Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Resting State Recording
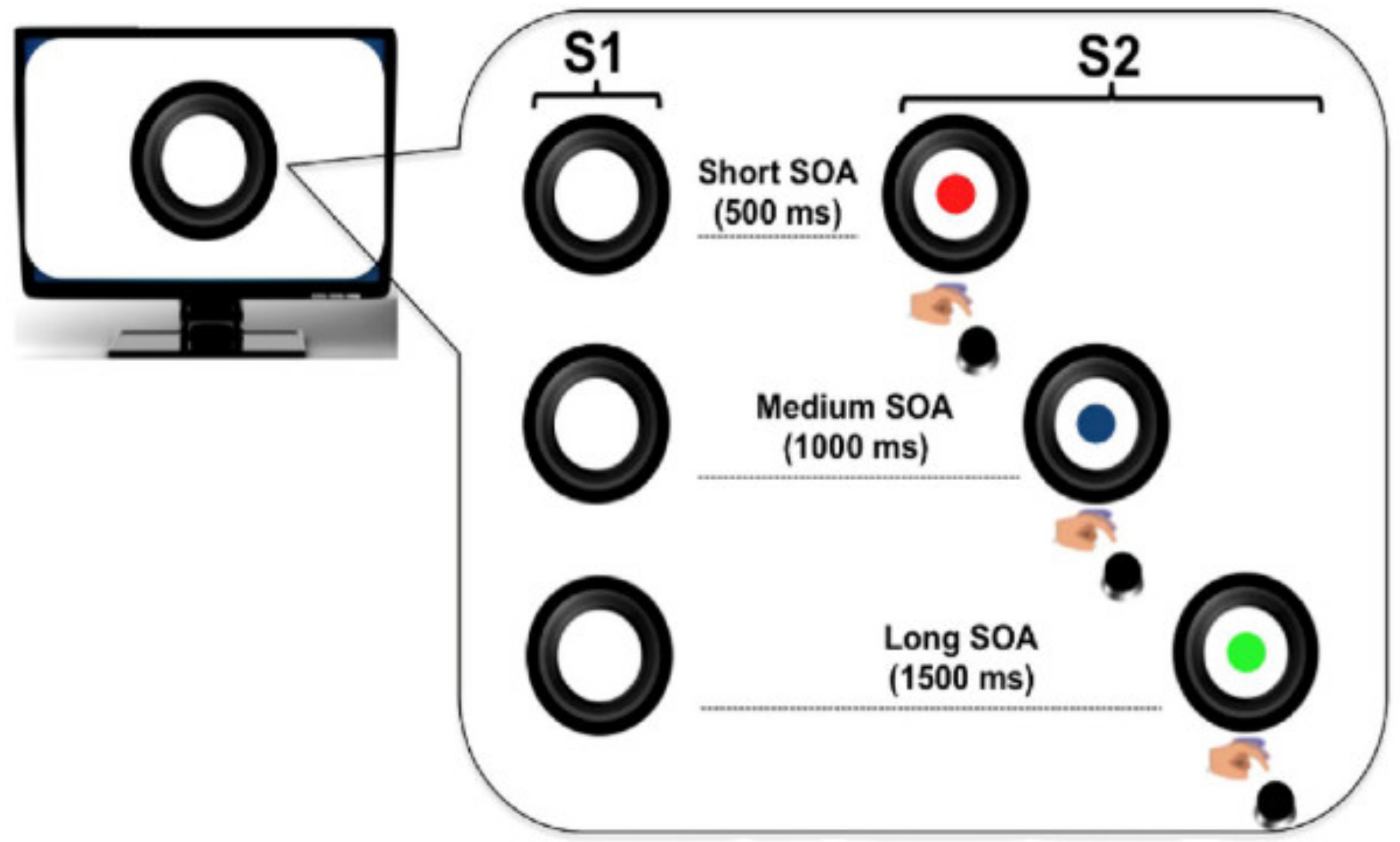
2.3. Experimental Task
2.4. Trial Structure
2.4.1. Local Predictive Context
2.4.2. Global Predictive Context
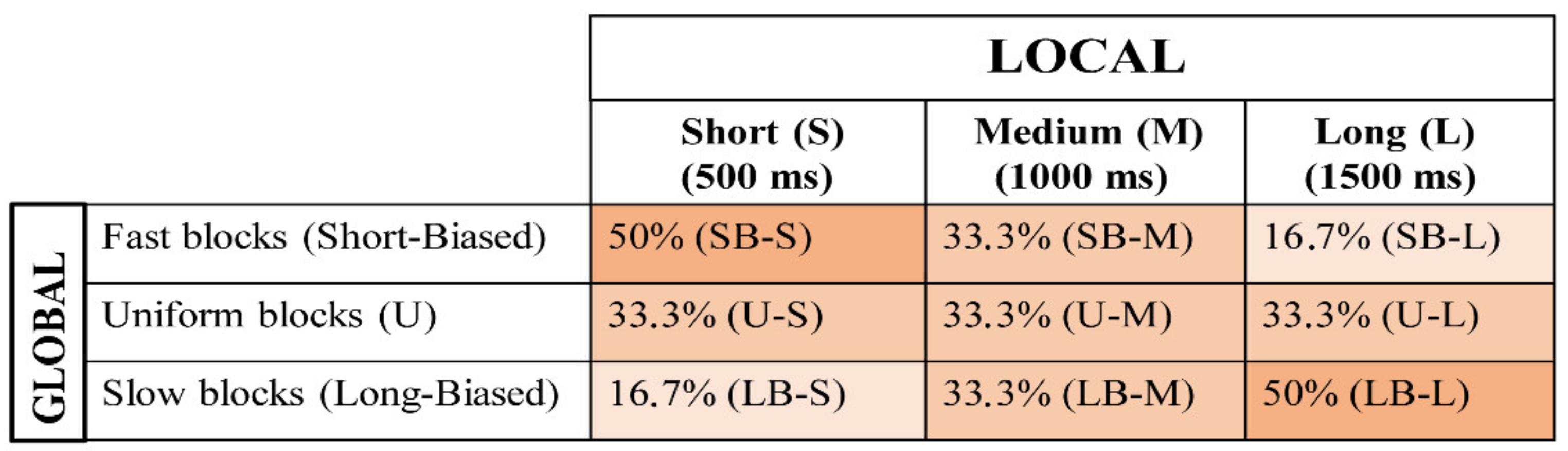
2.4.3. Uniform (U) Blocks
2.4.4. Fast Blocks (Short-Biased or SB)
2.4.5. Slow Blocks (Long-Biased or LB)
2.4.6. Experimental Design
2.5. Predicted Measures
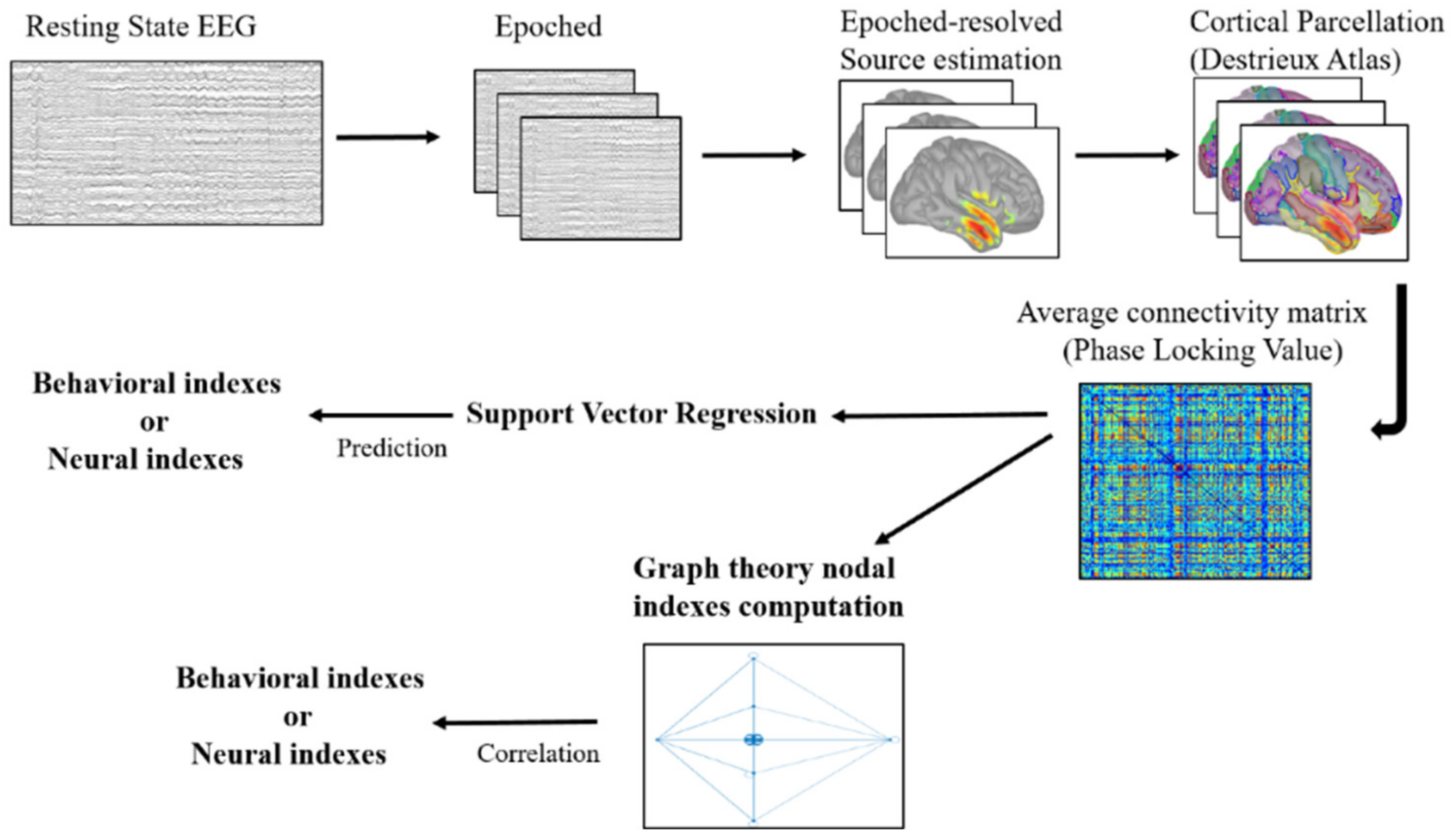
2.6. EEG Resting State Pre-Processing
2.7. Cortical Source Modelling
2.8. Network Definition and Functional Connectivity
2.9. Support Vector Regression
2.10. Graph Theoretical Analysis
2.10.1. Graph Construction
2.10.2. Graph Measures
2.11. Correlation Analysis
3. Results
3.1. Predicting Behavioral Effects from the Networks of Interest
3.2. Predicting Neural Effects from the Networks of Interest
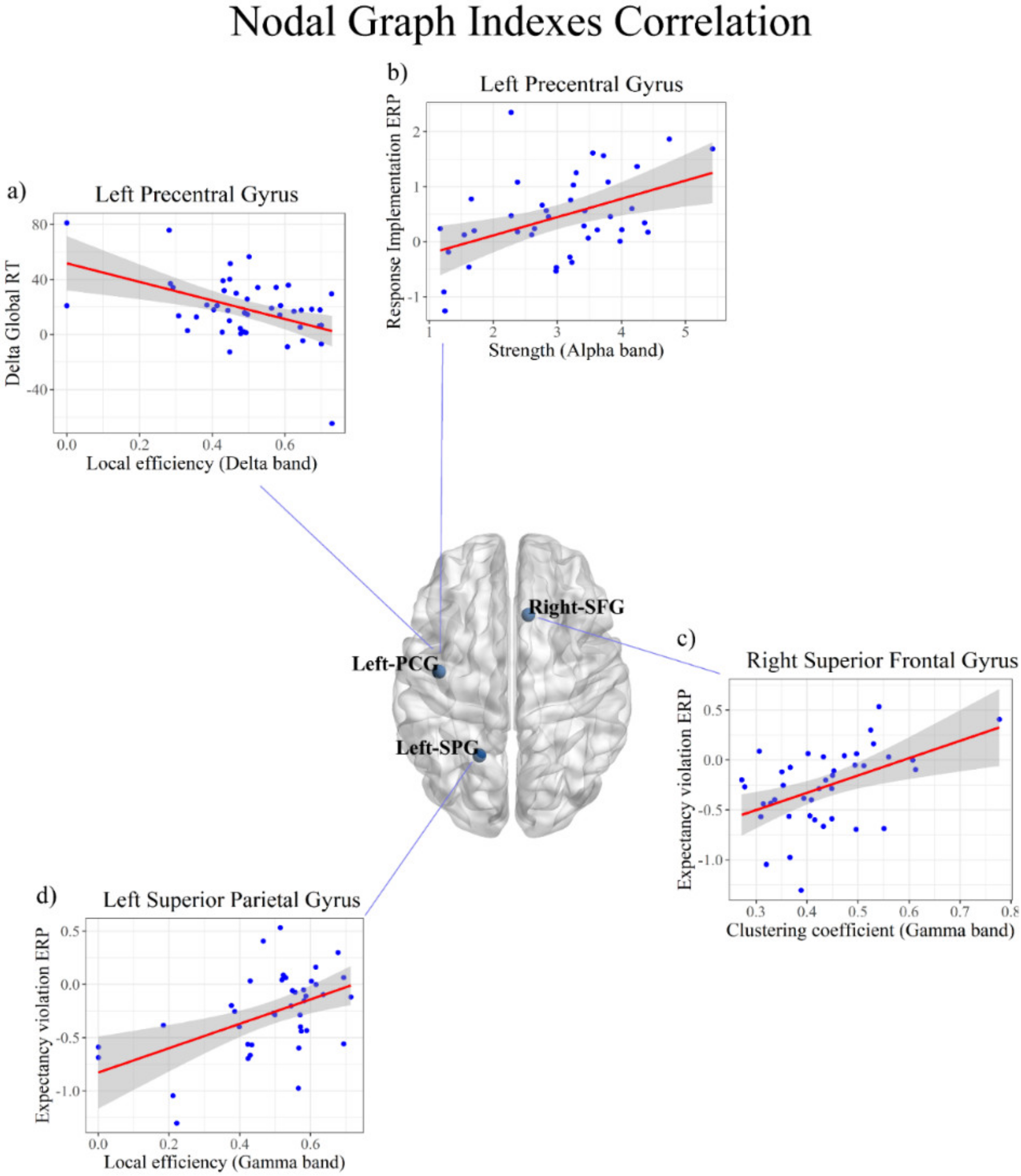
3.3. Correlations between the Local Graph Indexes and Behavioral Effects
3.4. Correlations between the Local Graph Indexes and Neural Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J. Voluntary Versus Automatic Control Over the Mind’s Eye’s Movement. In Attention and Performance; Long, J., Baddeley, A., Eds.; Erlbaum: Hillsdale, NJ, USA, 1981; Volume IX, pp. 187–203. [Google Scholar]
- Norman, D.A.; Shallice, T. Attention to Action. In Consciousness and Self-Regulation; Springer Science and Business Media LLC: Boston, MA, USA, 1986; pp. 1–18. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiffrin, R.M.; Schneider, W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol. Rev. 1977, 84, 127–190. [Google Scholar] [CrossRef]
- Abrahamse, E.; Braem, S.; Notebaert, W.; Verguts, T. Grounding cognitive control in associative learning. Psychol. Bull. 2016, 142, 693–728. [Google Scholar] [CrossRef]
- Crump, M.J.C.; Logan, G. Contextual control over task-set retrieval. Atten. Percept. Psychophys. 2010, 72, 2047–2053. [Google Scholar] [CrossRef]
- Sali, A.W.; Anderson, B.A.; Yantis, S. Learned states of preparatory attentional control. J. Exp. Psychol. Learn. Mem. Cogn. 2015, 41, 1790–1805. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Manly, T. Anticipatory Control Through Associative Learning of Subliminal Relations: Invisible May Be Better Than Visible. Psychol. Sci. 2015, 26, 325–334. [Google Scholar] [CrossRef]
