Resting-State Functional Connectivity following Phonological Component Analysis: The Combined Action of Phonology and Visual Orthographic Cues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.2.1. Language Assessment
2.2.2. Fr-PCA Therapy
2.2.3. Outcome Measures
2.3. Data Acquisition and Preprocessing
2.3.1. Functional Neuroimaging Parameters
2.3.2. Resting-State Acquisitions
2.3.3. Preprocessing
2.4. Data Analysis
2.4.1. Behavioral Responses to Therapy
2.4.2. Functional Connectivity Analysis
3. Results
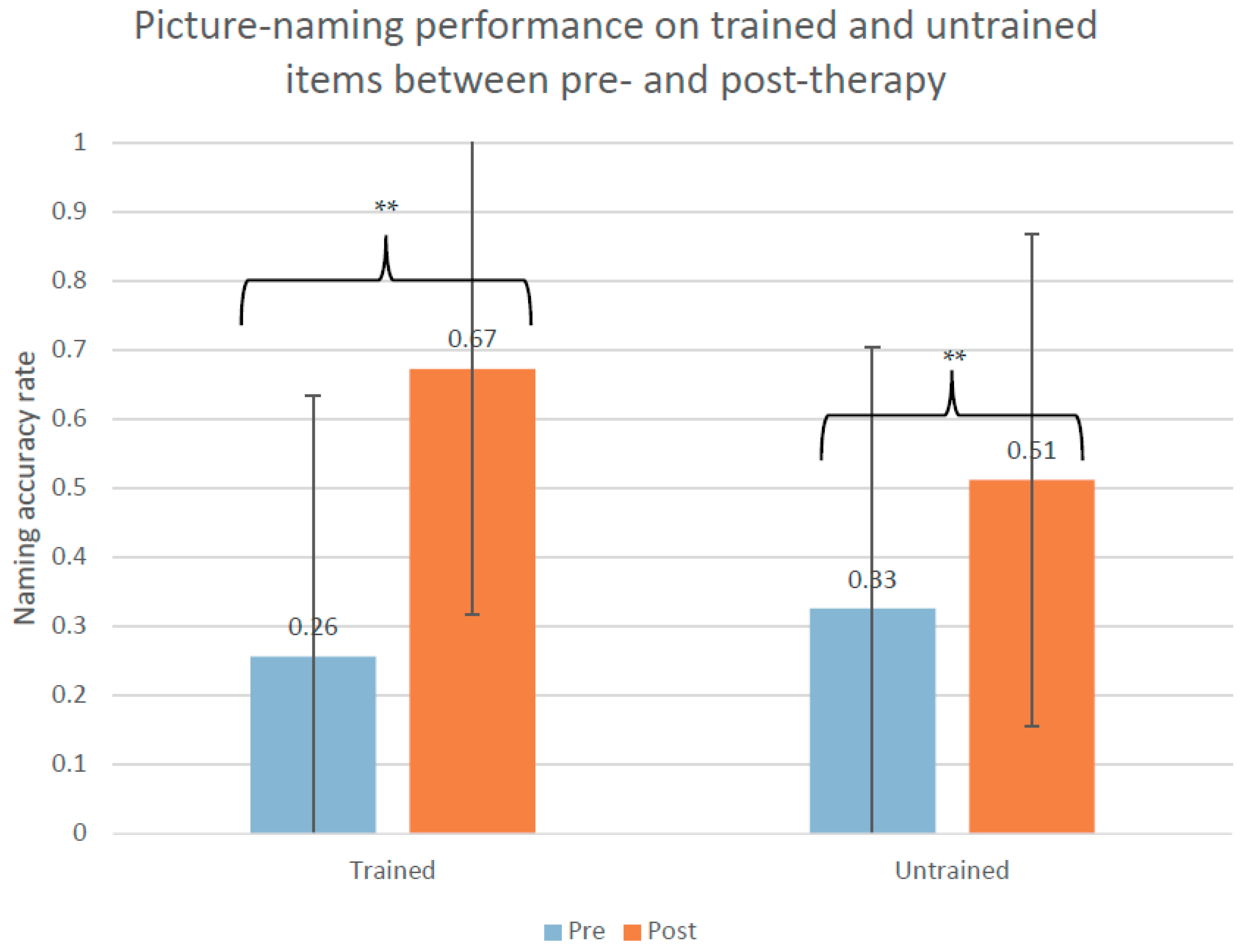
3.1. Behavioral Results
3.2. Functional Connectivity Results
Therapy-Induced rsFC Changes
3.3. Correlations between rsFC Changes and Naming Improvements following Therapy
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| PCA1 | PCA2 | PCA3 | PCA4 | PCA5 | PCA6 | PCA7 | PCA8 | PCA9 | PCA10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post | |
| TQD60 | −11.93 | −5.29 | −19.09 | −16.39 | −12.49 | −7.84 | −25.85 | −18.19 | 0.73 | 0.73 | −14.15 | −6.03 | −0.63 | −0.18 | −0.63 | 0.73 | −22.25 | −21.80 | −10.09 | −5.13 |
| variation | 6.64 | 2.70 | 4.65 | 7.66 | 0.00 | 8.12 | 0.45 | 1.35 | 0.45 | 4.95 | ||||||||||
