sound/Rain] [#18] |
| How is the loudness of the sound? Would you like to adjust the sound level? The new sound is good, clear and partly loud [Cicadas] [#1] Fine. I vary the volume when I feel it needs adjusting [Fan] [#2] I adjust if I need to [Water sound/Rain] [#3] It’s fine [Birds] [#4] It’s fine I just set the volume [Cicadas] [#5] The volume varies throughout the track, and is sometimes quite soft and other times loud, but it is no more than attention grabbing when the volume changes [Birds] [#6] Very quiet in the first few minutes, later its OK [Birds] [#7] I am able to adjust the sound if I wish [Cicadas] [#8] It is fine [Water sound/Rain] [#9] I can adjust the sound level through my headphones [Water and Bird] [#10] I need to turn it up at first to hear it then it seems to get louder after a while [Birds] [#11] Can hear it OK [Birds] [#12] I’m happy with the loudness. I adjust the volume depending on what earpieces I am using but find it matches the loudness of my tinnitus well [Water sound/Rain] [#13] OK [Water sound/Rain] [#14] No thank you [Water sound/Rain] [#15] It’s OK, I can adjust to suit [Water sound/Rain] [#17] It is OK, environmental sound content seems greater in volume than tinnitus [Water sound/Rain] [#18] |
References
- Wu, B.; Exeter, D.; Searchfield, G.D. Tinnitus in New Zealand. In Proceedings of the 8th TRI International Tinnitus Conference, Auckland, New Zealand, 10–13 March 2014. [Google Scholar]
- Baguley, D.; Andersson, G.; McFerran, N.; McKenna, L. Tinnitus: A Multidisciplinary Approach; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- De Ridder, D.; Vanneste, S.; Freeman, W. The Bayesian brain: Phantom percepts resolve sensory uncertainty. Neurosci. Biobehav. Rev. 2014, 44, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning Out the Noise: Limbic-Auditory Interactions in Tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, J.A. Tinnitus: Models and mechanisms. Hear. Res. 2011, 276, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Song, J.-J.; De Ridder, D. Tinnitus and musical hallucinosis: The same but more. NeuroImage 2013, 82, 373–383. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Kobayashi, K.; Sanders, M. An Adaptation Level Theory of Tinnitus Audibility. Front. Syst. Neurosci. 2012, 6, 46. [Google Scholar] [CrossRef]
- Searchfield, G.D. Tinnitus What and Where: An Ecological Framework. Front. Neurol. 2014, 5, 271. [Google Scholar] [CrossRef]
- De Ridder, D.; Elgoyhen, A.B.; Romo, R.; Langguth, B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8075–8080. [Google Scholar] [CrossRef]
- Feldmann, H. Tinnitus–reality or phantom? In Proceedings of the Fourth International Tinnitus Seminar, Bordeaux, France, 27–30 August 1991; Kugler: Amsterdam, The Netherlands, 1992; pp. 7–14. [Google Scholar]
- Parducci, A.; Helson, H. Adaptation-Level Theory. Am. J. Psychol. 1965, 78, 158. [Google Scholar] [CrossRef]
- Durai, M.; Sanders, M.; Kobayashi, K.; Searchfield, G.D. Auditory Streaming and Prediction in Tinnitus Sufferers. Ear Hear. 2019, 40, 345–357. [Google Scholar] [CrossRef]
- Rossiter, S.; Stevens, C.; Walker, G. Tinnitus and Its Effect on Working Memory and Attention. J. Speech Lang. Hear. Res. 2006, 49, 150–160. [Google Scholar] [CrossRef]
- Pierce, K.J.; Kallogjeri, D.; Piccirillo, J.F.; Garcia, K.S.; Nicklaus, J.E.; Burton, H. Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J. Clin. Exp. Neuropsychol. 2012, 34, 126–134. [Google Scholar] [CrossRef]
- Hallam, R.S.; McKenna, L.; Shurlock, L. Tinnitus impairs cognitive efficiency. Int. J. Audiol. 2004, 43, 218–226. [Google Scholar] [CrossRef]
- Andersson, G.; Ingerholt, C.; Jansson, M. Autobiographical Memory in Patients with Tinnitus. Psychol. Health 2003, 18, 667–675. [Google Scholar] [CrossRef]
- Andersson, G.; Hesser, H.; Cima, R.F.; Weise, C. Autobiographical Memory Specificity in Patients with Tinnitus Versus Patients with Depression and Normal Controls. Cogn. Behav. Ther. 2013, 42, 116–126. [Google Scholar] [CrossRef]
