Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Characteristics
2.3. Motion Analysis
2.4. Statistics
3. Results
3.1. Differences in Gait Parameters among the SGs
3.1.1. Quasi-Joint Stiffness
3.1.2. Spatiotemporal Parameters
3.1.3. Kinematic Parameters
3.1.4. Kinetic Parameters
3.2. Differences in Demographic Data and Clinical Features among the Subgroups
3.2.1. Demographic Data
3.2.2. Clinical Features
3.2.3. Use of AFOs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Lord, S.E.; McPherson, K.; McNaughton, H.K.; Rochester, L.; Weatherall, M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef]
- Barclay, R.E.; Stevenson, T.J.; Poluha, W.; Ripat, J.; Nett, C.; Srikesavan, C.S. Interventions for improving community ambulation in individuals with stroke. Cochrane Database Syst. Rev. 2015, Cd010200. [Google Scholar] [CrossRef]
- Awad, L.N.; Binder-Macleod, S.A.; Pohlig, R.T.; Reisman, D.S. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil. Neural Repair 2015, 29, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Fulk, G.D.; He, Y.; Boyne, P.; Dunning, K. Predicting Home and Community Walking Activity Poststroke. Stroke 2017, 48, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.; Knarr, B.A.; Higginson, J.S.; Binder-Macleod, S.A. Mechanisms to increase propulsive force for individuals poststroke. J. Neuroeng. Rehabil. 2015, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Muraki, T.; Kuramatsu, Y.; Furusawa, Y.; Izumi, S. The contribution of quasi-joint stiffness of the ankle joint to gait in patients with hemiparesis. Clin. Biomech. 2012, 27, 495–499. [Google Scholar] [CrossRef]
- Daryabor, A.; Yamamoto, S.; Orendurff, M.; Kobayashi, T. Effect of types of ankle-foot orthoses on energy expenditure metrics during walking in individuals with stroke: A systematic review. Disabil. Rehabil. 2020, 1–11. [Google Scholar] [CrossRef]
- Wada, Y.; Otaka, Y.; Mukaino, M.; Tsujimoto, Y.; Shiroshita, A.; Kawate, N.; Taito, S. The effect of ankle-foot orthosis on ankle kinematics in stroke individuals: A systematic review and meta-analysis. PMR J. Inj. Funct. Rehabil. 2021. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Effectiveness of an ankle-foot orthosis on walking in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef] [PubMed]
- Totah, D.; Menon, M.; Jones-Hershinow, C.; Barton, K.; Gates, D.H. The impact of ankle-foot orthosis stiffness on gait: A systematic literature review. Gait Posture 2019, 69, 101–111. [Google Scholar] [CrossRef]
- Bregman, D.J.; Harlaar, J.; Meskers, C.G.; de Groot, V. Spring-like Ankle Foot Orthoses reduce the energy cost of walking by taking over ankle work. Gait Posture 2012, 35, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daryabor, A.; Arazpour, M.; Aminian, G. Effect of different designs of ankle-foot orthoses on gait in patients with stroke: A systematic review. Gait Posture 2018, 62, 268–279. [Google Scholar] [CrossRef]
- Tyson, S.F.; Sadeghi-Demneh, E.; Nester, C.J. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin. Rehabil. 2013, 27, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Muraki, T.; Owaki, D.; Honda, K.; Izumi, S.I. Regulation of quasi-joint stiffness by combination of activation of ankle muscles in midstances during gait in patients with hemiparesis. Gait Posture 2018, 62, 378–383. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Muraki, T.; Tanaka, N.; Izumi, S. Relationship between activation of ankle muscles and quasi-joint stiffness in early and middle stances during gait in patients with hemiparesis. Gait Posture 2015, 42, 348–353. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Owaki, D.; Honda, K.; Fukushi, K.; Hiroi, N.; Nozaki, T.; Izumi, S.I. Ankle-foot orthosis with dorsiflexion resistance using spring-cam mechanism increases knee flexion in the swing phase during walking in stroke patients with hemiplegia. Gait Posture 2020, 81, 27–32. [Google Scholar] [CrossRef]
- Perry, J.; Schoneberger, B. GAIT ANALYSIS: Normal and Pathological Function; Slack Inc.: Thorofare, NJ, USA, 1992. [Google Scholar]