| DVL38 | 0.37 | 0.45 | −5.79 | −4.68 | −0.04 | 0.10 | −5.68 | −3.02 | 0.05 | 0.60 | −8.34 | −6.63 | −1.07 | 0.32 | 0.21 | 0.21 | −6.63 | −4.68 | −0.70 | −0.05 |
| variation | 0.08 | 1.11 | 0.14 | 2.66 | 0.56 | 1.71 | 1.39 | 0.00 | 1.95 | 0.65 | ||||||||||
| Oral Comp. | −4.60 | −3.32 | −5.30 | −7.58 | −4.54 | −5.08 | −9.86 | −8.95 | −3.56 | −3.56 | −10.78 | −7.12 | −3.56 | −4.06 | −5.05 | −4.06 | −9.86 | −10.32 | −3.93 | −3.01 |
| Variation | 1.28 | −2.28 | −0.54 | 0.91 | 0.00 | 3.65 | −0.50 | 0.99 | −0.46 | 0.91 | ||||||||||
| Repetition | 1.23 | 1.23 | 1.23 | 1.23 | 0.70 | 0.70 | −27.89 | −24.98 | 0.70 | 0.70 | −2.65 | −0.71 | 0.70 | 0.70 | −2.33 | −0.82 | −27.89 | −17.21 | 1.23 | 1.23 |
| variation | 0.00 | 0.00 | 0.00 | 2.91 | 0.00 | 1.94 | 0.00 | 1.52 | 10.68 | 0.00 | ||||||||||
| Verbal fluency | −3.83 | −2.21 | −3.68 | −3.13 | −4.52 | −4.52 | −4.23 | −3.50 | −0.43 | −0.27 | −3.86 | −3.50 | 2.21 | −1.40 | −1.72 | −0.92 | −2.95 | −2.95 | −2.21 | −2.03 |
| variation | 1.62 | 0.55 | 0.00 | 0.73 | 0.16 | 0.37 | 0.81 | 0.81 | 0.00 | 0.18 | ||||||||||
References
- Simmons-Mackie, N. White Paper: Frequency and Demographics; Aphasia Access. Available online: http://www.aphasiaaccess.org (accessed on 29 January 2021).