- Andersson, G.; Eriksson, J.; Lundh, L.-G.; Lyttkens, L. Tinnitus and Cognitive Interference: A Stroop Paradigm Study. J. Speech Lang. Hear. Res. 2000, 43, 1168. [Google Scholar] [CrossRef]
- Stevens, C.; Walker, G.; Boyer, M.; Gallagher, M. Severe tinnitus and its effect on selective and divided attention. Int. J. Audiol. 2007, 46, 208–216. [Google Scholar] [CrossRef]
- Andersson, G.; McKenna, L. The role of cognition in tinnitus. Acta Oto-Laryngol. 2006, 126, 39–43. [Google Scholar] [CrossRef]
- Searchfield, G.D. Sense and Sensibility: A Review of the Behavioral Neuroscience of Tinnitus Sound Therapy and a New Typology. In Neurotoxin Modeling of Brain Disorders—Life-Long Outcomes in Behavioral Teratology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–35. [Google Scholar]
- Stevens, K.; Libermann, A.; Studdert-Kennedy, M.; Öhman, S. Crosslanguage Study of Vowel Perception. Lang. Speech 1969, 12, 1–23. [Google Scholar] [CrossRef]
- Guenther, F.H.; Gjaja, M.N. The perceptual magnet effect as an emergent property of neural map formation. J. Acoust. Soc. Am. 1996, 100, 1111–1121. [Google Scholar] [CrossRef]
- Eimas, P.D.; Siqueland, E.R.; Jusczyk, P.; Vigorito, J. Speech Perception in Infants. Science 1971, 171, 303–306. [Google Scholar] [CrossRef]
- Streeter, L.A. Language perception of 2-month-old infants shows effects of both innate mechanisms and experience. Nat. Cell Biol. 1976, 259, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, R.L. Influences of categorization on perceptual discrimination. J. Exp. Psychol. Gen. 1994, 123, 178–200. [Google Scholar] [CrossRef] [PubMed]
- Eimas, P.D. Infants, speech, and language: A look at some connections. Cognition 1981, 10, 79–84. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Conboy, B.T.; Padden, D.; Nelson, T.; Pruitt, J. Early speech perception and later language development: Implications for the critical period. Lang. Learn. Dev. 2005, 1, 237–264. [Google Scholar]
- Kuhl, P.; Williams, K.; Lacerda, F.; Stevens, K.; Lindblom, B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science 1992, 255, 606–608. [Google Scholar] [CrossRef]
- Foroni, F.; Myron, R. Category Boundaries and Category Labels: When Does a Category Name Influence the Perceived Similarity of Category Members. Soc. Cogn. 2011, 29, 547–576. [Google Scholar] [CrossRef]
- Bergman, P.; Skold, A.; Vastfjall, D.; Fransson, N. Perceptual and emotional categorization of sound. J. Acoust. Soc. Am. 2009, 126, 3156–3167. [Google Scholar] [CrossRef]
- Burleigh, T.J.; Schoenherr, J.R. A reappraisal of the uncanny valley: Categorical perception or frequency-based sensitization? Front. Psychol. 2015, 5, 1488. [Google Scholar] [CrossRef]
- Barozzi, S.; Del Bo, L.; Crocetti, A.; Dyrlund, O.; Passoni, S.; Zolin, A.; Panicucci, E.; Mancuso, A.; Kaur, M.; Searchfield, G.D.; et al. A Comparison of Nature and Technical Sounds for Tinnitus Therapy. Acta Acust. United Acust. 2016, 102, 540–546. [Google Scholar] [CrossRef]
- Durai, M.; Searchfield, G.D. A Mixed-Methods Trial of Broad Band Noise and Nature Sounds for Tinnitus Therapy: Group and Individual Responses Modeled under the Adaptation Level Theory of Tinnitus. Front. Aging Neurosci. 2017, 9, 44. [Google Scholar] [CrossRef]
- Durai, M.; O’Keeffe, M.G.; Searchfield, G.D. Examining the short term effects of emotion under an Adaptation Level Theory model of tinnitus perception. Hear. Res. 2017, 345, 23–29. [Google Scholar] [CrossRef]
- Durai, M.; O’Keeffe, M.G.; Searchfield, G.D. The personality profile of tinnitus sufferers and a nontinnitus control group. J. Am. Acad. Audiol. 2017, 28, 271–282. [Google Scholar] [CrossRef]
- Durai, M.; Searchfield, G. Anxiety and depression, personality traits relevant to tinnitus: A scoping review. Int. J. Audiol. 2016, 55, 605–615. [Google Scholar] [CrossRef]
- Durai, M.; O’Keeffe, M.G.; Searchfield, G.D. A review of auditory prediction and its potential role in tinnitus perception. J. Am. Acad. Audiol. 2018, 29, 533–547. [Google Scholar] [CrossRef]
