Words as Visual Objects: Neural and Behavioral Evidence for High-Level Visual Impairments in Dyslexia
Abstract
:1. Introduction
2. The Role of Vision in Dyslexia
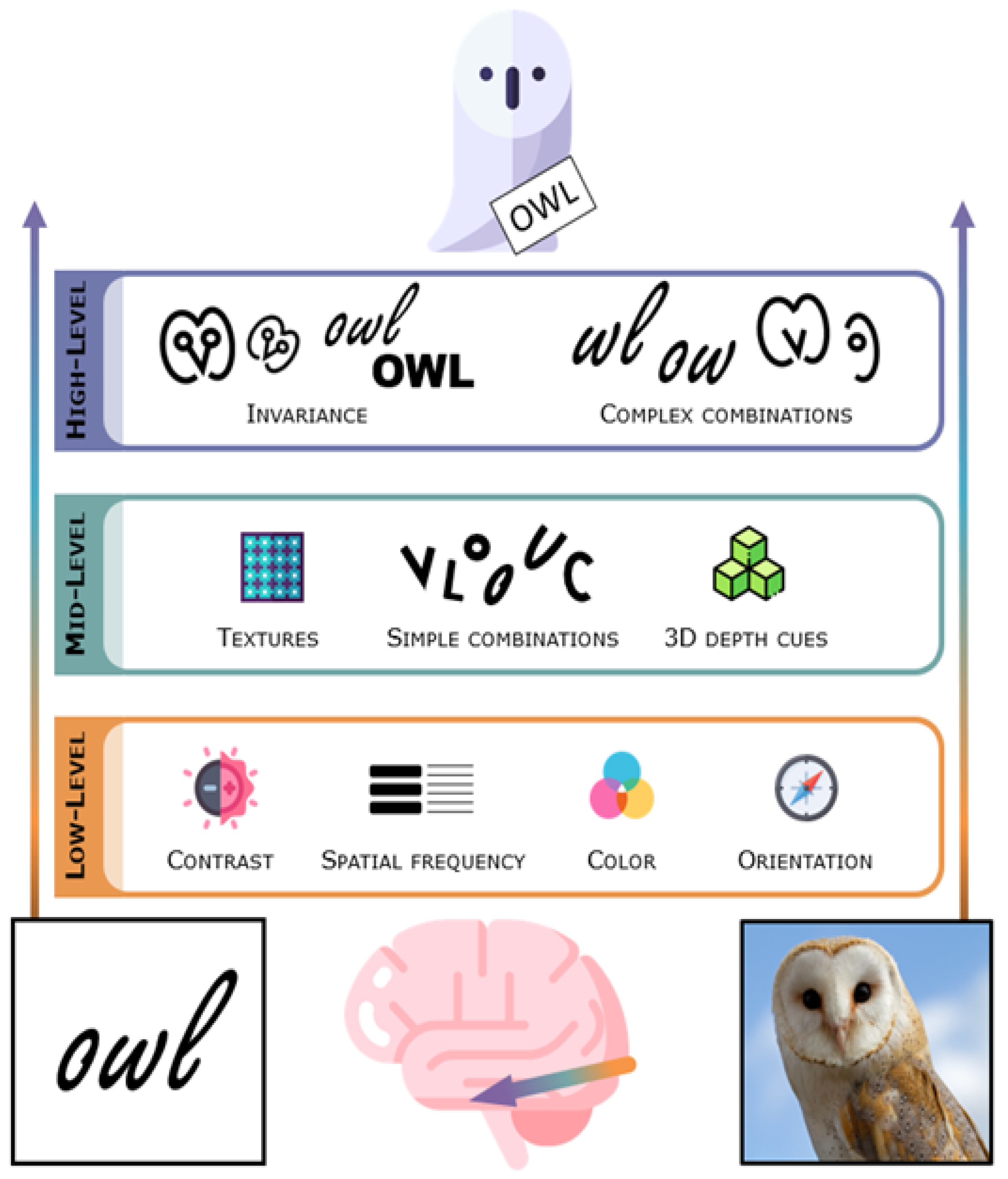
3. The Visual System
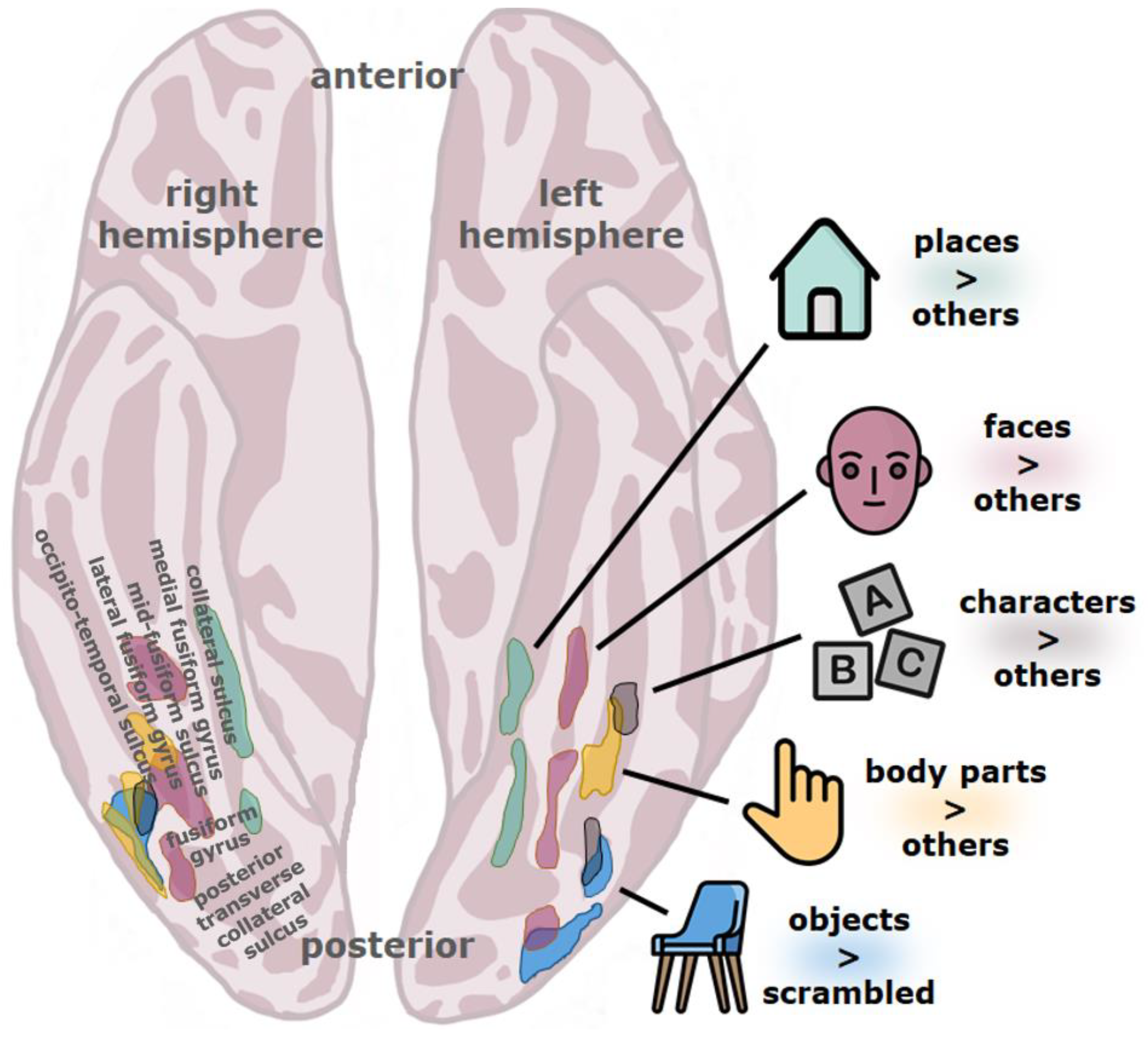
4. The Visual Word Form Area (VWFA)
5. Neural Evidence
5.1. Functional Neuroimaging
5.2. Structural
5.3. EEG and MEG
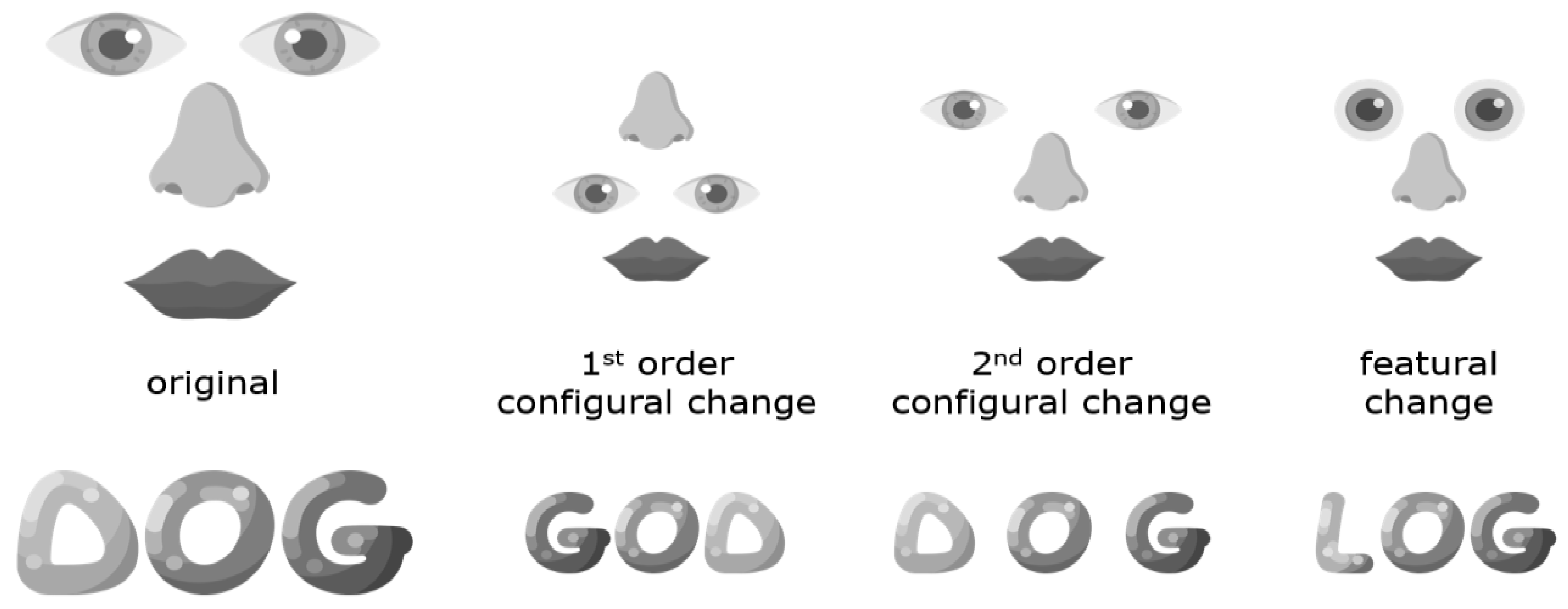
6. Behavioral Evidence
7. Practical Implications
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catts, H.W. Defining dyslexia as a developmental language disorder. Ann. Dyslexia 1989, 39, 50. [Google Scholar] [CrossRef]
- Pennington, B.F.; Van Orden, G.C.; Smith, S.D.; Green, P.A.; Haith, M.M. Phonological processing skills and deficits in adult dyslexics. Child Dev. 1990, 61, 1753–1778. [Google Scholar] [CrossRef]
- Shaywitz, S.E.; Shaywitz, B.A. Dyslexia (specific reading disability). Biol. Psychiatry 2005, 57, 1301–1309. [Google Scholar] [CrossRef]
- Snowling, M.J. From language to reading and dyslexia 1. Dyslexia 2001, 7, 37–46. [Google Scholar] [CrossRef]
- Vellutino, F.R.; Fletcher, J.M.; Snowling, M.J.; Scanlon, D.M. Specific reading disability (dyslexia): What have we learned in the past four decades? J. Child Psychol. Psychiatry 2004, 45, 2–40. [Google Scholar] [CrossRef]
- Lyon, G.R.; Shaywitz, S.E.; Shaywitz, B.A. A definition of dyslexia. Ann. Dyslexia 2003, 53, 1–14. [Google Scholar] [CrossRef]
- Shaywitz, B.A.; Shaywitz, S.E. The American experience: Towards a 21st century definition of dyslexia. Oxf. Rev. Educ. 2020, 46, 454–471. [Google Scholar] [CrossRef]
- De Martino, S.; Espesser, R.; Rey, V.; Habib, M. The “temporal processing deficit” hypothesis in dyslexia: New experimental evidence. Brain Cogn. 2001, 46, 104–108. [Google Scholar] [CrossRef]
- Farmer, M.E.; Klein, R.M. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychon. Bull. Rev. 1995, 2, 460–493. [Google Scholar] [CrossRef] [Green Version]
- Goswami, U. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 2011, 15, 3–10. [Google Scholar] [CrossRef]
- Giofrè, D.; Toffalini, E.; Provazza, S.; Calcagnì, A.; Altoè, G.; Roberts, D.J. Are children with developmental dyslexia all the same? A cluster analysis with more than 300 cases. Dyslexia 2019, 25, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychologia 2019, 130, 66–77. [Google Scholar] [CrossRef]
- Valdois, S.; Bosse, M.L.; Tainturier, M.J. The cognitive deficits responsible for developmental dyslexia: Review of evidence for a selective visual attentional disorder. Dyslexia 2004, 10, 339–363. [Google Scholar] [CrossRef]
- Norton, E.S.; Beach, S.D.; Gabrieli, J.D. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015, 30, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Denckla, M.B.; Rudel, R.G. Rapid ‘automatized’naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia 1976, 14, 471–479. [Google Scholar] [CrossRef]
- Reid, A.A. Neuroimaging reveals heterogeneous neural correlates of reading deficit in individuals with dyslexia consistent with a multiple deficit model. In Neuroimaging-Structure, Function and Mind; IntechOpen: London, UK, 2018. [Google Scholar]
- O’Brien, G.; Yeatman, J.D. Bridging sensory and language theories of dyslexia: Toward a multifactorial model. Dev. Sci. 2021, 24, e13039. [Google Scholar] [CrossRef]
- Ziegler, J.C.; Bertrand, D.; Tóth, D.; Csépe, V.; Reis, A.; Faísca, L.; Saine, N.; Lyytinen, H.; Vaessen, A.; Blomert, L. Orthographic depth and its impact on universal predictors of reading: A cross-language investigation. Psychol. Sci. 2010, 21, 551–559. [Google Scholar] [CrossRef]
- Cox, D.D. Do we understand high-level vision? Curr. Opin. Neurobiol. 2014, 25, 187–193. [Google Scholar] [CrossRef]
- Cox, D.D.; Dean, T. Neural networks and neuroscience-inspired computer vision. Curr. Biol. 2014, 24, R921–R929. [Google Scholar] [CrossRef] [Green Version]
- Kussmaul, A. Disturbance of speech. In Cyclopedia of the Practice of Medicine; William Wood and company: New York, NY, USA, 1877; pp. 581–875. [Google Scholar]
- Hinshelwood, J. Word-blindness and visual memory. Lancet 1895, 146, 1564–1570. [Google Scholar] [CrossRef]
- Hinshelwood, J. Congenital word-blindness. Lancet 1900, 155, 1506–1508. [Google Scholar] [CrossRef] [Green Version]
- Morgan, W.P. A case of congenital word blindness. Br. Med. J. 1896, 2, 1378. [Google Scholar] [CrossRef] [Green Version]
- Papert, S.A. The Summer Vision Project. Available online: https://dspace.mit.edu/handle/1721.1/6125 (accessed on 20 September 2021).