- Kobayashi, T.; Leung, A.K.; Akazawa, Y.; Hutchins, S.W. The effect of varying the plantarflexion resistance of an ankle-foot orthosis on knee joint kinematics in patients with stroke. Gait Posture 2013, 37, 457–459. [Google Scholar] [CrossRef]
- Kobayashi, T.; Singer, M.L.; Orendurff, M.S.; Gao, F.; Daly, W.K.; Foreman, K.B. The effect of changing plantarflexion resistive moment of an articulated ankle-foot orthosis on ankle and knee joint angles and moments while walking in patients post stroke. Clin. Biomech. 2015, 30, 775–780. [Google Scholar] [CrossRef] [Green Version]
- De Quervain, I.A.; Simon, S.R.; Leurgans, S.; Pease, W.S.; McAllister, D. Gait pattern in the early recovery period after stroke. J. Bone Joint Surg. Am. 1996, 78, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsella, S.; Moran, K. Gait pattern categorization of stroke participants with equinus deformity of the foot. Gait Posture 2008, 27, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, S.; Gronley, J.; Weiss, W.; Newsam, C.; Perry, J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture 2003, 18, 114–125. [Google Scholar] [CrossRef]
- Little, V.L.; McGuirk, T.E.; Perry, L.A.; Patten, C. Pelvic excursion during walking post-stroke: A novel classification system. Gait Posture 2018, 62, 395–404. [Google Scholar] [CrossRef]
- Wang, F.C.; Chen, S.F.; Lin, C.H.; Shih, C.J.; Lin, A.C.; Yuan, W.; Li, Y.C.; Kuo, T.Y. Detection and Classification of Stroke Gaits by Deep Neural Networks Employing Inertial Measurement Units. Sensors 2021, 21, 1864. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Liu, M.; Sonoda, S.; Domen, K.; Chino, N. The stroke impairment assessment set: Its internal consistency and predictive validity. Arch. Phys. Med. Rehabil. 2000, 81, 863–868. [Google Scholar] [CrossRef]
- Dumas, R.; Cheze, L.; Verriest, J.P. Adjustments to McConville et al. and Young et al. body segment inertial parameters. J. Biomech. 2007, 40, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Sekiguchi, Y.; Muraki, T.; Izumi, S.I. The differences in sagittal plane whole-body angular momentum during gait between patients with hemiparesis and healthy people. J. Biomech. 2019, 86, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Luc-Harkey, B.A.; Franz, J.R.; Blackburn, J.T.; Padua, D.A.; Hackney, A.C.; Pietrosimone, B. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. J. Biomech. 2018, 76, 94–102. [Google Scholar] [CrossRef]
- Robertson, G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S. Research Methods in Biomechanics; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Benedetti, M.G.; Catani, F.; Leardini, A.; Pignotti, E.; Giannini, S. Data management in gait analysis for clinical applications. Clin. Biomech. 1998, 13, 204–215. [Google Scholar] [CrossRef]
- Kerrigan, D.C.; Frates, E.P.; Rogan, S.; Riley, P.O. Hip hiking and circumduction: Quantitative definitions. Am. J. Phys. Med. Rehabil. 2000, 79, 247–252. [Google Scholar] [CrossRef]
- Jauhiainen, S.; Pohl, A.J.; Äyrämö, S.; Kauppi, J.P.; Ferber, R. A hierarchical cluster analysis to determine whether injured runners exhibit similar kinematic gait patterns. Scand. J. Med. Sci. Sports 2020, 30, 732–740. [Google Scholar] [CrossRef]
- Lydersen, S.; Pradhan, V.; Senchaudhuri, P.; Laake, P. Choice of test for association in small sample unordered r × c tables. Stat. Med. 2007, 26, 4328–4343. [Google Scholar] [CrossRef] [PubMed]
- Lydersen, S.; Fagerland, M.W.; Laake, P. Recommended tests for association in 2 × 2 tables. Stat. Med. 2009, 28, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsen, S.; Flå, T.; Willassen, N.P. DeltaProt: A software toolbox for comparative genomics. BMC Bioinform. 2010, 11, 573. [Google Scholar] [CrossRef] [Green Version]
- Thorvaldsen, S. Fisher’s Exact with Mid-P Method. 2010. Available online: https://jp.mathworks.com/matlabcentral/fileexchange/29819-fisher-s-exact-with-mid-p-method (accessed on 15 August 2021).