- Braem, S.; Egner, T. Getting a Grip on Cognitive Flexibility. Curr. Dir. Psychol. Sci. 2018, 27, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Duma, G.; Granziol, U.; Mento, G. Should I stay or should I go? How local-global implicit temporal expectancy shapes proactive motor control: An hdEEG study. NeuroImage 2020, 220, 117071. [Google Scholar] [CrossRef] [PubMed]
- Mento, G.; Granziol, U. The developing predictive brain: How implicit temporal expectancy induced by local and global prediction shapes action preparation across development. Dev. Sci. 2020, 23, e12954. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, A.; Lewis, C.; Committeri, G.; Snyder, A.Z.; Romani, G.L.; Corbetta, M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc. Natl. Acad. Sci. USA 2012, 109, 3516–3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, M.D.; Scheinost, D.; Greene, A.S.; Avery, E.; Kwon, Y.H.; Finn, E.S.; Ramani, R.; Qiu, M.; Constable, R.T.; Chun, M.M. Functional connectivity predicts changes in attention observed across minutes, days, and months. Proc. Natl. Acad. Sci. USA 2020, 117, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- González, G.F.; Smit, D.J.A.; van der Molen, M.J.W.; Tijms, J.; Stam, C.J.; de Geus, E.J.C.; van der Molen, M.W. EEG Resting State Functional Connectivity in Adult Dyslexics Using Phase Lag Index and Graph Analysis. Front. Hum. Neurosci. 2018, 12, 341. [Google Scholar] [CrossRef]
- Rogala, J.; Kublik, E.; Krauz, R.; Wróbel, A. Resting-state EEG activity predicts frontoparietal network reconfiguration and improved attentional performance. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, C.; Billinger, M.; Seeber, M.; Mullen, T.R.; Makeig, S. Volume conduction influences scalp-based connectivity estimates. Front. Comput. Neurosci. 2016, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Vanderwal, T.; Kelly, C.; Eilbott, J.; Mayes, L.C.; Castellanos, F. Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. NeuroImage 2015, 122, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Mento, G.; Tarantino, V. Developmental Trajectories of Internally and Externally Driven Temporal Prediction. PLoS ONE 2015, 10, e0135098. [Google Scholar] [CrossRef]