- Lam, J.M.; Wodchis, W.P. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Med. Care 2010, 48, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, M.L.; Korpelainen, J.T.; Hiltunen, P.; Määttä, R.; Mononen, H.; Brusin, E.; Sotaniemi, K.A.; Myllylä, V.V. Aphasia, Depression, and Non-Verbal Cognitive Impairment in Ischaemic Stroke. Cerebrovasc. Dis. 2000, 10, 455–461. [Google Scholar] [CrossRef]
- Brady, M.C.; Kelly, H.; Godwin, J.; Enderby, P.; Campbell, P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst. Rev. 2016, 6, CD000425. [Google Scholar] [CrossRef] [Green Version]
- Benghanem, S.; Rosso, C.; Arbizu, C.; Moulton, E.; Dormont, D.; Leger, A.; Pires, C.; Samson, Y. Aphasia outcome: The interactions between initial severity, lesion size and location. J. Neurol. 2019, 266, 1303–1309. [Google Scholar] [CrossRef]
- Lazar, R.M.; Minzer, B.; Antoniello, D.; Festa, J.R.; Krakauer, J.W.; Marshall, R.S. Improvement in Aphasia Scores After Stroke Is Well Predicted by Initial Severity. Stroke 2010, 41, 1485–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiran, S.; Meier, E.L.; Johnson, J.P. Neuroplasticity in Aphasia: A Proposed Framework of Language Recovery. J. Speech Lang Hear. Res. 2019, 62, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Kiran, S. What is the nature of poststroke language recovery and reorganization? ISRN Neurol. 2012, 2012, 786872. [Google Scholar] [CrossRef] [Green Version]
- Hickok, G.; Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kummerer, D.; Kellmeyer, P.; Vry, M.S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef] [Green Version]
- Friederici, A.D. White-matter pathways for speech and language processing. Handb. Clin. Neurol. 2015, 129, 177–186. [Google Scholar] [CrossRef]
- Price, C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef] [Green Version]
- Saur, D.; Lange, R.; Baumgaertner, A.; Schraknepper, V.; Willmes, K.; Rijntjes, M.; Weiller, C. Dynamics of language reorganization after stroke. Brain 2006, 129, 1371–1384. [Google Scholar] [CrossRef] [Green Version]
- Anglade, C.; Thiel, A.; Ansaldo, A.I. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: A critical review of literature. Brain Inj. 2014, 28, 138–145. [Google Scholar] [CrossRef]
- Watila, M.M.; Balarabe, S.A. Factors predicting post-stroke aphasia recovery. J. Neurol. Sci. 2015, 352, 12–18. [Google Scholar] [CrossRef]
- Boyle, M. Semantic feature analysis treatment for anomia in two fluent aphasia syndromes. Am. J. Speech Lang. Pathol. 2004, 13, 236–249. [Google Scholar] [CrossRef]
- Boyle, M.; Coelho, C.A. Application of Semantic Feature Analysis as a Treatment for Aphasic Dysnomia. Am. J. Speech Lang. Pathol. 1995, 4, 94–98. [Google Scholar] [CrossRef]
- Kristensson, J.; Saldert, C. Naming of Objects and Actions after Treatment with Phonological Components Analysis in Aphasia. Clin. Arch. Commun. Disord. 2018, 3, 137–150. [Google Scholar] [CrossRef]
- Leonard, C.; Rochon, E.; Laird, L. Treating naming impairments in aphasia: Findings from a phonological components analysis treatment. Aphasiology 2008, 22, 923–947. [Google Scholar] [CrossRef]