- Cichy, R.M.; Kaiser, D. Deep neural networks as scientific models. Trends Cogn. Sci. 2019, 23, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M. A multi-modal parcellation of human cerebral cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felleman, D.J.; Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1991, 1, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Sigurdardottir, H.M.; Sheinberg, D.L. The neurophysiology of attention and object recognition in visual scenes. In Scene Vision; MIT Press: Cambridge, MA, USA, 2014; pp. 85–104. [Google Scholar]
- Foster, K.; Gaska, J.P.; Nagler, M.; Pollen, D. Spatial and temporal frequency selectivity of neurones in visual cortical areas V1 and V2 of the macaque monkey. J. Physiol. 1985, 365, 331–363. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968, 195, 215–243. [Google Scholar] [CrossRef]
- Shapley, R.; Kaplan, E.; Soodak, R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature 1981, 292, 543–545. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Pitcher, D.; Ungerleider, L.G. Evidence for a third visual pathway specialized for social perception. Trends Cogn. Sci. 2021, 25, 100–110. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Haxby, J.V. ‘What’and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994, 4, 157–165. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Mishkin, M. Two cortical visual systems. In Analysis of Visual Behavior; Goodale, M., Ingle, D.J., Mansfield, R.J.W., Eds.; MIT Press: Cambridge, MA, USA, 1982; pp. 549–586. [Google Scholar]
- Grill-Spector, K.; Weiner, K.S. The functional architecture of the ventral temporal cortex and its role in categorization. Nature Rev. Neurosci. 2014, 15, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Desimone, R.; Albright, T.D.; Gross, C.G.; Bruce, C. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 1984, 4, 2051–2062. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.G.; Rocha-Miranda, C.d.; Bender, D. Visual properties of neurons in inferotemporal cortex of the macaque. J. Neurophysiol. 1972, 35, 96–111. [Google Scholar] [CrossRef]
- Milner, D.; Goodale, M. The Visual Brain in Action; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Logothetis, N.K.; Sheinberg, D.L. Visual object recognition. Annu. Rev. Neurosci. 1996, 19, 577–621. [Google Scholar] [CrossRef]
- Palmeri, T.J.; Gauthier, I. Visual object understanding. Nat. Rev. Neurosci. 2004, 5, 291–303. [Google Scholar] [CrossRef]
- Tanaka, K.; Saito, H.-a.; Fukada, Y.; Moriya, M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 1991, 66, 170–189. [Google Scholar] [CrossRef] [Green Version]
- Kar, K.; DiCarlo, J.J. Fast recurrent processing via ventrolateral prefrontal cortex is needed by the primate ventral stream for robust core visual object recognition. Neuron 2021, 109, 164–176.e165. [Google Scholar] [CrossRef]
- Kietzmann, T.C.; Spoerer, C.J.; Sörensen, L.K.; Cichy, R.M.; Hauk, O.; Kriegeskorte, N. Recurrence is required to capture the representational dynamics of the human visual system. Proc. Natl. Acad. Sci. USA 2019, 116, 21854–21863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehaene, S.; Cohen, L.; Sigman, M.; Vinckier, F. The neural code for written words: A proposal. Trends Cogn. Sci. 2005, 9, 335–341. [Google Scholar] [CrossRef]
- Anzai, A.; Peng, X.; Van Essen, D.C. Neurons in monkey visual area V2 encode combinations of orientations. Nat. Neurosci. 2007, 10, 1313–1321. [Google Scholar] [CrossRef]
- Pegado, F.; Nakamura, K.; Cohen, L.; Dehaene, S. Breaking the symmetry: Mirror discrimination for single letters but not for pictures in the Visual Word Form Area. Neuroimage 2011, 55, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Vilis, T.; Strother, L. Functionally separable font-invariant and font-sensitive neural populations in occipitotemporal cortex. J. Cogn. Neurosci. 2019, 31, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, H.M.; Danielsdottir, H.B.; Gudmundsdottir, M.; Hjartarson, K.H.; Thorarinsdottir, E.A.; Kristjánsson, Á. Problems with visual statistical learning in developmental dyslexia. Sci. Rep. 2017, 7, 606. [Google Scholar] [CrossRef] [Green Version]
- Groen, I.I.; Silson, E.H.; Baker, C.I. Contributions of low-and high-level properties to neural processing of visual scenes in the human brain. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160102. [Google Scholar] [CrossRef] [Green Version]