- Mento, G.; Scerif, G.; Granziol, U.; Franzoi, M.; Lanfranchi, S. The Effect of Probabilistic Context on Implicit Temporal Expectations in Down Syndrome. Front. Psychol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Los, S.A. Foreperiod and sequential effects: Theory and data. Atten. Time 2010, 289, 289–302. [Google Scholar] [CrossRef]
- Niemi, P.; Näätänen, R. Foreperiod and simple reaction time. Psychol. Bull. 1981, 89, 133. [Google Scholar] [CrossRef]
- Woodrow, H. The measurement of attention. Psychol. Monogr. 1914, 17, i-158. [Google Scholar] [CrossRef]
- Luce, R.D. Response Times: Their Role in Inferring Elementary Mental Organization (No. 8); Oxford University Press on Demand: Oxford, UK, 1986. [Google Scholar]
- Mento, G. The role of the P3 and CNV components in voluntary and automatic temporal orienting: A high spatial-resolution ERP study. Neuropsychology 2017, 107, 31–40. [Google Scholar] [CrossRef]
- Mento, G.; Tarantino, V.; Vallesi, A.; Bisiacchi, P.S. Spatiotemporal Neurodynamics Underlying Internally and Externally Driven Temporal Prediction: A High Spatial Resolution ERP Study. J. Cogn. Neurosci. 2015, 27, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Vallesi, A.; Shallice, T. Developmental dissociations of preparation over time: Deconstructing the variable foreperiod phenomena. J. Exp. Psychol. Hum. Percept. Perform. 2007, 33, 1377. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Burrowes, E.; Coull, J.T. Children Can Implicitly, but Not Voluntarily, Direct Attention in Time. PLoS ONE 2015, 10, e0123625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mento, G.; Vallesi, A. Spatiotemporally dissociable neural signatures for generating and updating expectation over time in children: A High Density-ERP study. Dev. Cogn. Neurosci. 2016, 19, 98–106. [Google Scholar] [CrossRef]
- Los, S.A.; Kruijne, W.; Meeter, M. Hazard versus history: Temporal preparation is driven by past experience. J. Exp. Psychol. Hum. Percept. Perform. 2017, 43, 78–88. [Google Scholar] [CrossRef]