- Kaczmarczyk, K.; Wit, A.; Krawczyk, M.; Zaborski, J. Gait classification in post-stroke patients using artificial neural networks. Gait Posture 2009, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Shorter, A.L.; Richardson, J.K.; Finucane, S.B.; Joshi, V.; Gordon, K.; Rouse, E.J. Characterization and clinical implications of ankle impedance during walking in chronic stroke. Sci. Rep. 2021, 11, 16726. [Google Scholar] [CrossRef]
- Cooper, A.; Alghamdi, G.A.; Alghamdi, M.A.; Altowaijri, A.; Richardson, S. The relationship of lower limb muscle strength and knee joint hyperextension during the stance phase of gait in hemiparetic stroke patients. Physiother. Res. Int. 2012, 17, 150–156. [Google Scholar] [CrossRef]
- Kuo, A.; Donelan, J.; Ruina, A. Energetic consequences of walking like an inverted pendulum: Step-to-step transitions. Exerc. Sport Sci. Rev. 2005, 33, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ohata, K.; Yasui, T.; Tsuboyama, T.; Ichihashi, N. Effects of an ankle-foot orthosis with oil damper on muscle activity in adults after stroke. Gait Posture 2011, 33, 102–107. [Google Scholar] [CrossRef]
- Kobayashi, T.; Orendurff, M.S.; Hunt, G.; Lincoln, L.S.; Gao, F.; LeCursi, N.; Foreman, K.B. An articulated ankle-foot orthosis with adjustable plantarflexion resistance, dorsiflexion resistance and alignment: A pilot study on mechanical properties and effects on stroke hemiparetic gait. Med. Eng. Phys. 2017, 44, 94–101. [Google Scholar] [CrossRef]
- Campanini, I.; Merlo, A.; Damiano, B. A method to differentiate the causes of stiff-knee gait in stroke patients. Gait Posture 2013, 38, 165–169. [Google Scholar] [CrossRef]
- Anderson, F.C.; Goldberg, S.R.; Pandy, M.G.; Delp, S.L. Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: An induced position analysis. J. Biomech. 2004, 37, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Orendurff, M.S.; Singer, M.L.; Gao, F.; Hunt, G.; Foreman, K.B. Effect of plantarflexion resistance of an ankle-foot orthosis on ankle and knee joint power during gait in individuals post-stroke. J. Biomech. 2018, 75, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, K.; Mukaino, M.; Ohtsuka, K.; Matsuda, F.; Tanikawa, H.; Yamada, J.; Pongpipatpaiboon, K.; Kanada, Y.; Saitoh, E.; Otaka, Y. Effects of ankle-foot orthoses on the stability of post-stroke hemiparetic gait. Eur. J. Phys. Rehabil. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
