Recall and Self-Relevance of Emotional Words Predict Subjective Self-Evaluation of Cognition in Patients with MTLE with or without Depressive Symptoms
Abstract
:Highlights
- Depression specific self-relevance bias is observed in MTLE patients with depressive symptoms.
- Behavioral variables are predictors of subjective self-evaluation of cognition.
- Epilepsy duration is a predictor of subjective self-evaluation of cognition.
1. Introduction
1.1. The Presence of Depressive Symptoms
1.2. The Presence of Depression-Specific Cognitive Bias without Current Depressive Symptoms
1.3. The Objective Memory Ability Assessment Method
2. Methods
2.1. Participants
2.2. Experimental Procedure
2.2.1. Inventories
Beck Depression Inventory—II (BDI-II)
Quality of Life in Epilepsy Inventory—31 (QOLIE-31)
2.2.2. Experimental Behavioral Paradigm
Self-Relevance Ratings Task
Experimental Stimuli
2.3. Data Analysis
3. Results
3.1. Self-Evaluation of Cognition in Participants with MTLE
3.2. Task Performance
3.2.1. Overall Free Recall
3.2.2. Self-Relevance Ratings
3.3. Correlation Analysis
3.4. Hierarchical Regression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tinson, D.; Crockford, C.; Gharooni, S.; Russell, H.; Zoeller, S.; Leavy, Y.; Lloyd, R.; Duncan, S. Memory complaints in epilepsy: An examination of the role of mood and illness perceptions. Epilepsy Behav. 2018, 80, 221–228. [Google Scholar] [CrossRef]
- Rayner, G.; Wrench, J.M.; Wilson, S.J. Differential contributions of objective memory and mood to subjective memory complaints in refractory focal epilepsy. Epilepsy Behav. 2010, 12, 359–364. [Google Scholar] [CrossRef]
- Sawrie, S.M.; Martin, R.C.; Kuzniecky, R.; Faught, E.; Morawetz, R.; Jamil, F.; Viikinsalo, M.; Gilliam, F. Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology 1999, 53, 1511–1517. [Google Scholar] [CrossRef]
- Vermeulen, J.; Aldenkamp, A.P.; Alpherts, W.C.J. Memory complaints in epilepsy: Correlations with cognitive performance and neuroticism. Epilepsy Res. 1993, 15, 157–170. [Google Scholar] [CrossRef]
- Munera, C.P.; Lomlomdjian, C.; Terpiluk, V.; Medel, N.; Solis, P.; Kochen, S. Memory for emotional material in temporal lobe epilepsy. Epilepsy Behav. 2015, 52, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Kang, H.; You, L.; Rastogi, P.; Venkatesh, D.; Chandra, M. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann. Indian Acad. Neurol. 2014, 17, 374–382. [Google Scholar] [CrossRef]
- Guedj, E.; Barbeau, E.J.; Liégois-Chauvel, C.; Confort Gouny, S.; Bartolomei, F.; Chauvel, P.; Cozzone, P.J.; Ranjeva, J.P.; Mundler, O.; Guye, M. Performance in recognition memory is correlated with entorhinal/perirhinal interictal metabolism in temporal lobe epilepsy. Epilepsy Behav. 2010, 19, 612–617. [Google Scholar] [CrossRef]
- Galioto, R.; Blum, A.S.; Tremont, G. Subjective cognitive complaints versus objective neuropsychological performance in older adults with epilepsy. Epilepsy Behav. 2015, 51, 48–52. [Google Scholar] [CrossRef]
- Marino, S.E.; Meador, K.J.; Loring, D.W.; Okun, M.S.; Fernandez, H.H.; Fessler, A.J.; Kustra, R.P.; Miller, J.M.; Ray, P.G.; Roy, A.; et al. Subjective perception of cognition is related to mood and not performance. Epilepsy Behav. 2009, 14, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liik, M.; Vahter, L.; Gross-Paju, K.; Haldre, S. Subjective complaints compared to the results of neuropsychological assessment in patients with epilepsy: The influence of comorbid depression. Epilepsy Res. 2009, 84, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Srisurapanont, M.; Suttajit, S.; Eurviriyanukul, K.; Varnado, P. Discrepancy between objective and subjective cognition in adults with major depressive disorder. Sci. Rep. 2017, 7, 3901. [Google Scholar] [CrossRef] [PubMed]