- Madden, E.; Robinson, R.; Kendall, D. Phonological Treatment Approaches for Spoken Word Production in Aphasia. Semin. Speech Lang. 2017, 38, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcotte, K.; Laird, L.; Bitan, T.; Meltzer, J.A.; Graham, S.J.; Leonard, C.; Rochon, E. Therapy-Induced Neuroplasticity in Chronic Aphasia After Phonological Component Analysis: A Matter of Intensity. Front. Neurol. 2018, 9, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simic, T.; Leonard, C.; Laird, L.; Stewart, S.; Rochon, E. The effects of intensity on a phonological treatment for anomia in post-stroke aphasia. J. Commun. Disord. 2021, 93, 106125. [Google Scholar] [CrossRef] [PubMed]
- Goldrick, M.; Rapp, B. A restricted interaction account (RIA) of spoken word production: The best of both worlds. Aphasiology 2002, 16, 20–55. [Google Scholar] [CrossRef]
- Simic, T.; Chambers, C.; Bitan, T.; Stewart, S.; Goldberg, D.; Laird, L.; Leonard, C.; Rochon, E. Mechanisms underlying anomia treatment outcomes. J. Commun. Disord. 2020, 88, 106048. [Google Scholar] [CrossRef] [PubMed]
- Bose, A. Phonological therapy in jargon aphasia: Effects on naming and neologisms. Int. J. Lang. Commun. Disord. 2013, 48, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Haentjens, K.; Auclair-Ouellet, N. Naming gains and within-intervention progression following semantic feature analysis (SFA) and phonological components analysis (PCA) in adults with chronic post-stroke aphasia. Aphasiology 2020, 1–24. [Google Scholar] [CrossRef]
- van Hees, S.; Angwin, A.; McMahon, K.; Copland, D. A comparison of semantic feature analysis and phonological components analysis for the treatment of naming impairments in aphasia. Neuropsychol. Rehabil. 2013, 23, 102–132. [Google Scholar] [CrossRef]
- Rochon, E.; Leonard, C.; Burianova, H.; Laird, L.; Soros, P.; Graham, S.; Grady, C. Neural changes after phonological treatment for anomia: An fMRI study. Brain Lang. 2010, 114, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, S.; McMahon, K.; Angwin, A.; De Zubicaray, G.; Copland, D.A. Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain Lang. 2014, 129, 47–57. [Google Scholar] [CrossRef]
- Wilson, S.M.; Schneck, S.M. Neuroplasticity in post-stroke aphasia: A systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol. Lang. 2021, 2, 22–82. [Google Scholar] [CrossRef]
- Geranmayeh, F.; Brownsett, S.L.; Wise, R.J. Task-induced brain activity in aphasic stroke patients: What is driving recovery? Brain 2014, 137, 2632–2648. [Google Scholar] [CrossRef]
- Klingbeil, J.; Wawrzyniak, M.; Stockert, A.; Saur, D. Resting-state functional connectivity: An emerging method for the study of language networks in post-stroke aphasia. Brain Cogn. 2019, 131, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Masson-Trottier, M.; Sontheimer, A.; Ansaldo, A.I. Increased links between language and motor areas: A proof-of-concept study on resting-state functional connectivity following Personalized Observation, Execution and Mental imagery therapy in chronic aphasia. Brain Cogn. 2021, 148, 105659. [Google Scholar] [CrossRef]
- Duncan, E.S.; Small, S.L. Changes in dynamic resting state network connectivity following aphasia therapy. Brain Imaging Behav. 2018, 12, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Gili, T.; Fiori, V.; De Pasquale, G.; Sabatini, U.; Caltagirone, C.; Marangolo, P. Right sensory-motor functional networks subserve action observation therapy in aphasia. Brain Imaging Behav. 2017, 11, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P.; Fiori, V.; Sabatini, U.; De Pasquale, G.; Razzano, C.; Caltagirone, C.; Gili, T. Bilateral Transcranial Direct Current Stimulation Language Treatment Enhances Functional Connectivity in the Left Hemisphere: Preliminary Data from Aphasia. J. Cogn. Neurosci. 2016, 28, 724–738. [Google Scholar] [CrossRef] [PubMed]