- Vinckier, F.; Dehaene, S.; Jobert, A.; Dubus, J.P.; Sigman, M.; Cohen, L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron 2007, 55, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.; Henry, C.; Dehaene, S.; Martinaud, O.; Lehéricy, S.; Lemer, C.; Ferrieux, S. The pathophysiology of letter-by-letter reading. Neuropsychologia 2004, 42, 1768–1780. [Google Scholar] [CrossRef] [Green Version]
- Leff, A.; Spitsyna, G.; Plant, G.; Wise, R. Structural anatomy of pure and hemianopic alexia. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1004–1007. [Google Scholar] [CrossRef]
- Pflugshaupt, T.; Gutbrod, K.; Wurtz, P.; von Wartburg, R.; Nyffeler, T.; de Haan, B.; Karnath, H.-O.; Mueri, R.M. About the role of visual field defects in pure alexia. Brain 2009, 132, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Diehl, B.; Piao, Z.; Schuele, S.; Lapresto, E.; Liu, P.; Nair, D.; Dinner, D.; Lüders, H. Evidence for a basal temporal visual language center: Cortical stimulation producing pure alexia. Neurology 2008, 71, 1621–1627. [Google Scholar] [CrossRef]
- Dehaene, S.; Cohen, L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011, 15, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Dundas, E.M.; Plaut, D.C.; Behrmann, M. The joint development of hemispheric lateralization for words and faces. J. Exp. Psychol. Gen. 2013, 142, 348. [Google Scholar] [CrossRef] [Green Version]
- Dundas, E.M.; Plaut, D.C.; Behrmann, M. An ERP investigation of the co-development of hemispheric lateralization of face and word recognition. Neuropsychologia 2014, 61, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Monzalvo, K.; Fluss, J.; Billard, C.; Dehaene, S.; Dehaene-Lambertz, G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage 2012, 61, 258–274. [Google Scholar] [CrossRef]
- Dehaene, S.; Cohen, L.; Morais, J.; Kolinsky, R. Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015, 16, 234–244. [Google Scholar] [CrossRef]
- Caspers, J.; Zilles, K.; Eickhoff, S.B.; Schleicher, A.; Mohlberg, H.; Amunts, K. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct. Funct. 2013, 218, 511–526. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.; Dehaene, S.; Naccache, L.; Lehéricy, S.; Dehaene-Lambertz, G.; Hénaff, M.-A.; Michel, F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 2000, 123, 291–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.; Lehéricy, S.; Chochon, F.; Lemer, C.; Rivaud, S.; Dehaene, S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain 2002, 125, 1054–1069. [Google Scholar] [CrossRef]
- Price, C.J.; Devlin, J.T. The myth of the visual word form area. Neuroimage 2003, 19, 473–481. [Google Scholar] [CrossRef]
- Binder, J.R.; Medler, D.A.; Westbury, C.F.; Liebenthal, E.; Buchanan, L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage 2006, 33, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Güçlü, U.; van Gerven, M.A. Deep neural networks reveal a gradient in the complexity of neural representations across the ventral stream. J. Neurosci. 2015, 35, 10005–10014. [Google Scholar] [CrossRef] [Green Version]
- Stigliani, A.; Weiner, K.S.; Grill-Spector, K. Temporal processing capacity in high-level visual cortex is domain specific. J. Neurosci. 2015, 35, 12412–12424. [Google Scholar] [CrossRef]
- Dien, J. A tale of two recognition systems: Implications of the fusiform face area and the visual word form area for lateralized object recognition models. Neuropsychologia 2009, 47, 1–16. [Google Scholar] [CrossRef]
- Barton, J.J.; Fox, C.J.; Sekunova, A.; Iaria, G. Encoding in the visual word form area: An fMRI adaptation study of words versus handwriting. J. Cogn. Neurosci. 2010, 22, 1649–1661. [Google Scholar] [CrossRef]
- Bouhali, F.; de Schotten, M.T.; Pinel, P.; Poupon, C.; Mangin, J.-F.; Dehaene, S.; Cohen, L. Anatomical connections of the visual word form area. J. Neurosci. 2014, 34, 15402–15414. [Google Scholar] [CrossRef]
- Song, Y.; Bu, Y.; Hu, S.; Luo, Y.; Liu, J. Short-term language experience shapes the plasticity of the visual word form area. Brain Res. 2010, 1316, 83–91. [Google Scholar] [CrossRef]
- Turk-Browne, N.B.; Scholl, B.J.; Chun, M.M.; Johnson, M.K. Neural evidence of statistical learning: Efficient detection of visual regularities without awareness. J. Cogn. Neurosci. 2009, 21, 1934–1945. [Google Scholar] [CrossRef] [Green Version]
- Reinke, K.; Fernandes, M.; Schwindt, G.; O’Craven, K.; Grady, C.L. Functional specificity of the visual word form area: General activation for words and symbols but specific network activation for words. Brain Lang. 2008, 104, 180–189. [Google Scholar] [CrossRef]