- Trillenberg, P.; Verleger, R.; Wascher, E.; Wauschkuhn, B.; Wessel, K. CNV and temporal uncertainty with ’ageing’and ’non-ageing’S1–S2 intervals. Clin. Neurophysiol. 2000, 111, 1216–1226. [Google Scholar] [CrossRef]
- Townsend, J.T.; Ashby, F.G. Methods of modeling capacity in simple processing systems. In Cognitive Theory; Castellan, J., Restle, F., Eds.; Erlbaum Associate: Hillsdale, NJ, USA, 1978; Volume III, pp. 200–239. [Google Scholar]
- Vandierendonck, A. A comparison of methods to combine speed and accuracy measures of performance: A rejoinder on the binning procedure. Behav. Res. Methods 2016, 49, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.V. Independent component analysis: An introduction. Trends Cogn. Sci. 2002, 6, 59–64. [Google Scholar] [CrossRef]
- Bell, A.J.; Sejnowski, T.J. An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef]
- Perrin, F.; Pernier, J.; Bertrand, O.; Echallier, J. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 184–187. [Google Scholar] [CrossRef]
- Ferree, T.C. Spherical Splines and Average Referencing in Scalp Electroencephalography. Brain Topogr. 2006, 19, 43–52. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Evans, A.C.; Janke, A.; Collins, D.L.; Baillet, S. Brain templates and atlases. NeuroImage 2012, 62, 911–922. [Google Scholar] [CrossRef]
- Kybic, J.; Clerc, M.; Faugeras, O.; Keriven, R.; Papadopoulo, T.; Papadopoulo, T. Fast multipole acceleration of the MEG/EEG boundary element method. Phys. Med. Biol. 2005, 50, 4695–4710. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. Forward Field Computation with OpenMEEG. Comput. Intell. Neurosci. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Niso, G.; Tadel, F.; Bock, E.; Cousineau, M.; Santos, A.; Baillet, S. Brainstorm Pipeline Analysis of Resting-State Data from the Open MEG Archive. Front. Neurosci. 