Striatal Dopamine Transporter Availability Is Not Associated with Food Craving in Lean and Obese Humans; a Molecular Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Brain Single-Photon Emission Computed Tomography (SPECT) Imaging
2.4. Region-of-Interest (ROI) Analysis
2.5. General Food Craving Questionnaire-Trait
2.6. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. BMI and Craving
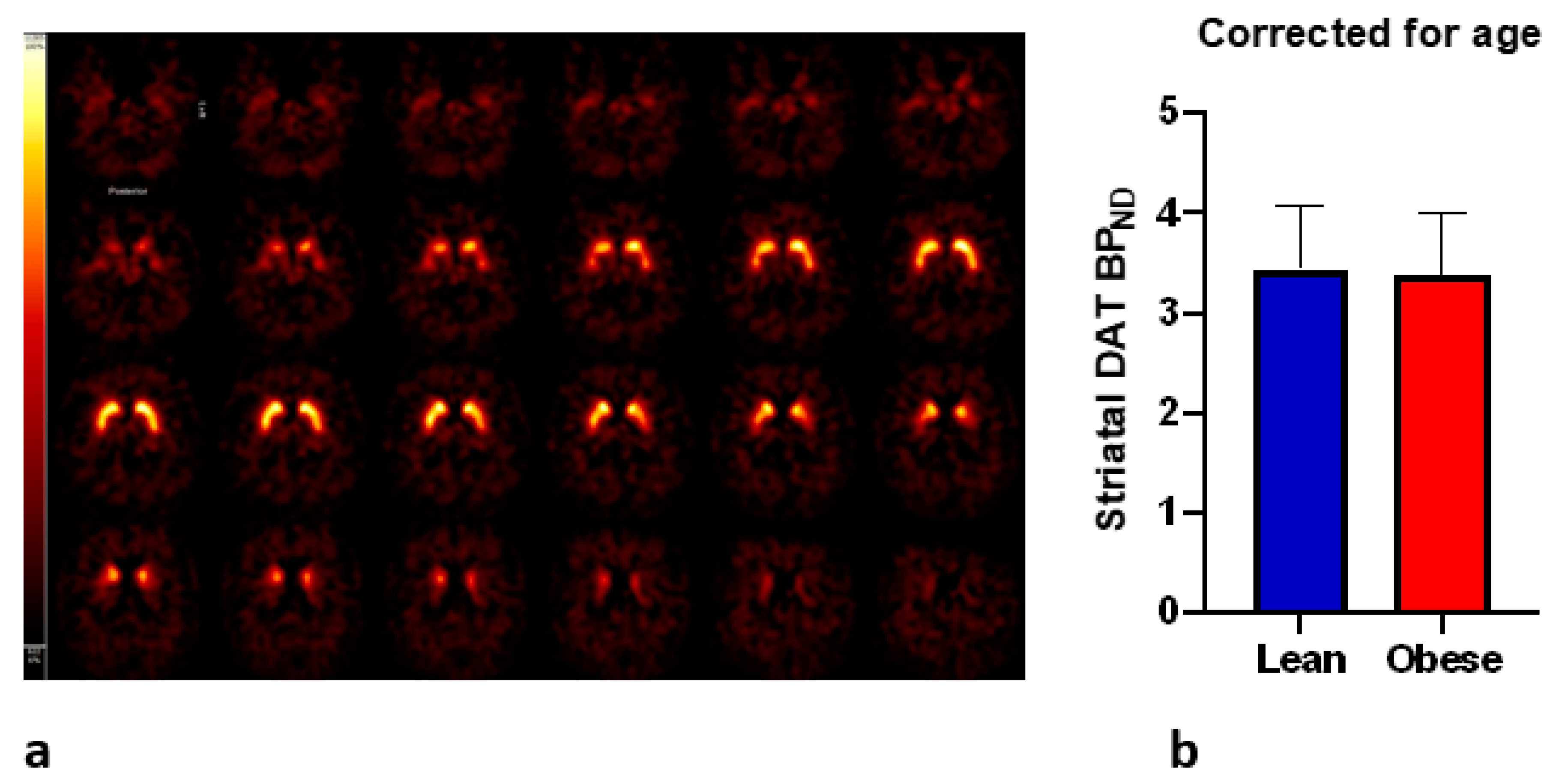
3.3. DAT and Craving
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cambridge Dictionary. Available online: https://dictionary.cambridge.org/dictionary/english/craving (accessed on 1 August 2021).