- Dehaene, S.; Pegado, F.; Braga, L.; Ventura Filho, P.; GN, J. Impact of literacy on the cortical networks for vision and language. Science 2010, 3, 1359–1364. [Google Scholar] [CrossRef] [Green Version]
- Nestor, A.; Behrmann, M.; Plaut, D.C. The neural basis of visual word form processing: A multivariate investigation. Cereb. Cortex 2013, 23, 1673–1684. [Google Scholar] [CrossRef] [Green Version]
- Starrfelt, R.; Gerlach, C. The visual what for area: Words and pictures in the left fusiform gyrus. Neuroimage 2007, 35, 334–342. [Google Scholar] [CrossRef]
- Gauthier, I.; Tarr, M.J.; Moylan, J.; Anderson, A.W.; Skudlarski, P.; Gore, J.C. Does visual subordinate-level categorisation engage the functionally defined fusiform face area? Cogn. Neuropsychol. 2000, 17, 143–164. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Alpert, N.M.; Thompson, W.L. Identifying objects at different levels of hierarchy: A positron emission tomography study. Hum. Brain Mapp. 1995, 3, 107–132. [Google Scholar] [CrossRef]
- Rogers, T.T.; Hocking, J.; Mechelli, A.; Patterson, K.; Price, C. Fusiform activation to animals is driven by the process, not the stimulus. J. Cogn. Neurosci. 2005, 17, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Borghesani, V.; Wang, C.; Watson, C.; Bouhali, F.; Caverzasi, E.; Battistella, G.; Bogley, R.; Yabut, N.A.; Deleon, J.; Miller, Z.A. Functional and morphological correlates of developmental dyslexia: A multimodal investigation of the ventral occipitotemporal cortex. J. Neuroimaging 2021, 31, 962–972. [Google Scholar] [CrossRef]
- Brem, S.; Maurer, U.; Kronbichler, M.; Schurz, M.; Richlan, F.; Blau, V.; Reithler, J.; van der Mark, S.; Schulz, E.; Bucher, K. Visual word form processing deficits driven by severity of reading impairments in children with developmental dyslexia. Sci. Rep. 2020, 10, 18728. [Google Scholar] [CrossRef]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 2011, 56, 1735–1742. [Google Scholar] [CrossRef]
- Kronbichler, L.; Kronbichler, M. The importance of the left occipitotemporal cortex in developmental dyslexia. Curr. Dev. Disord. Rep. 2018, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, C.; Koschutnig, K.; Moll, K.; Schulte-Körne, G.; Fink, A.; Landerl, K. Reading-related functional activity in children with isolated spelling deficits and dyslexia. Lang. Cogn. Neurosci. 2021, 36, 543–561. [Google Scholar] [CrossRef]
- Dębska, A.; Banfi, C.; Chyl, K.; Dzięgiel-Fivet, G.; Kacprzak, A.; Łuniewska, M.; Plewko, J.; Grabowska, A.; Landerl, K.; Jednoróg, K. Neural patterns of word processing differ in children with dyslexia and isolated spelling deficit. Brain Struct. Funct. 2021, 226, 1467–1478. [Google Scholar] [CrossRef]
- Cutting, L.E.; Clements-Stephens, A.; Pugh, K.R.; Burns, S.; Cao, A.; Pekar, J.J.; Davis, N.; Rimrodt, S.L. Not all reading disabilities are dyslexia: Distinct neurobiology of specific comprehension deficits. Brain Connect. 2013, 3, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Kronbichler, M.; Richlan, F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Hum. Brain Mapp. 2016, 37, 2676–2699. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, W.; You, W.; Li, Y.; Awati, N.; Zhao, X.; Booth, J.R.; Peng, D. Similar alterations in brain function for phonological and semantic processing to visual characters in Chinese dyslexia. Neuropsychologia 2012, 50, 2224–2232. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Lee, H.L.; Zhang, Q.; Liu, T.; Geng, L.B.; Seghier, M.L.; Shakeshaft, C.; Twomey, T.; Green, D.W.; Yang, Y.M. Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain 2010, 133, 1694–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschle, N.M.; Zuk, J.; Gaab, N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. USA 2012, 109, 2156–2161. [Google Scholar] [CrossRef] [Green Version]
- Centanni, T.M.; Norton, E.S.; Ozernov-Palchik, O.; Park, A.; Beach, S.D.; Halverson, K.; Gaab, N.; Gabrieli, J.D. Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage Clin. 2019, 22, 101715. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Kushnir, T.; Hendler, T.; Malach, R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat. Neurosci. 2000, 3, 837–843. [Google Scholar] [CrossRef]
- Vocks, S.; Busch, M.; Grönemeyer, D.; Schulte, D.; Herpertz, S.; Suchan, B. Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. Cogn. Affect. Behav. Neurosci. 2010, 10, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Sigurdardottir, H.M.; Ívarsson, E.; Kristinsdóttir, K.; Kristjánsson, Á. Impaired recognition of faces and objects in dyslexia: Evidence for ventral stream dysfunction? Neuropsychology 2015, 29, 739. [Google Scholar] [CrossRef] [Green Version]