2019, 13, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 2010, 53, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Albouy, P.; Weiss, A.; Baillet, S.; Zatorre, R.J. Selective Entrainment of Theta Oscillations in the Dorsal Stream Causally Enhances Auditory Working Memory Performance. Neuron 2017, 94, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Duma, G.M.; Mento, G.; Cutini, S.; Sessa, P.; Baillet, S.; Brigadoi, S.; Dell’Acqua, R. Functional dissociation of anterior cingulate cortex and intraparietal sulcus in visual working memory. Cortex 2019, 121, 277–291. [Google Scholar] [CrossRef]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Sain, S.R.; Vapnik, V.N. The Nature of Statistical Learning Theory. Technometrics 1996, 38, 409. [Google Scholar] [CrossRef]
- Yadav, S.; Shukla, S. Analysis of k-Fold Cross-Validation over Hold-Out Validation on Colossal Datasets for Quality Classification. In Proceedings of the 2016 IEEE 6th International Conference on Advanced Computing (IACC), Bhimavaram, India, 27–28 February 2016; pp. 78–83. [Google Scholar]
- Arlot, S.; Celisse, A. A survey of cross-validation procedures for model selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Di Bono, M.G.; Zorzi, M. Decoding Cognitive States from fMRI Data Using Support Vector Regression. PsychNology J. 2008, 6, 189–201. [Google Scholar]
- Jeffreys, H. The Theory of Probability; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Langer, N.; Pedroni, A.; Jancke, L. The Problem of Thresholding in Small-World Network Analysis. PLoS ONE 2013, 8, e53199. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Cainelli, E.; di Bono, M.G.; Bisiacchi, P.S.; Suppiej, A. Electroencephalographic functional connectivity in extreme prematurity: A pilot study based on graph theory. Pediatr. Res. 2019, 87, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.D.; Gurney, K. Network ’Small-World-Ness’: A Quantitative Method for Determining Canonical Network Equivalence. PLoS ONE 2008, 3, e0002051. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Zwi, J.D. The Small World of the Cerebral Cortex. Neuroinformatics 2004, 2, 145–162. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Di Bono, M.G.; Priftis, K.; Umiltà, C. Bridging the Gap between Brain Activity and Cognition: Beyond the Different Tales of fMRI Data Analysis. Front. Neurosci. 2017, 11, 31. [Google Scholar] [CrossRef]