- Chao, A.; Grilo, C.M.; White, M.A.; Sinha, R. Food cravings, food intake, and weight status in a community-based sample. Eat. Behav. 2014, 15, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Meule, A.; Lutz, A.; Vogele, C.; Kubler, A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite 2012, 58, 88–97. [Google Scholar] [CrossRef]
- Meule, A.; Kuppers, C.; Harms, L.; Friederich, H.C.; Schmidt, U.; Blechert, J.; Brockmeyer, T. Food cue-induced craving in individuals with bulimia nervosa and binge-eating disorder. PLoS ONE 2018, 13, e0204151. [Google Scholar] [CrossRef] [PubMed]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Smithson, E.F.; Hill, A.J. It is not how much you crave but what you do with it that counts: Behavioural responses to food craving during weight management. Eur. J. Clin. Nutr. 2017, 71, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagniard, B.; Balsam, P.D.; Brunner, D.; Zhuang, X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 2006, 31, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Wise, R.A.; Schwartz, H.V. Pimozide attenuates acquisition of lever-pressing for food in rats. Pharmacol. Biochem. Behav. 1981, 15, 655–656. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Jayne, M.; Franceschi, D.; Wong, C.; Gatley, S.J.; Gifford, A.N.; Ding, Y.S.; et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002, 44, 175–180. [Google Scholar] [CrossRef]
- Szczypka, M.S.; Kwok, K.; Brot, M.D.; Marck, B.T.; Matsumoto, A.M.; Donahue, B.A.; Palmiter, R.D. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 2001, 30, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Sotak, B.N.; Hnasko, T.S.; Robinson, S.; Kremer, E.J.; Palmiter, R.D. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005, 1061, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Zhao, W.; Sun, L.; Zhang, R.; Zhu, S.; Xie, K.; Feng, X.; Wu, X.; Sun, Z.; et al. Food reward depends on TLR4 activation in dopaminergic neurons. Pharmacol. Res. 2021, 169, 105659. [Google Scholar] [CrossRef] [PubMed]
- Roitman, M.F.; Stuber, G.D.; Phillips, P.E.; Wightman, R.M.; Carelli, R.M. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 2004, 24, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U.; Ljungberg, T.; Steg, G. Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv. Neurol. 1974, 5, 421–426. [Google Scholar]
- Trifilieff, P.; Feng, B.; Urizar, E.; Winiger, V.; Ward, R.D.; Taylor, K.M.; Martinez, D.; Moore, H.; Balsam, P.D.; Simpson, E.H.; et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 2013, 18, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamada, T.; Hata, T. Striatal dopamine D1 receptors control motivation to respond, but not interval timing, during the timing task. Learn Mem. 2021, 28, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Nagai, Y.; Mimura, K.; Suhara, T.; Higuchi, M.; Bouret, S.; Minamimoto, T. D1- and D2-like receptors differentially mediate the effects of dopaminergic transmission on cost-benefit evaluation and motivation in monkeys. PLoS Biol. 2021, 19, e3001055. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Ferreira, J.G.; Tellez, L.A.; Ren, X.; Yeckel, C.W. The gut-brain dopamine axis: A regulatory system for caloric intake. Physiol. Behav. 2012, 106, 394–399. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Jones-Gotman, M.; Dagher, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef]
- Thanarajah, S.E.; Backes, H.; DiFeliceantonio, A.G.; Albus, K.; Cremer, A.L.; Hanssen, R.; Lippert, R.N.; Cornely, O.A.; Small, D.M.; Bruning, J.C.; et al. Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metab. 2019, 29, 695–706.e4. [Google Scholar] [CrossRef] [Green Version]
- de Weijer, B.A.; van de Giessen, E.; van Amelsvoort, T.A.; Boot, E.; Braak, B.; Janssen, I.M.; van de Laar, A.; Fliers, E.; Serlie, M.J.; Booij, J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011, 1, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.J.; Tomasi, D.; Convit, A.; Logan, J.; Wong, C.T.; Shumay, E.; Fowler, J.S.; Volkow, N.D. BMI modulates calorie-dependent dopamine changes in accumbens from glucose intake. PLoS ONE 2014, 9, e101585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Galen, K.A.; Ter Horst, K.W.; Booij, J.; la Fleur, S.E.; Serlie, M.J. The role of central dopamine and serotonin in human obesity: Lessons learned from molecular neuroimaging studies. Metab. Clin. Exp. 2018, 85, 325–339. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. NPJ Parkinsons Dis. 2021, 7, 22. [Google Scholar] [CrossRef]