- McCrory, E.J.; Mechelli, A.; Frith, U.; Price, C.J. More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain 2005, 128, 261–267. [Google Scholar] [CrossRef]
- Rüsseler, J.; Ye, Z.; Gerth, I.; Szycik, G.R.; Münte, T.F. Audio-visual speech perception in adult readers with dyslexia: An fMRI study. Brain Imaging Behav. 2018, 12, 357–368. [Google Scholar] [CrossRef]
- Boros, M.; Anton, J.-L.; Pech-Georgel, C.; Grainger, J.; Szwed, M.; Ziegler, J.C. Orthographic processing deficits in developmental dyslexia: Beyond the ventral visual stream. NeuroImage 2016, 128, 316–327. [Google Scholar] [CrossRef]
- Perrachione, T.K.; Del Tufo, S.N.; Winter, R.; Murtagh, J.; Cyr, A.; Chang, P.; Halverson, K.; Ghosh, S.S.; Christodoulou, J.A.; Gabrieli, J.D. Dysfunction of rapid neural adaptation in dyslexia. Neuron 2016, 92, 1383–1397. [Google Scholar] [CrossRef] [Green Version]
- Sigurdardottir, H.M.; Jozranjbar, B. Laterality effect (face perception). In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Altarelli, I.; Monzalvo, K.; Iannuzzi, S.; Fluss, J.; Billard, C.; Ramus, F.; Dehaene-Lambertz, G. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: Evidence for differential effects in boys and girls. J. Neurosci. 2013, 33, 11296–11301. [Google Scholar] [CrossRef] [PubMed]
- Adrián-Ventura, J.; Soriano-Ferrer, M.; Fuentes-Claramonte, P.; Morte-Soriano, M.; Parcet, M.A.; Avila, C. Grey matter reduction in the occipitotemporal cortex in Spanish children with dyslexia: A voxel-based morphometry study. J. Neurolinguistics 2020, 53, 100873. [Google Scholar] [CrossRef]
- Ma, Y.; Koyama, M.S.; Milham, M.P.; Castellanos, F.X.; Quinn, B.T.; Pardoe, H.; Wang, X.; Kuzniecky, R.; Devinsky, O.; Thesen, T. Cortical thickness abnormalities associated with dyslexia, independent of remediation status. NeuroImage Clin. 2015, 7, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamboer, P.; Vorst, H.; Ghebreab, S.; Scholte, H. Machine learning and dyslexia: Classification of individual structural neuro-imaging scans of students with and without dyslexia. NeuroImage Clin. 2016, 11, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkersdörfer, J.; Lonnemann, J.; Lindberg, S.; Hasselhorn, M.; Fiebach, C.J. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS ONE 2012, 7, e43122. [Google Scholar]
- Ulfarsson, M.; Walters, G.; Gustafsson, O.; Steinberg, S.; Silva, A.; Doyle, O.; Brammer, M.; Gudbjartsson, D.; Arnarsdottir, S.; Jonsdottir, G. 15q11. 2 CNV affects cognitive, structural and functional correlates of dyslexia and dyscalculia. Transl. Psychiatry 2017, 7, e1109. [Google Scholar] [CrossRef] [Green Version]
- Jednorog, K.; Marchewka, A.; Altarelli, I.; Monzalvo Lopez, A.K.; van Ermingen-Marbach, M.; Grande, M.; Grabowska, A.; Heim, S.; Ramus, F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? Insights from a large-scale voxel-based morphometry study. Hum. Brain Mapp. 2015, 36, 1741–1754. [Google Scholar] [CrossRef]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Hum. Brain Mapp. 2013, 34, 3055–3065. [Google Scholar] [CrossRef]
- Carreiras, M.; Seghier, M.L.; Baquero, S.; Estévez, A.; Lozano, A.; Devlin, J.T.; Price, C.J. An anatomical signature for literacy. Nature 2009, 461, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Krafnick, A.J.; Flowers, D.L.; Luetje, M.M.; Napoliello, E.M.; Eden, G.F. An investigation into the origin of anatomical differences in dyslexia. J. Neurosci. 2014, 34, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Beelen, C.; Blockmans, L.; Wouters, J.; Ghesquière, P.; Vandermosten, M. Brain-behavior dynamics between the left fusiform and reading. Brain Struct. Funct. 2021. [Google Scholar] [CrossRef]
- Raschle, N.M.; Chang, M.; Gaab, N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage 2011, 57, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Beelen, C.; Vanderauwera, J.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Atypical gray matter in children with dyslexia before the onset of reading instruction. Cortex 2019, 121, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M. The face-specific N170 component reflects late stages in the structural encoding of faces. Neuroreport 2000, 11, 2319–2324. [Google Scholar] [CrossRef]