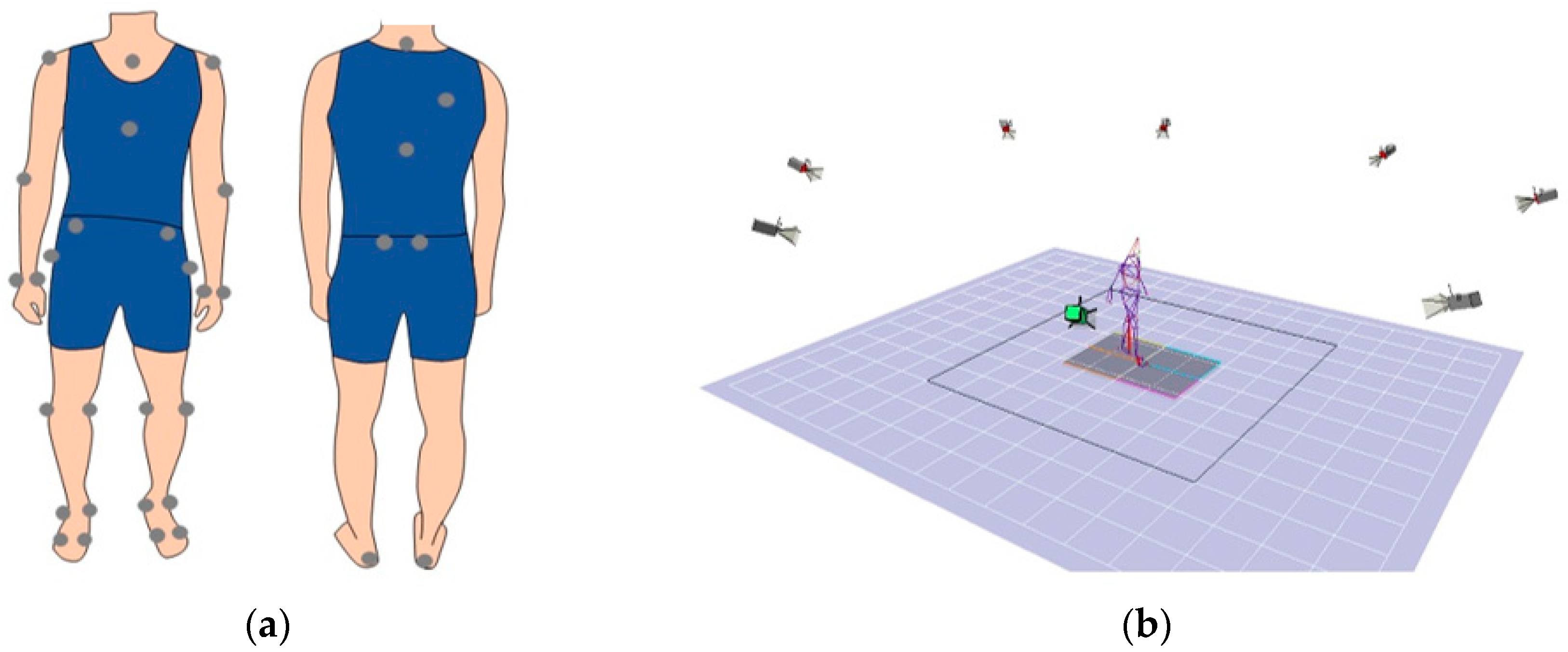


| Segment | Placement of Markers |
|---|---|
| Trunk | Spinous process of the 7th cervical vertebrae, spinous process of the 10th thoracic vertebrae, jugular notch where the clavicles meet the sternum, xiphoid process of the sternum, and the position in the middle of the right scapula |
| Upper arm | Both acromions and both lateral epicondyles of elbow |
| Forearm | Both lateral epicondyles of the elbow and both styloid processes of the ulna and radius |
| Pelvis | Both anterior superior iliac spines and both posterior superior iliac spines |
| Thigh | Both greater trochanters and both lateral and medial epicondyles of knee |
| Shank | Both lateral epicondyles of knee and both lateral and medial malleolus |
| Foot | Both the first and fifth metatarsal heads, both lateral and medial malleolus, and both calcaneus |
| Group | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG1 | SG2 | SG3 | ANOVA | p-Values of the Post Hoc Test | ||||||
| F-Value | p-Value | η2 | SG1 vs. SG2 | SG1 vs. SG3 | SG2 vs. SG3 | |||||
| Stance time (s) | Mean | 0.78 | 0.84 | 1.31 | 9.084 | <0.001 | 0.208 | 1.000 | 0.019 | 0.001 |
| SD | 0.13 | 0.14 | 0.66 | |||||||
| Swing time (s) | Mean | 0.50 | 0.51 | 0.60 | 6.695 | 0.002 | 0.163 | 1.000 | 0.069 | 0.004 |
| SD | 0.06 | 0.09 | 0.12 | |||||||
| Single stance time (s) | Mean | 0.38 | 0.43 | 0.34 | 5.057 | 0.009 | 0.128 | 0.763 | 1.000 | 0.007 |
| SD | 0.08 | 0.05 | 0.14 | |||||||
| Initial double stance time (s) | SD | 0.17 | 0.20 | 0.53 | 11.263 | <0.001 | 0.246 | 1.000 | 0.010 | <0.001 |
| SD | 0.07 | 0.07 | 0.42 | |||||||
| Late double stance time (s) | Mean | 0.24 | 0.22 | 0.44 | 8.613 | <0.001 | 0.200 | 1.000 | 0.065 | 0.001 |
| SD | 0.16 | 0.09 | 0.29 | |||||||
| Walking speed (m/s) | Mean | 0.70 | 0.67 | 0.31 | 25.99 | <0.001 | 0.430 | 1.000 | <0.001 | <0.001 |
| SD | 0.31 | 0.23 | 0.18 | |||||||
| Step length (m) | ||||||||||
| Paretic side | Mean | 0.44 | 0.43 | 0.27 | 18.525 | <0.001 | 0.349 | 1.000 | 0.001 | <0.001 |
| SD | 0.12 | 0.11 | 0.13 | |||||||
| Non-paretic side | Mean | 0.39 | 0.42 | 0.22 | 25.974 | <0.001 | 0.430 | 1.000 | 0.001 | <0.001 |