- van Hees, S.; McMahon, K.; Angwin, A.; de Zubicaray, G.; Read, S.; Copland, D.A. A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Human Brain Mapp. 2014, 35, 3919–3931. [Google Scholar] [CrossRef] [PubMed]
- Durand, E. Développement D’une Nouvelle Thérapie Ciblant L’anomie des Verbes D’action: Validation Comportementale et Exploration des Corrélats Neurofonctionnels de ses Effets dans les cas D’aphasie. Ph.D. Thesis, Université de Montréal, Montréal, QC, Canada, 2019. [Google Scholar]
- Nespoulous, J.L.; Lecours, A.R.; Lafond, D.; Parente, M. Protocole Montréal-Tolouse MT-86 d’examen linguistique de l’aphasie-version Beta. Lab. Théophile Alajouanine Montréal 1986. [Google Scholar]
- Macoir, J.; Beaudoin, C.; Bluteau, J.; Potvin, O.; Wilson, M.A. TDQ-60–a color picture-naming test for adults and elderly people: Validation and normalization data. Aging Neuropsychol. Cogn. 2017, 25, 753–766. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Berroir, P.; Ansaldo, A.I. The Neural and Behavioral Correlates of Anomia Recovery following Personalized Observation, Execution, and Mental Imagery Therapy: A Proof of Concept. Neural. Plast. 2018, 2018, 5943759. [Google Scholar] [CrossRef] [Green Version]
- Marcotte, K.; Adrover-Roig, D.; Damien, B.; de Preaumont, M.; Genereux, S.; Hubert, M.; Ansaldo, A.I. Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia 2012, 50, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, K.; Ansaldo, A.I. The neural correlates of semantic feature analysis in chronic aphasia: Discordant patterns according to the etiology. Semin. Speech Lang. 2010, 31, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Marcotte, K.; Perlbarg, V.; Marrelec, G.; Benali, H.; Ansaldo, A.I. Default-mode network functional connectivity in aphasia: Therapy-induced neuroplasticity. Brain Lang. 2013, 124, 45–55. [Google Scholar] [CrossRef]
- Webster, J.; Whitworth, A.; Morris, J. Is it time to stop “fishing”? A review of generalisation following aphasia intervention. Aphasiology 2015, 29, 1240–1264. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.D.; Dalton, S.G. Main concepts for three different discourse tasks in a large non-clinical sample. Aphasiology 2016, 30, 45–73. [Google Scholar] [CrossRef]
- Dalton, S.G.; Richardson, J.D. Core-lexicon and main-concept production during picture-sequence description in adults without brain damage and adults with aphasia. Am. J. Speech Lang. Pathol. 2015, 24, S923–S938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodeur, M.; Dion-Lessard, G.; Chauret, M.; Dionne-Dostie, E.; Montreuil, T.; Lepage, M. The Bank of Standardized Stimuli (BOSS): A new normative dataset of 480 visual stimuli to be used in visual cognition research. J. Vis. 2011, 11, 825. [Google Scholar] [CrossRef]
- Masson-Trottier, M.; Marcotte, K.; Leonard, C.; Rochon, E.; Ansaldo, A.I. Validation de la version francophone de stimuli et indices nécessaires pour l’administration de la thérapie par Analyse des Composantes Phonologiques. In Proceedings of the International Conference on Speech-language Pathology and Audiology, Montreal, QC, Canada, 14–15 November 2016. [Google Scholar]