- Eimer, M.; Gosling, A.; Nicholas, S.; Kiss, M. The N170 component and its links to configural face processing: A rapid neural adaptation study. Brain Res. 2011, 1376, 76–87. [Google Scholar] [CrossRef]
- Sagiv, N.; Bentin, S. Structural encoding of human and schematic faces: Holistic and part-based processes. J. Cogn. Neurosci. 2001, 13, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.; Nakayama, K. Rapid adaptation of the M170 response: Importance of face parts. Cereb. Cortex 2008, 18, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, G.; Petit, L.; Bernard, C.; Rebaï, M. N170 ERPs could represent a logographic processing strategy in visual word recognition. Behav. Brain Funct. 2007, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Maurer, D.; Le Grand, R.; Mondloch, C.J. The many faces of configural processing. Trends Cogn. Sci. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Richler, J.; Palmeri, T.J.; Gauthier, I. Meanings, mechanisms, and measures of holistic processing. Front. Psychol. 2012, 3, 553. [Google Scholar] [CrossRef] [Green Version]
- Sigurdardottir, H.M.; Arnardottir, A.; Halldorsdottir, E.T.; Omarsdottir, H.R.; Valgeirsdottir, A.S. Faces and words are both associated and dissociated: Evidence from visual problems in dyslexia. PsyArXiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Pelli, D.G.; Tillman, K.A. Parts, wholes, and context in reading: A triple dissociation. PLoS ONE 2007, 2, e680. [Google Scholar] [CrossRef]
- Bentin, S.; Allison, T.; Puce, A.; Perez, E.; McCarthy, G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996, 8, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentin, S.; Mouchetant-Rostaing, Y.; Giard, M.-H.; Echallier, J.-F.; Pernier, J. ERP manifestations of processing printed words at different psycholinguistic levels: Time course and scalp distribution. J. Cogn. Neurosci. 1999, 11, 235–260. [Google Scholar] [CrossRef]
- Brem, S.; Bucher, K.; Halder, P.; Summers, P.; Dietrich, T.; Martin, E.; Brandeis, D. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage 2006, 29, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Maurer, U.; McCandliss, B.D. The development of visual expertise for words: The contribution of electrophysiology. In Single-Word Reading; Psychology Press: Hove, UK, 2007; pp. 57–77. [Google Scholar]
- Rossion, B. Understanding face perception by means of human electrophysiology. Trends Cogn. Sci. 2014, 18, 310–318. [Google Scholar] [CrossRef]
- Rossion, B.; Jacques, C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage 2008, 39, 1959–1979. [Google Scholar] [CrossRef]
- Rossion, B.; Joyce, C.A.; Cottrell, G.W.; Tarr, M.J. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage 2003, 20, 1609–1624. [Google Scholar] [CrossRef]
- van de Walle de Ghelcke, A.; Rossion, B.; Schiltz, C.; Lochy, A. Developmental changes in neural letter-selectivity: A 1-year follow-up of beginning readers. Dev. Sci. 2021, 24, e12999. [Google Scholar] [PubMed]
- Kast, M.; Elmer, S.; Jancke, L.; Meyer, M. ERP differences of pre-lexical processing between dyslexic and non-dyslexic children. Int. J. Psychophysiol. 2010, 77, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Deffke, I.; Sander, T.; Heidenreich, J.; Sommer, W.; Curio, G.; Trahms, L.; Lueschow, A. MEG/EEG sources of the 170-ms response to faces are co-localized in the fusiform gyrus. Neuroimage 2007, 35, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Itier, R.J.; Taylor, M.J. Source analysis of the N170 to faces and objects. Neuroreport 2004, 15, 1261–1265. [Google Scholar] [CrossRef]
- James, T.W.; Culham, J.; Humphrey, G.K.; Milner, A.D.; Goodale, M.A. Ventral occipital lesions impair object recognition but not object-directed grasping: An fMRI study. Brain 2003, 126, 2463–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahé, G.; Bonnefond, A.; Gavens, N.; Dufour, A.; Doignon-Camus, N. Impaired visual expertise for print in French adults with dyslexia as shown by N170 tuning. Neuropsychologia 2012, 50, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Helenius, P.; Tarkiainen, A.; Cornelissen, P.; Hansen, P.C.; Salmelin, R. Dissociation of normal feature analysis and deficient processing of letter-strings in dyslexic adults. Cereb. Cortex 1999, 9, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Maurer, U.; Schulz, E.; Brem, S.; van der Mark, S.; Bucher, K.; Martin, E.; Brandeis, D. The development of print tuning in children with dyslexia: Evidence from longitudinal ERP data supported by fMRI. Neuroimage 2011, 57, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Maurer, U.; Brem, S.; Kranz, F.; Bucher, K.; Benz, R.; Halder, P.; Steinhausen, H.-C.; Brandeis, D. Coarse neural tuning for print peaks when children learn to read. Neuroimage 2006, 33, 749–758. [Google Scholar] [CrossRef]