- Braem, S. Conditioning task switching behavior. Cognition 2017, 166, 272–276. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-C.; Egner, T. Cueing cognitive flexibility: Item-specific learning of switch readiness. J. Exp. Psychol. Hum. Percept. Perform. 2017, 43, 1950–1960. [Google Scholar] [CrossRef]
- Mento, G.; Astle, D.E.; Scerif, G. Cross-frequency Phase–Amplitude Coupling as a Mechanism for Temporal Orienting of Attention in Childhood. J. Cogn. Neurosci. 2018, 30, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Attention and Time; Coull, J.T. (Ed.) Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Triviño, M.; Correa, Á.; Lupiáñez, J.; Funes, M.J.; Catena, A.; He, X.; Humphreys, G.W. Brain networks of temporal preparation: A multiple regression analysis of neuropsychological data. NeuroImage 2016, 142, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantini, D.; Perrucci, M.G.; del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Eichele, T.; Calhoun, V.D. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: A concurrent EEG-fMRI study. NeuroImage 2010, 52, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Arcara, G.; Porcaro, C.; Mantini, D. Hemodynamic Correlates of Electrophysiological Activity in the Default Mode Network. Front. Neurosci. 2019, 13, 1060. [Google Scholar] [CrossRef]
- Rohenkohl, G.; Nobre, A.C. Alpha Oscillations Related to Anticipatory Attention Follow Temporal Expectations. J. Neurosci. 2011, 31, 14076–14084. [Google Scholar] [CrossRef] [Green Version]
- De Lange, F.P.; Rahnev, D.A.; Donner, T.H.; Lau, H. Prestimulus Oscillatory Activity over Motor Cortex Reflects Perceptual Expectations. J. Neurosci. 2013, 33, 1400–1410. [Google Scholar] [CrossRef]
- Jann, K.; Koenig, T.; Dierks, T.; Boesch, C.; Federspiel, A. Association of individual resting state EEG alpha frequency and cerebral blood flow. NeuroImage 2010, 51, 365–372. [Google Scholar] [CrossRef]
- Mennes, M.; Kelly, C.; Zuo, X.-N.; di Martino, A.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. NeuroImage 2010, 50, 1690–1701. [Google Scholar] [CrossRef] [Green Version]