- New, B.; Pallier, C.; Brysbaert, M.; Ferrand, L. Manuel de Lexique 3. Behav. Res. Methods Instrum. Comput. 2004, 36, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Hickin, J.; Best, W.; Herbert, R.; Howard, D.; Osborne, F. Phonological therapy for word-finding difficulties: A re-evaluation. Aphasiology 2002, 16, 981–999. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Evans, W.S.; Hula, W.D.; Quique, Y.; Starns, J.J. How Much Time Do People With Aphasia Need to Respond During Picture Naming? Estimating Optimal Response Time Cutoffs Using a Multinomial Ex-Gaussian Approach. J. Speech Lang. Hear. Res. 2020, 63, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press, 2020. [Google Scholar]
- Porcu, M.; Cocco, L.; Puig, J.; Mannelli, L.; Yang, Q.; Suri, J.S.; Defazio, G.; Saba, L. Global Fractional Anisotropy: Effect on Resting-state Neural Activity and Brain Networking in Healthy Participants. Neuroscience 2021, 472, 103–115. [Google Scholar] [CrossRef]
- Sontheimer, A.; Pontier, B.; Claise, B.; Chassain, C.; Coste, J.; Lemaire, J.-J. Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness. Brain Sci. 2021, 11, 356. [Google Scholar] [CrossRef]
- Martinez-Molina, N.; Siponkoski, S.T.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Resting-State Network Plasticity Induced by Music Therapy after Traumatic Brain Injury. Neural. Plast. 2021, 2021, 6682471. [Google Scholar] [CrossRef]
- Rosenthal, R.; Cooper, H.; Hedges, L. Parametric measures of effect size. Handb. Res. Synth. 1994, 621, 231–244. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meteyard, L.; Bose, A. What Does a Cue Do? Comparing Phonological and Semantic Cues for Picture Naming in Aphasia. J. Speech Lang. Hear. Res. 2018, 61, 658–674. [Google Scholar] [CrossRef]
- Sze, W.P.; Hameau, S.; Warren, J.; Best, W. Identifying the components of a successful spoken naming therapy: A meta-analysis of word-finding interventions for adults with aphasia. Aphasiology 2020, 35, 33–72. [Google Scholar] [CrossRef]
- Thomas, L.; Lander, L.; Cox, N.; Romani, C. Speech and language therapy for aphasia: Parameters and outcomes. Aphasiology 2020, 34, 603–642. [Google Scholar] [CrossRef]
- Leonard, C.; Laird, L.; Burianová, H.; Graham, S.; Grady, C.; Simic, T.; Rochon, E. Behavioural and neural changes after a “choice” therapy for naming deficits in aphasia: Preliminary findings. Aphasiology 2015, 29, 506–525. [Google Scholar] [CrossRef]
- Ischebeck, A.; Indefrey, P.; Usui, N.; Nose, I.; Hellwig, F.; Taira, M. Reading in a regular orthography: An FMRI study investigating the role of visual familiarity. J. Cogn. Neurosci. 2004, 16, 727–741. [Google Scholar] [CrossRef] [Green Version]
- Papathanassiou, D.; Etard, O.; Mellet, E.; Zago, L.; Mazoyer, B.; Tzourio-Mazoyer, N. A common language network for comprehension and production: A contribution to the definition of language epicenters with PET. Neuroimage 2000, 11, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ardila, A.; Bernal, B.; Rosselli, M. How Extended Is Wernicke’s Area? Meta-Analytic Connectivity Study of BA20 and Integrative Proposal. Neurosci. J. 2016, 2016, 4962562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schevenels, K.; Price, C.J.; Zink, I.; De Smedt, B.; Vandermosten, M. A Review on Treatment-Related Brain Changes in Aphasia. Neurobiol. Lang. 2020, 1, 402–433. [Google Scholar] [CrossRef]
- Salo, E.; Rinne, T.; Salonen, O.; Alho, K. Brain activity during auditory and visual phonological, spatial and simple discrimination tasks. Brain Res. 2013, 1496, 55–69. [Google Scholar] [CrossRef]
- Heiss, W.D.; Thiel, A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006, 98, 118–123. [Google Scholar] [CrossRef]