- Zhuang, X.; Oosting, R.S.; Jones, S.R.; Gainetdinov, R.R.; Miller, G.W.; Caron, M.G.; Hen, R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1982–1987. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Toyoshima, O.; Kunimatsu, J.; Yamada, H.; Matsumoto, M. Tonic firing mode of midbrain dopamine neurons continuously tracks reward values changing moment-by-moment. Elife 2021, 10, e63166. [Google Scholar] [CrossRef]
- Pecina, S.; Cagniard, B.; Berridge, K.C.; Aldridge, J.W.; Zhuang, X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J. Neurosci. 2003, 23, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Koopman, K.E.; Roefs, A.; Elbers, D.C.; Fliers, E.; Booij, J.; Serlie, M.J.; la Fleur, S.E. Brain dopamine and serotonin transporter binding are associated with visual attention bias for food in lean men. Psychol. Med. 2016, 46, 1707–1717. [Google Scholar] [CrossRef]
- Koopman, K.E.; Booij, J.; Fliers, E.; Serlie, M.J.; la Fleur, S.E. Diet-induced changes in the Lean Brain: Hypercaloric high-fat-high-sugar snacking decreases serotonin transporters in the human hypothalamic region. Mol. Metab. 2013, 2, 417–422. [Google Scholar] [CrossRef] [PubMed]
- van Galen, K.A.; Booij, J.; Schrantee, A.; Adriaanse, S.M.; Unmehopa, U.A.; Fliers, E.; Schwartz, G.J.; DiLeone, R.J.; Ter Horst, K.W.; la Fleur, S.E.; et al. The response to prolonged fasting in hypothalamic serotonin transporter availability is blunted in obesity. Metabolism 2021, 123, 154839. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, R.I.; Koopman, K.E.; Booij, J.; Ackermans, M.T.; Unmehopa, U.A.; Fliers, E.; la Fleur, S.E.; Serlie, M.J. Serotonin Transporter Binding in the Diencephalon Is Reduced in Insulin-Resistant Obese Humans. Neuroendocrinology 2017, 105, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, R.I.; Schrantee, A.; Adriaanse, S.M.; Unmehopa, U.A.; Booij, J.; Reneman, L.; Fliers, E.; la Fleur, S.E.; Serlie, M.J. Timing of caloric intake during weight loss differentially affects striatal dopamine transporter and thalamic serotonin transporter binding. FASEB J. 2017, 31, 4545–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 1 October 2021).
- Booij, J.; Hemelaar, T.G.; Speelman, J.D.; de Bruin, K.; Janssen, A.G.; van Royen, E.A. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J. Nucl. Med. 1999, 40, 753–761. [Google Scholar]
- Adriaanse, S.M.; de Wit, T.C.; Stam, M.; Verwer, E.; de Bruin, K.M.; Booij, J. Clinical evaluation of [(123)I]FP-CIT SPECT scans on the novel brain-dedicated InSPira HD SPECT system: A head-to-head comparison. EJNMMI Res. 2018, 8, 85. [Google Scholar] [CrossRef]
- Booij, J.; Tissingh, G.; Boer, G.J.; Speelman, J.D.; Stoof, J.C.; Janssen, A.G.; Wolters, E.C.; van Royen, E.A. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joutsa, J.; Johansson, J.; Kaasinen, V. Is Occipital Cortex a Valid Reference Region in 123I-FP-CIT SPECT Imaging? Clin. Nucl. Med. 2015, 40, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.; Franken, I.H.; Muris, P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite 2007, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Booij, J.; Kapucu, O.L.; Kluge, A.; Knudsen, G.M.; Koulibaly, P.M.; et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): Age-related effects, gender differences and evaluation of different methods of analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 213–227. [Google Scholar] [CrossRef]
- van de Giessen, E.; Hesse, S.; Caan, M.W.; Zientek, F.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Guignard, R.; Akdemir, U.O.; et al. No association between striatal dopamine transporter binding and body mass index: A multi-center European study in healthy volunteers. Neuroimage 2013, 64, 61–67. [Google Scholar] [CrossRef]
- Koskela, A.K.; Kaurijoki, S.; Pietilainen, K.H.; Karhunen, L.; Pesonen, U.; Kuikka, J.T.; Kaprio, J.; Rissanen, A. Serotonin transporter binding and acquired obesity—An imaging study of monozygotic twin pairs. Physiol. Behav. 2008, 93, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, G.; Ziebell, M.; Jensen, P.S.; da Cuhna-Bang, S.; Knudsen, G.M.; Pinborg, L.H. No correlation between body mass index and striatal dopamine transporter availability in healthy volunteers using SPECT and [123I]PE2I. Obesity 2013, 21, 1803–1806. [Google Scholar] [CrossRef]
- Meule, A. Twenty Years of the Food Cravings Questionnaires: A Comprehensive Review. Curr. Addict. Rep. 2020, 7, 30–43. [Google Scholar] [CrossRef] [Green Version]