- Zou, Q.; Ross, T.J.; Gu, H.; Geng, X.; Zuo, X.-N.; Hong, L.E.; Gao, J.-H.; Stein, E.A.; Zang, Y.-F.; Yang, Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum. Brain Mapp. 2013, 34, 3204–3215. [Google Scholar] [CrossRef] [Green Version]
- Bruns, A.; Eckhorn, R. Task-related coupling from high- to low-frequency signals among visual cortical areas in human subdural recordings. Int. J. Psychophysiol. 2004, 51, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- Putman, P. Resting state EEG delta–beta coherence in relation to anxiety, behavioral inhibition, and selective attentional processing of threatening stimuli. Int. J. Psychophysiol. 2011, 80, 63–68. [Google Scholar] [CrossRef]
- Fell, J.; Klaver, P.; Elfadil, H.; Schaller, C.; Elger, C.E.; Fernández, G. Rhinal-hippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? Eur. J. Neurosci. 2003, 17, 1082–1088. [Google Scholar] [CrossRef]
- Kaiser, J.; Lutzenberger, W. Induced Gamma-Band Activity and Human Brain Function. Neuroscience 2003, 9, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Fries, P.; Nikolic, D.; Singer, W. The gamma cycle. Trends Neurosci. 2007, 30, 309–316. [Google Scholar] [CrossRef]
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009, 32, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Maffei, A. Spectrally resolved EEG intersubject correlation reveals distinct cortical oscillatory patterns during free-viewing of affective scenes. Psychophysiology 2020, 57, e13652. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duma, G.M.; Di Bono, M.G.; Mento, G. Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation. Brain Sci. 2021, 11, 1513. https://doi.org/10.3390/brainsci11111513
Duma GM, Di Bono MG, Mento G. Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation. Brain Sciences. 2021; 11(11):1513. https://doi.org/10.3390/brainsci11111513
Chicago/Turabian StyleDuma, Gian Marco, Maria Grazia Di Bono, and Giovanni Mento. 2021. "Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation" Brain Sciences 11, no. 11: 1513. https://doi.org/10.3390/brainsci11111513
APA StyleDuma, G. M., Di Bono, M. G., & Mento, G. (2021). Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation. Brain Sciences, 11(11), 1513. https://doi.org/10.3390/brainsci11111513