| SD | 0.17 | 0.10 | 0.11 | |||||||
| Stride length (m) | Mean | 0.86 | 0.87 | 0.51 | 25.410 | <0.001 | 0.424 | 1.000 | <0.001 | <0.001 |
| SD | 0.28 | 0.21 | 0.20 | |||||||
| Cadence (steps/min) | Mean | 95.55 | 91.05 | 69.23 | 16.969 | <0.001 | 0.330 | 1.000 | <0.001 | <0.001 |
| SD | 14.74 | 13.93 | 18.93 | |||||||
| Step width (m) | Mean | 0.15 | 0.14 | 0.18 | 6.944 | 0.002 | 0.168 | 1.000 | 0.120 | 0.002 |
| SD | 0.04 | 0.03 | 0.05 | |||||||
| Group | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG1 | SG2 | SG3 | ANOVA | p-Values of the Post Hoc Test | ||||||
| F-Value | p-Value | η2 | SG1 vs. SG2 | SG1 vs. SG3 | SG2 vs. SG3 | |||||
| Maximum ankle plantarflexion in early stance (°) | Mean | 1.49 | 3.32 | 6.01 | 2.304 | 0.107 | 0.063 | 1.000 | 0.236 | 0.312 |
| SD | 5.93 | 5.18 | 7.42 | |||||||
| Maximum ankle dorsiflexion in stance (°) | Mean | −12.01 | −15.14 | −12.45 | 1.461 | 0.239 | 0.041 | 0.752 | 1.000 | 0.353 |
| SD | 7.73 | 4.63 | 7.82 | |||||||
| Ankle plantarflexion at toe off (°) | Mean | 1.82 | 1.00 | −1.12 | 0.756 | 0.473 | 0.021 | 1.000 | 1.000 | 0.912 |
| SD | 9.54 | 7.56 | 8.17 | |||||||
| Maximum ankle dorsiflexion in swing (°) | Mean | −5.09 | −5.17 | −2.77 | 1.027 | 0.364 | 0.029 | 1.000 | 1.000 | 0.544 |
| SD | 7.20 | 5.85 | 7.84 | |||||||
| Ankle angle excursion (°) | Mean | 17.51 | 21.99 | 23.84 | 2.255 | 0.113 | 0.061 | 0.460 | 0.121 | 1.000 |
| SD | 6.72 | 6.74 | 8.62 | |||||||
| Knee flexion at heel contact (°) | Mean | −10.97 | −12.26 | −7.50 | 3.417 | 0.038 | 0.090 | 1.000 | 0.696 | 0.037 |
| SD | 5.08 | 4.81 | 9.17 | |||||||
| Maximum knee flexion in early stance (°) | Mean | −15.63 | −17.61 | −11.83 | 3.085 | 0.052 | 0.082 | 1.000 | 0.904 | 0.050 |
| SD | 5.26 | 6.08 | 11.75 | |||||||
| Maximum knee extension in stance (°) | Mean | −1.21 | −6.26 | −1.82 | 2.856 | 0.064 | 0.076 | 0.346 | 1.000 | 0.087 |
| SD | 5.47 | 6.08 | 9.40 | |||||||
| Knee flexion at toe off (°) | Mean | −27.59 | −31.26 | −19.17 | 10.849 | <0.001 | 0.236 | 1.000 | 0.129 | <0.001 |
| SD | 10.23 | 7.26 | 12.41 | |||||||
| Maximum knee flexion in swing (°) | Mean | −39.07 | −43.64 | −26.36 | 14.455 | <0.001 | 0.295 | 1.000 | 0.044 | <0.001 |
| SD | 13.55 | 10.74 | 14.40 | |||||||
| Knee angle excursion (°) | Mean | 38.21 | 38.48 | 25.57 | 11.205 | <0.001 | 0.245 | 1.000 | 0.019 | <0.001 |