- Bertet, S.; Baskind, A.; Londero, A.; Bonfils, L.; Viaud-Delmon, I.; Warusfel, O. Design and evaluation of tinnitus synthesis methods: From spectral to spatial matching. Am. J. Otolaryngol. 2013, 34, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Doborjeh, Z.; Doborjeh, M.; Crook-Rumsey, M.; Taylor, T.; Wang, G.Y.; Moreau, D.; Krägeloh, C.; Wrapson, W.; Siegert, R.J.; Kasabov, N.; et al. Interpretability of Spatiotemporal Dynamics of the Brain Processes Followed by Mindfulness Intervention in a Brain-Inspired Spiking Neural Network Architecture. Sensors 2020, 20, 7354. [Google Scholar] [CrossRef]
- Doborjeh, M.; Kasabov, N.; Doborjeh, Z.; Enayatollahi, R.; Tu, E.; Gandomi, A.H. Personalised modelling with spiking neural networks integrating temporal and static information. Neural Netw. 2019, 119, 162–177. [Google Scholar] [CrossRef]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The Tinnitus Functional Index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012, 33, 153–167. [Google Scholar] [CrossRef]
- Chandra, N.; Lee, A.; Searchfield, G.D. Validation of the Tinnitus Functional Index in New Zealand. In Proceedings of the 8th International TRI Tinnitus Conference, Auckland, New Zealand, 10–13 March 2014; p. 31. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegan, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales (DASS) in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Onwuegbuzie, A.J.; Leech, N.L. On Becoming a Pragmatic Researcher: The Importance of Combining Quantitative and Qualitative Research Methodologies. Int. J. Soc. Res. Methodol. 2005, 8, 375–387. [Google Scholar] [CrossRef]
- Rossman, G.B.; Wilson, B.L. Numbers and Words: Combining Quantitative and Qualitative Methods in a Single Large-Scale Evaluation Study. Eval. Rev. 1985, 9, 627–643. [Google Scholar] [CrossRef]
- Pouyanfar, S.; Sadiq, A.; Yan, Y.; Tian, H.; Tao, Y.; Reyes, M.P.; Shyu, M.-L.; Chen, S.-C.; Iyengar, S.S. A survey on deep learning: Algorithms, techniques, and applications. ACM Comput. Surv. 2018, 51, 1–36. [Google Scholar] [CrossRef]
- Kasabov, N.K. NeuCube: A spiking neural network architecture for mapping, learning and understanding of spatio-temporal brain data. Neural Netw. 2014, 52, 62–76. [Google Scholar] [CrossRef]
- Doborjeh, Z.G.; Kasabov, N.; Doborjeh, M.G.; Sumich, A. Modelling Peri-Perceptual Brain Processes in a Deep Learning Spiking Neural Network Architecture. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Doborjeh, Z.G.; Doborjeh, M.G.; Kasabov, N. Attentional Bias Pattern Recognition in Spiking Neural Networks from Spatio-Temporal EEG Data. Cogn. Comput. 2018, 10, 35–48. [Google Scholar] [CrossRef]
- Doborjeh, Z.; Doborjeh, M.; Taylor, T.; Kasabov, N.; Wang, G.Y.; Siegert, R.; Sumich, A. Spiking Neural Network Modelling Approach Reveals How Mindfulness Training Rewires the Brain. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Capecci, E.; Doborjeh, Z.; Mammone, N.; la Foresta, F.; Morabito, F.C.; Kasabov, N. Longitudinal study of Alzheimer’s disease degeneration through EEG data analysis with a NeuCube spiking neural network model. In Proceedings of the 2016 International Joint Conference on Neural Networks (IJCNN), Vancouver, BC, Canada, 24–29 July 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1360–1366. [Google Scholar]
- Petro, B.; Kasabov, N.; Kiss, R.M. Selection and Optimization of Temporal Spike Encoding Methods for Spiking Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2019, 31, 358–370. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging: An Approach to Cerebral Imaging; Thieme: Stuttgart, Germany, 1988. [Google Scholar]
- Liao, X.; Vasilakos, A.V.; He, Y. Small-world human brain networks: Perspectives and challenges. Neurosci. Biobehav. Rev. 2017, 77, 286–300. [Google Scholar] [CrossRef]
- Doborjeh, M.G.; Kasabov, N.; Doborjeh, Z.G. Evolving, dynamic clustering of spatio/spectro-temporal data in 3D spiking neural network models and a case study on EEG data. Evol. Syst. 2018, 9, 195–211. [Google Scholar] [CrossRef]