- Fraga-González, G.; Pleisch, G.; Di Pietro, S.V.; Neuenschwander, J.; Walitza, S.; Brandeis, D.; Karipidis, I.I.; Brem, S. The rise and fall of rapid occipito-temporal sensitivity to letters: Transient specialization through elementary school. Dev. Cogn. Neurosci. 2021, 49, 100958. [Google Scholar] [CrossRef]
- Eberhard-Moscicka, A.K.; Jost, L.B.; Raith, M.; Maurer, U. Neurocognitive mechanisms of learning to read: Print tuning in beginning readers related to word-reading fluency and semantics but not phonology. Dev. Sci. 2015, 18, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Liberman, I.Y.; Shankweiler, D.; Liberman, A.M. The alphabetic principle and learning to read. In Phonology and Reading Disability: Solving the Reading Puzzle; Shankweiler, D., Liberman, I.Y., Eds.; University of Michigan Press: Ann Arbor, MI, USA, 1989. [Google Scholar]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307. [Google Scholar] [CrossRef]
- Wagner, R.K.; Torgesen, J.K. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol. Bull. 1987, 101, 192. [Google Scholar] [CrossRef]
- Zoubrinetzky, R.; Bielle, F.; Valdois, S. New insights on developmental dyslexia subtypes: Heterogeneity of mixed reading profiles. PLoS ONE 2014, 9, e99337. [Google Scholar] [CrossRef]
- Sigurdardottir, H.M.; Gauthier, I. Expertise and object recognition. Brain Mapp. Encycl. Ref. 2015, 2, 523–527. [Google Scholar]
- Mahé, G.; Bonnefond, A.; Doignon-Camus, N. Is the impaired N170 print tuning specific to developmental dyslexia? A matched reading-level study with poor readers and dyslexics. Brain Lang. 2013, 127, 539–544. [Google Scholar] [CrossRef]
- van de Walle de Ghelcke, A.; Rossion, B.; Schiltz, C.; Lochy, A. Impact of learning to read in a mixed approach on neural tuning to words in beginning readers. Front. Psychol. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Júnior, R.d.M.; Marinho de Sousa, B.; Fukusima, S. Hemispheric specialization in face recognition: From spatial frequencies to holistic/analytic cognitive processing. Psychol. Neurosci. 2014, 7, 503. [Google Scholar] [CrossRef]
- Mayseless, N.; Breznitz, Z. Brain activity during processing objects and pseudo-objects: Comparison between adult regular and dyslexic readers. Clin. Neurophysiol. 2011, 122, 284–298. [Google Scholar] [CrossRef]
- Rüsseler, J.; Gerth, I.; Heldmann, M.; Münte, T. Audiovisual perception of natural speech is impaired in adult dyslexics: An ERP study. Neuroscience 2015, 287, 55–65. [Google Scholar] [CrossRef]
- Rüsseler, J.; Johannes, S.; Münte, T.F. Recognition memory for unfamiliar faces does not differ for adult normal and dyslexic readers: An event-related brain potential study. Clin. Neurophysiol. 2003, 114, 1285–1291. [Google Scholar] [CrossRef]
- Tarkiainen, A.; Helenius, P.; Salmelin, R. Category-specific occipitotemporal activation during face perception in dyslexic individuals: An MEG study. Neuroimage 2003, 19, 1194–1204. [Google Scholar] [CrossRef]
- Calder, A.J.; Young, A.W. Understanding the recognition of facial identity and facial expression. Nat. Rev. Neurosci. 2005, 6, 641–651. [Google Scholar] [CrossRef]
- Sinha, P.; Balas, B.; Ostrovsky, Y.; Russell, R. Face recognition by humans: Nineteen results all computer vision researchers should know about. Proc. IEEE 2006, 94, 1948–1962. [Google Scholar] [CrossRef]
- Collins, E.; Dundas, E.; Gabay, Y.; Plaut, D.C.; Behrmann, M. Hemispheric organization in disorders of development. Vis. Cogn. 2017, 25, 416–429. [Google Scholar] [CrossRef]
- Raman, I. The role of age of acquisition in picture and word naming in dyslexic adults. Br. J. Psychol. 2011, 102, 328–339. [Google Scholar] [CrossRef]
- Denckla, M.B.; Rudel, R.G. Naming of object-drawings by dyslexic and other learning disabled children. Brain Lang. 1976, 3, 1–15. [Google Scholar] [CrossRef]
- McGugin, R.W.; Richler, J.J.; Herzmann, G.; Speegle, M.; Gauthier, I. The Vanderbilt Expertise Test reveals domain-general and domain-specific sex effects in object recognition. Vis. Res. 2012, 69, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Huestegge, L.; Rohrßen, J.; van Ermingen-Marbach, M.; Pape-Neumann, J.; Heim, S. Devil in the details? Developmental dyslexia and visual long-term memory for details. Front. Psychol. 2014, 5, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Z. Brachacki, G.W.; Nicolson, R.I.; Fawcett, A.J. Impaired recognition of traffic signs in adults with dyslexia. J. Learn. Disabil. 1995, 28, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Gabay, Y.; Dundas, E.; Plaut, D.; Behrmann, M. Atypical perceptual processing of faces in developmental dyslexia. Brain Lang. 2017, 173, 41–51. [Google Scholar] [CrossRef]