- Meyer, M.; Jancke, L. Involvement of the left and right frontal operculum in speech and nonspeech perception and production. Broca’s Region. 2006, 218–241. [Google Scholar] [CrossRef]
- Meyer, M.; Steinhauer, K.; Alter, K.; Friederici, A.D.; von Cramon, D.Y. Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang. 2004, 89, 277–289. [Google Scholar] [CrossRef]
- Fridriksson, J.; Morrow-Odom, L.; Moser, D.; Fridriksson, A.; Baylis, G. Neural recruitment associated with anomia treatment in aphasia. Neuroimage 2006, 32, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Yourganov, G.; Fridriksson, J.; Stark, B.; Rorden, C. Removal of artifacts from resting-state fMRI data in stroke. Neuroimage. Clin. 2018, 17, 297–305. [Google Scholar] [CrossRef] [PubMed]
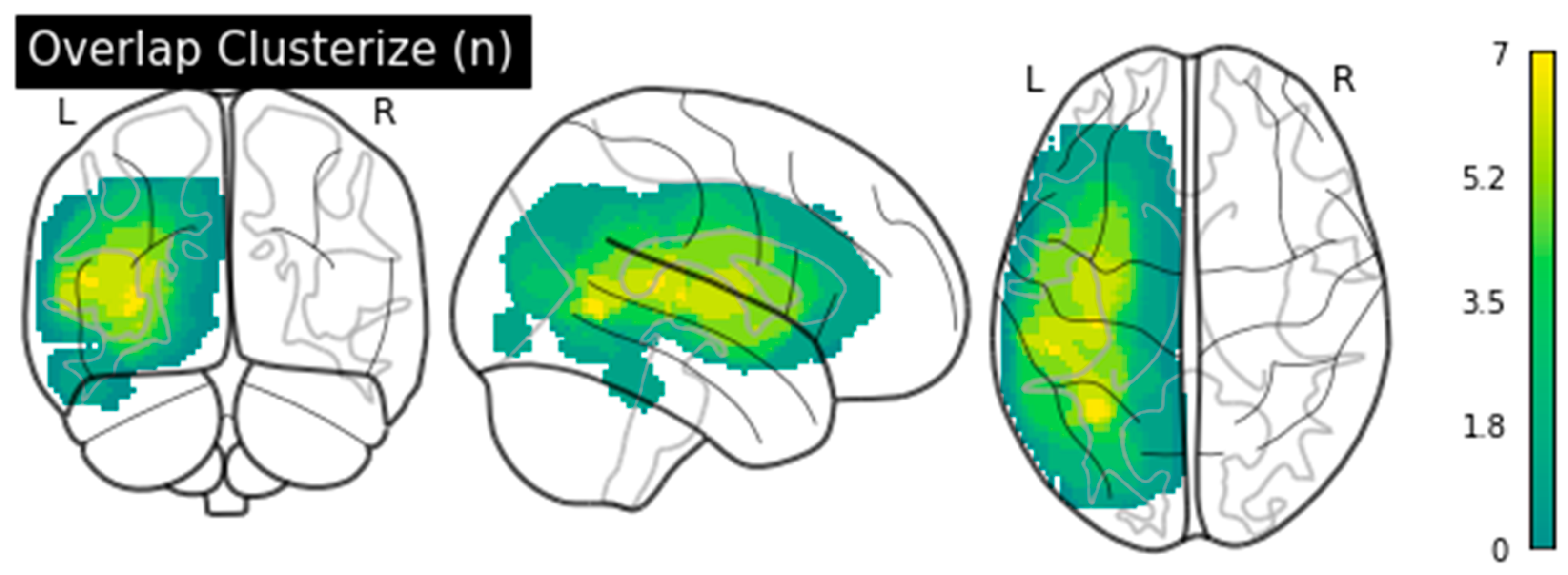
| ID | Sex | Age | Years of Education | Time Post-Onset (Months) | Lesion Size (mm3) | Aphasia Type | Aphasia Severity (BDAE Scale) | % Noun Naming (TDQ60) |
|---|---|---|---|---|---|---|---|---|
| PCA1 | M | 73 | 8 | 36 | 3188 | Transcortical motor | 4 | 0.40 |
| PCA2 | M | 82 | 15 | 24 | 138,096 | Transcortical mixed | 2 | 0.27 |
| PCA3 | M | 48 | 15 | 22 | 26,833 | Transcortical motor | 3 | 0.72 |
| PCA4 | W | 70 | 15 | 41 | 124,217 | Global | 1 | 0.02 |
| PCA5 | M | 60 | 12 | 172 | 223,253 | Anomic | 4 | 1.00 |
| PCA6 | W | 72 | 12 | 47 | 95,672 | Broca | 2 | 0.30 |
| PCA7 | M | 65 | 15 | 57 | 104,924 | Anomic | 2 | 0.95 |
| PCA8 | W | 63 | 18 | 11 | 66,573 | Broca | 2 | 0.95 |
| PCA9 | M | 79 | 20 | 12 | 43,121 | Global | 1 | 0.15 |
| PCA10 | M | 77 | 17 | 11 | 12,874 | Anomic | 3 | 0.60 |
| Region A | Region B | T(9) | p-FDR |
|---|---|---|---|
| ant. Temporal Fusiform Cortex L | Supracalcarine Cortex L | 7.20 | 0.0053 |
| Supracalcarine Cortex R | 4.83 | 0.0488 | |
| Supracalcarine Cortex L | ant. Inferior Temporal Gyrus L | 5.07 | 0.0443 |
| Lingual Gyrus R | Superior Frontal Gyrus R | −5.73 | 0.0298 |
| Region A | Region B | T(8) | p-FDR | R2 |
|---|---|---|---|---|
| post. Temporal Fusiform Cortex L | ant. Superior temporal gyrus R | 10.82 | 0.0005 | 0.94 |
| Insular cortex R | 5.23 | 0.0413 | 0.77 | |
| Frontal operculum cortex R | Pallidum R | 9.24 | 0.0016 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masson-Trottier, M.; Sontheimer, A.; Durand, E.; Ansaldo, A.I. Resting-State Functional Connectivity following Phonological Component Analysis: The Combined Action of Phonology and Visual Orthographic Cues. Brain Sci. 2021, 11, 1458. https://doi.org/10.3390/brainsci11111458
Masson-Trottier M, Sontheimer A, Durand E, Ansaldo AI. Resting-State Functional Connectivity following Phonological Component Analysis: The Combined Action of Phonology and Visual Orthographic Cues. Brain Sciences. 2021; 11(11):1458. https://doi.org/10.3390/brainsci11111458
Chicago/Turabian StyleMasson-Trottier, Michèle, Anna Sontheimer, Edith Durand, and Ana Inés Ansaldo. 2021. "Resting-State Functional Connectivity following Phonological Component Analysis: The Combined Action of Phonology and Visual Orthographic Cues" Brain Sciences 11, no. 11: 1458. https://doi.org/10.3390/brainsci11111458
APA StyleMasson-Trottier, M., Sontheimer, A., Durand, E., & Ansaldo, A. I. (2021). Resting-State Functional Connectivity following Phonological Component Analysis: The Combined Action of Phonology and Visual Orthographic Cues. Brain Sciences, 11(11), 1458. https://doi.org/10.3390/brainsci11111458