- Doborjeh, Z. Modelling of Spatiotemporal EEG and ERP Brain Data for Dynamic Pattern Recognition and Brain State Prediction using Spiking Neural Networks: Methods and Applications in Psychology. Ph.D. Thesis, Auckland University of Technology, Auckland, New Zealand, 2019. [Google Scholar]
- Kasabov, N.K.; Doborjeh, M.G.; Doborjeh, Z.G. Mapping, Learning, Visualization, Classification, and Understanding of fMRI Data in the NeuCube Evolving Spatiotemporal Data Machine of Spiking Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2016, 28, 887–899. [Google Scholar] [CrossRef]
- Kasabov, N.; Zhou, L.; Doborjeh, M.G.; Doborjeh, Z.G.; Yang, J. New Algorithms for Encoding, Learning and Classification of fMRI Data in a Spiking Neural Network Architecture: A Case on Modeling and Understanding of Dynamic Cognitive Processes. IEEE Trans. Cogn. Dev. Syst. 2016, 9, 293–303. [Google Scholar] [CrossRef]
- Kaiser, D.A. Cortical Cartography. Biofeedback 2010, 38, 9–12. [Google Scholar] [CrossRef]
- Rossion, B.; Collins, D.; Goffaux, V.; Curran, T. Long-term Expertise with Artificial Objects Increases Visual Competition with Early Face Categorization Processes. J. Cogn. Neurosci. 2007, 19, 543–555. [Google Scholar] [CrossRef][Green Version]
- Grill-Spector, K.; Weiner, K.S. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci. 2014, 15, 536–548. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Durai, M.; Linford, T. A State-of-the-Art Review: Personalization of Tinnitus Sound Therapy. Front. Psychol. 2017, 8, 1599. [Google Scholar] [CrossRef]
- Durai, M.; Sanders, P.; Doborjeh, Z.; Doborjeh, M.; Wendt, A.; Kasabov, N.; Searchfield, G.D. Prediction of tinnitus masking benefit within a case series using a spiking neural network model. In Neuroendocrinology—Pathological Situations and Diseases; Elsevier BV: Amsterdam, The Netherlands, 2021; Volume 260, pp. 129–165. [Google Scholar]
- Sanders, P.; Doborjeh, Z.; Doborjeh, M.; Kasabov, N.; Searchfield, G. Prediction of Acoustic Residual Inhibition of Tinnitus Using a Brain-Inspired Spiking Neural Network Model. Brain Sci. 2021, 11, 52. [Google Scholar] [CrossRef]
- Husain, F.T.; Schmidt, S.A. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 2014, 307, 153–162. [Google Scholar] [CrossRef]
- Schecklmann, M.; Landgrebe, M.; Poeppl, T.B.; Kreuzer, P.; Männer, P.; Marienhagen, J.; Wack, D.S.; Kleinjung, T.; Hajak, G.; Langguth, B. Neural correlates of tinnitus duration and Distress: A positron emission tomography study. Hum. Brain Mapp. 2011, 34, 233–240. [Google Scholar] [CrossRef]
- Geven, L.; de Kleine, E.; Willemsen, A.; van Dijk, P. Asymmetry in primary auditory cortex activity in tinnitus patients and controls. Neuroscience 2014, 256, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hofmeier, B.; Wolpert, S.; Aldamer, E.S.; Walter, M.; Thiericke, J.; Braun, C.; Zelle, D.; Rüttiger, L.; Klose, U.; Knipper, M. Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. NeuroImage Clin. 2018, 20, 637–649. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durai, M.; Doborjeh, Z.; Sanders, P.J.; Vajsakovic, D.; Wendt, A.; Searchfield, G.D. Behavioral Outcomes and Neural Network Modeling of a Novel, Putative, Recategorization Sound Therapy. Brain Sci. 2021, 11, 554. https://doi.org/10.3390/brainsci11050554
Durai M, Doborjeh Z, Sanders PJ, Vajsakovic D, Wendt A, Searchfield GD. Behavioral Outcomes and Neural Network Modeling of a Novel, Putative, Recategorization Sound Therapy. Brain Sciences. 2021; 11(5):554. https://doi.org/10.3390/brainsci11050554
Chicago/Turabian StyleDurai, Mithila, Zohreh Doborjeh, Philip J. Sanders, Dunja Vajsakovic, Anne Wendt, and Grant D. Searchfield. 2021. "Behavioral Outcomes and Neural Network Modeling of a Novel, Putative, Recategorization Sound Therapy" Brain Sciences 11, no. 5: 554. https://doi.org/10.3390/brainsci11050554
APA StyleDurai, M., Doborjeh, Z., Sanders, P. J., Vajsakovic, D., Wendt, A., & Searchfield, G. D. (2021). Behavioral Outcomes and Neural Network Modeling of a Novel, Putative, Recategorization Sound Therapy. Brain Sciences, 11(5), 554. https://doi.org/10.3390/brainsci11050554