- Sigurdardottir, H.M.; Fridriksdottir, L.E.; Gudjonsdottir, S.; Kristjánsson, Á. Specific problems in visual cognition of dyslexic readers: Face discrimination deficits predict dyslexia over and above discrimination of scrambled faces and novel objects. Cognition 2018, 175, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; McKeever, W.F. Material specific serial memory deficit in adolescent dyslexics. Cortex 1979, 15, 51–62. [Google Scholar] [CrossRef]
- Liberman, I.Y.; Mann, V.A.; Shankweiler, D.; Werfelman, M. Children’s memory for recurring linguistic and nonlinguistic material in relation to reading ability. Cortex 1982, 18, 367–375. [Google Scholar] [CrossRef]
- Korinth, S.P.; Sommer, W.; Breznitz, Z. Does silent reading speed in normal adult readers depend on early visual processes? Evidence from event-related brain potentials. Brain Lang. 2012, 120, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kühn, C.D.; Gerlach, C.; Andersen, K.B.; Poulsen, M.; Starrfelt, R. Face recognition in developmental dyslexia: Evidence for dissociation between faces and words. Cogn. Neuropsychol. 2021, 38, 107–115. [Google Scholar] [CrossRef]
- Benton, A.L.; Varney, N.R.; Hamsher, K.d. Visuospatial judgment: A clinical test. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef]
- Sigurdardottir, H.M.; Hjartarson, K.H.; Gudmundsson, G.L.; Kristjánsson, Á. Own-race and other-race face recognition problems without visual expertise problems in dyslexic readers. Vis. Res. 2019, 158, 146–156. [Google Scholar] [CrossRef]
- Pontius, A.A. Dyslexia and specifically distorted drawings of the face—A new subgroup with prosopagnosia-like signs. Experientia 1976, 32, 1432–1435. [Google Scholar] [CrossRef]
- Pontius, A.A. Links between literacy skills and accurate spatial relations in representations of the face: Comparison of preschoolers, school children, dyslexics, and mentally retarded. Percept. Mot. Ski. 1983, 57, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Aaron, P. Dyslexia, an imbalance in cerebral information-processing strategies. Percept. Mot. Ski. 1978, 47, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.; Nakayama, K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 2006, 44, 576–585. [Google Scholar] [CrossRef]
- Farah, M.J. Visual Agnosia; MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Peterson, M.A.; Rhodes, G. Perception of Faces, Objects, and Scenes: Analytic and Holistic Processes; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Jozranjbar, B.; Kristjansson, A.; Sigurdardottir, H.M. Featural and configural processing of faces and houses in matched dyslexic and typical readers. Neuropsychologia 2021, 62, 108059. [Google Scholar] [CrossRef] [PubMed]
- Norcia, A.M.; Appelbaum, L.G.; Ales, J.M.; Cottereau, B.R.; Rossion, B. The steady-state visual evoked potential in vision research: A review. J. Vis. 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochy, A.; Van Belle, G.; Rossion, B. A robust index of lexical representation in the left occipito-temporal cortex as evidenced by EEG responses to fast periodic visual stimulation. Neuropsychologia 2015, 66, 18–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigurdardottir, H.M.; Ólafsdóttir, I.M.; Devillez, H. Words as Visual Objects: Neural and Behavioral Evidence for High-Level Visual Impairments in Dyslexia. Brain Sci. 2021, 11, 1427. https://doi.org/10.3390/brainsci11111427
Sigurdardottir HM, Ólafsdóttir IM, Devillez H. Words as Visual Objects: Neural and Behavioral Evidence for High-Level Visual Impairments in Dyslexia. Brain Sciences. 2021; 11(11):1427. https://doi.org/10.3390/brainsci11111427
Chicago/Turabian StyleSigurdardottir, Heida Maria, Inga María Ólafsdóttir, and Hélène Devillez. 2021. "Words as Visual Objects: Neural and Behavioral Evidence for High-Level Visual Impairments in Dyslexia" Brain Sciences 11, no. 11: 1427. https://doi.org/10.3390/brainsci11111427
APA StyleSigurdardottir, H. M., Ólafsdóttir, I. M., & Devillez, H. (2021). Words as Visual Objects: Neural and Behavioral Evidence for High-Level Visual Impairments in Dyslexia. Brain Sciences, 11(11), 1427. https://doi.org/10.3390/brainsci11111427