| SD | 13.20 | 11.11 | 11.47 | |||||||
| Hip flexion at heel contact (°) | Mean | −28.88 | −28.64 | −23.84 | 3.312 | 0.042 | 0.088 | 1.000 | 0.337 | 0.060 |
| SD | 4.21 | 7.91 | 8.65 | |||||||
| Maximum hip extension in stance (°) | Mean | −2.37 | −1.85 | −9.91 | 8.726 | <0.001 | 0.202 | 1.000 | 0.059 | 0.001 |
| SD | 8.19 | 7.62 | 8.38 | |||||||
| Hip flexion at toe off (°) | Mean | −9.79 | −9.67 | −15.07 | 3.731 | 0.029 | 0.098 | 1.000 | 0.330 | 0.037 |
| SD | 7.87 | 8.15 | 8.59 | |||||||
| Maximum hip flexion during swing (°) Hip angle excursion (°) | Mean | −33.35 | −32.72 | −28.89 | 2.238 | 0.114 | 0.061 | 1.000 | 0.475 | 0.184 |
| SD | 4.95 | 7.37 | 8.91 | |||||||
| Mean | 31.04 | 31.13 | 19.74 | 20.642 | <0.001 | 0.374 | 1.000 | 0.001 | <0.001 | |
| SD | 7.99 | 6.45 | 8.14 | |||||||
| Pelvis hiking (°) | Mean | 5.06 | 3.10 | 5.30 | 3.280 | 0.044 | 0.087 | 0.499 | 1.000 | 0.044 |
| SD | 2.28 | 3.40 | 3.75 | |||||||
| Leg circumduction (°) | Mean | 11.54 | 9.58 | 13.01 | 4.985 | 0.010 | 0.126 | 0.786 | 1.000 | 0.007 |
| SD | 5.19 | 3.83 | 4.49 | |||||||
| Group | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG1 | SG2 | SG3 | ANOVA | p-Values of the Post Hoc Test | ||||||
| F-Value | p-Value | η2 | SG1 vs. SG2 | SG1 vs. SG3 | SG2 vs. SG3 | |||||
| Maximum hip extension moment in early stance (Nm/kg) | Mean | 0.84 | 0.61 | 0.47 | 7.235 | 0.001 | 0.173 | 0.085 | 0.002 | 0.123 |
| SD | 0.12 | 0.30 | 0.25 | |||||||
| Maximum hip flexion moment in stance phase (Nm/kg) | Mean | −0.52 | −0.60 | −0.33 | 12.048 | <0.001 | 0.259 | 1.000 | 0.098 | <0.001 |
| SD | 0.30 | 0.21 | 0.20 | |||||||
| First maximum knee extension moment in stance phase (Nm/kg) | Mean | 0.27 | 0.33 | 0.18 | 3.607 | 0.032 | 0.095 | 1.000 | 0.798 | 0.029 |
| SD | 0.23 | 0.18 | 0.23 | |||||||
| Maximum knee flexion moment in stance phase (Nm/kg) | Mean | −0.36 | −0.05 | −0.13 | 2.265 | 0.112 | 0.062 | 0.112 | 0.336 | 1.000 |
| SD | 0.31 | 0.29 | 0.43 | |||||||
| Second maximum knee extension moment in stance phase (Nm/kg) | Mean | 0.17 | 0.28 | 0.22 | 1.272 | 0.287 | 0.036 | 0.530 | 1.000 | 0.642 |
| SD | 0.14 | 0.16 | 0.23 | |||||||
| Maximum ankle dorsiflexion moment in early stance (Nm/kg) | Mean | −0.06 | −0.04 | −0.01 | 6.024 | 0.004 | 0.149 | 0.487 | 0.010 | 0.048 |