- van der Zwaal, E.M.; de Weijer, B.A.; van de Giessen, E.M.; Janssen, I.; Berends, F.J.; van de Laar, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Booij, J.; et al. Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss. Eur. Neuropsychopharmacol. 2016, 26, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Woods, C.; Zhen, J.; Antonio, T.; Carr, K.D.; Reith, M.E. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem. 2017, 140, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Bello, N.T.; Sweigart, K.L.; Lakoski, J.M.; Norgren, R.; Hajnal, A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1260–R1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meule, A.; Hermann, T.; Kubler, A. A short version of the Food Cravings Questionnaire-Trait: The FCQ-T-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Giessen, E.; Celik, F.; Schweitzer, D.H.; van den Brink, W.; Booij, J. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 2014, 28, 866–873. [Google Scholar] [CrossRef]
- Wang, G.J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Mercer, M.E.; Holder, M.D. Food cravings, endogenous opioid peptides, and food intake: A review. Appetite 1997, 29, 325–352. [Google Scholar] [CrossRef]
- Kirkham, T.C. Cannabinoids and appetite: Food craving and food pleasure. Int. Rev. Psychiatry 2009, 21, 163–171. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Morris, J.K.; Bomhoff, G.L.; Gorres, B.K.; Davis, V.A.; Kim, J.; Lee, P.P.; Brooks, W.M.; Gerhardt, G.A.; Geiger, P.C.; Stanford, J.A. Insulin resistance impairs nigrostriatal dopamine function. Exp. Neurol. 2011, 231, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Treadway, M.T.; Cooper, J.A.; Miller, A.H. Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends Cogn. Sci. 2019, 23, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Warthen, K.G.; Boyse-Peacor, A.; Jones, K.G.; Sanford, B.; Love, T.M.; Mickey, B.J. Sex differences in the human reward system: Convergent behavioral, autonomic and neural evidence. Soc. Cogn. Affect. Neurosci. 2020, 15, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.E.; McNaughton, K.A.; Hou, L.; Anderson, L.C.; Long, K.H.; Chesler, E.J. Sex and strain influence attribution of incentive salience to reward cues in mice. Behav. Brain Res. 2015, 292, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreckelmeyer, K.N.; Krach, S.; Kohls, G.; Rademacher, L.; Irmak, A.; Konrad, K.; Kircher, T.; Grunder, G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 2009, 4, 158–165. [Google Scholar] [CrossRef]
- Calipari, E.S.; Juarez, B.; Morel, C.; Walker, D.M.; Cahill, M.E.; Ribeiro, E.; Roman-Ortiz, C.; Ramakrishnan, C.; Deisseroth, K.; Han, M.H.; et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017, 8, 13877. [Google Scholar] [CrossRef] [Green Version]
- Diekhof, E.K.; Keil, M.; Obst, K.U.; Henseler, I.; Dechent, P.; Falkai, P.; Gruber, O. A functional neuroimaging study assessing gender differences in the neural mechanisms underlying the ability to resist impulsive desires. Brain Res. 2012, 1473, 63–77. [Google Scholar] [CrossRef]


| Lean Subjects n = 32 | Subjects with Obesity n = 34 | p-Value | |
|---|---|---|---|
| Sex, male (%) | 32 (100) | 27 (79.4) | 0.007 |
| Age (years) | 23 [21,58] | 54 [48,67] | <0.001 |
| BMI (kg/m2) | 22.9 [21.4–23.9] | 33.1 [32.3–36.2] | <0.001 |
| Fat percentage (%) | 16.5 ± 5.8 | 33.4 ± 8.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Son, J.; van Galen, K.A.; Bruijn, A.M.; Koopman, K.E.; Versteeg, R.I.; la Fleur, S.E.; Serlie, M.J.; Booij, J. Striatal Dopamine Transporter Availability Is Not Associated with Food Craving in Lean and Obese Humans; a Molecular Imaging Study. Brain Sci. 2021, 11, 1428. https://doi.org/10.3390/brainsci11111428
van Son J, van Galen KA, Bruijn AM, Koopman KE, Versteeg RI, la Fleur SE, Serlie MJ, Booij J. Striatal Dopamine Transporter Availability Is Not Associated with Food Craving in Lean and Obese Humans; a Molecular Imaging Study. Brain Sciences. 2021; 11(11):1428. https://doi.org/10.3390/brainsci11111428
Chicago/Turabian Stylevan Son, Jamie, Katy A. van Galen, Anne Marijn Bruijn, Karin E. Koopman, Ruth I. Versteeg, Susanne E. la Fleur, Mireille J. Serlie, and Jan Booij. 2021. "Striatal Dopamine Transporter Availability Is Not Associated with Food Craving in Lean and Obese Humans; a Molecular Imaging Study" Brain Sciences 11, no. 11: 1428. https://doi.org/10.3390/brainsci11111428
APA Stylevan Son, J., van Galen, K. A., Bruijn, A. M., Koopman, K. E., Versteeg, R. I., la Fleur, S. E., Serlie, M. J., & Booij, J. (2021). Striatal Dopamine Transporter Availability Is Not Associated with Food Craving in Lean and Obese Humans; a Molecular Imaging Study. Brain Sciences, 11(11), 1428. https://doi.org/10.3390/brainsci11111428