| SD | 0.07 | 0.04 | 0.04 | |||||||
| Maximum ankle plantarflexion moment in stance phase (Nm/kg) | Mean | 1.01 | 0.97 | 0.53 | 47.293 | <0.001 | 0.578 | 1.000 | <0.001 | <0.001 |
| SD | 0.16 | 0.20 | 0.19 | |||||||
| Group | |||
|---|---|---|---|
| SG1 1 | SG2 | SG3 | |
| Number 2 | 8 | 28 | 36 |
| Gender (Male/Female) 2 | 6/2 | 22/6 | 25/11 |
| Age (years) 3 | 52.6(9.5) | 55.7(12.2) | 58.5(11.4) |
| Height (cm) 3 | 162.1(5.6) | 167.8(9.0) | 164.9(7.6) |
| Weight (kg) 3 | 62.1(8.4) | 65.8(10.6) | 61.7(11.9) |
| Diagnosis (Hemorrhage/Infarction) 2 | 6/2 | 18/10 | 18/18 |
| Paretic side (Right/Left) 2 | 3/5 | 17/11 | 21/15 |
| Time since neurologic event (month) 3 | 11.25(18.35) | 35.82(40.98) | 36.31(49.49) |
| SIAS 2,4 | |||
| Motor function (0/1/2/3/4/5) | |||
| Ankle joint | 1/2/0/2/3/0 | 1/3/1/7/13/3 | 5/9/5/11/5/1 |
| Knee joint | 0/0/0/3/4/1 | 0/0/2/7/15/4 | 0/2/3/21/8/2 |
| Hip joint | 0/0/0/3/5/0 | 0/0/1/4/19/4 | 0/1/3/16/15/1 |
| Deep tendon reflex (lower limb) (0/1/2/3) | 0/5/2/1 | 1/10/8/9 | 1/13/18/4 |
| Range of motion (ankle dorsiflexion) (0/1/2/3) | 1/5/2/0 | 2/11/13/2 | 1/19/14/2 |
| SG1 (n = 8) | SG2 (n = 28) | SG3 (n = 36) | p-Value SG 1 vs. 2 | SG 1 vs. 3 | SG 2 vs. 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Non-use of AFO | 3 | 38 | 15 | 54 | 7 | 19 | 0.689 | 0.475 | 0.008 |
| Use of articulate AFO | 5 | 63 | 5 | 18 | 14 | 39 | 0.040 | 0.387 | 0.113 |
| Use of non-articulate AFO | 0 | 0 | 8 | 29 | 15 | 42 | 0.154 | 0.036 | 0.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekiguchi, Y.; Honda, K.; Owaki, D.; Izumi, S.-I. Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke. Brain Sci. 2021, 11, 1512. https://doi.org/10.3390/brainsci11111512
Sekiguchi Y, Honda K, Owaki D, Izumi S-I. Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke. Brain Sciences. 2021; 11(11):1512. https://doi.org/10.3390/brainsci11111512
Chicago/Turabian StyleSekiguchi, Yusuke, Keita Honda, Dai Owaki, and Shin-Ichi Izumi. 2021. "Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke" Brain Sciences 11, no. 11: 1512. https://doi.org/10.3390/brainsci11111512
APA StyleSekiguchi, Y., Honda, K., Owaki, D., & Izumi, S.-I. (2021). Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke. Brain Sciences, 11(11), 1512. https://doi.org/10.3390/brainsci11111512