- Rayner, G. The Contribution of Cognitive Networks to Depression in Epilepsy. Epilepsy Curr. 2017, 17, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Laxer, K.D.; Trinka, E.; Lawrence, J.H.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S.R. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Vhalente, K.D.R.; Filho, G.B. Depression and temporal lobe epilepsy represent an epiphenomenon sharing similar neural networks: Clinical and brain structural evidences. Arq. Neuro-Psiquiatr. 2013, 71, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.S. Depression in temporal lobe epilepsy: A review of prevalence, clinical features, and management considerations. Epilepsy Res. Treat. 2012, 2012, 809843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewe, P.; Nikstat, A.; Koch, O.; Koch-Stoecker, S.; Bien, C.G. Subjective memory complaints in patients with epilepsy: The role of depression, psychological distress, and attentional functions. Epilepsy Res. 2016, 127, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Seeathalakshmi, R.; Krishnamoorthy, E.S. Depression in epilepsy: Phenomenology, diagnosis and management. Epileptic Disord. 2007, 9, 1–10. [Google Scholar]
- McCagh, J.; Fisk, J.E.; Baker, G.A. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009, 86, 1–14. [Google Scholar] [CrossRef]
- Gilliam, F.; Hećimović, H.; Sheline, Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003, 4, 26–30. [Google Scholar] [CrossRef]
- Thapar, A.; Roland, M.; Harold, G. Do depression symptoms predict seizure frequency-or vice versa? J. Psychosom. Res. 2005, 59, 269–274. [Google Scholar] [CrossRef]
- Mula, M.; Monaco, F. Antiepileptic drugs and psychopathology of epilepsy: An update. Epileptic Disord. 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vanegas, M.A.; Cisneros-Franco, J.M.; Castillo-Montoya, C.; Martinez-Rosas, A.R.; Gomez-Perez, M.E.; Rubio-Donnadieu, F. Self-reported quality of life in pharmacoresistant temporal lobe epilepsy: Correlation with clinical variables and memory evaluation. Epileptic Disord. 2013, 15, 263–271. [Google Scholar] [CrossRef]
- Damasceno, B.P. Methodological issues and controversies in research on cognitive disorders. Dement. Neuropsychol. 2010, 4, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Joormann, J.; Talbot, L.; Gotlib, I.H. Biased processing of emotional information in girls at risk for depression. J. Abnorm. Psychol. 2007, 116, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beevers, C.G. Cognitive vulnerability to depression: A dual process model. Clin. Psychol. Rev. 2005, 25, 975–1002. [Google Scholar] [CrossRef]
- Arnold, J.F.; Fitzgerald, D.A.; Fernández, G.; Rijpkema, M.; Rinck, M.; Eling, P.A.T.M.; Becker, E.S.; Speckens, A.; Tendolkar, I. Rose or black glasses? Altered neural processing of positive events during memory formation is a trait marker of depression. J. Affect. Disord. 2011, 131, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramponi, C.; Murphy, F.C.; Calder, A.J.; Barnard, P.J. Recognition memory for pictorial material in subclinical depression. Acta Psychol. 2010, 135, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ridout, N.; Noreen, A.; Johal, J. Memory for emotional faces in naturally occurring dysphoria and induced sadness. Behav. Res. Ther. 2009, 47, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Direnfeld, D.M.; Roberts, J.E. Mood-congruent memory in dysphoria: The roles of state affect and cognitive style. Behav. Res. Ther. 2006, 44, 1275–1285. [Google Scholar] [CrossRef]
- Fitzgerald, D.A.; Arnold, J.F.; Becker, E.S.; Speckens, A.E.M.; Rinck, M.; Rijpkema, M.; Fernández, G.; Tendolkar, I. How mood challenges emotional memory formation. Neuroimage 2011, 56, 1783–1790. [Google Scholar] [CrossRef]
- Koster, E.H.W.; De Raedt, R.; Leyman, L.; Lissnyder, E.D. Mood-congruent attention and memory bias in dysphoria: Exploring the coherence among information-processing biases. Behav. Res. Ther. 2010, 48, 219–225. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; Ruis, C.; Kappelle, L.J. The impact of self-reported depressive symptoms on memory function in neurological outpatients. Clin. Neurol. Neurosurg. 2007, 109, 323–326. [Google Scholar] [CrossRef]
- Tarsia, M.; Power, M.J.; Sanavio, E. Implicit and explicit memory biases in mixed anxiety- depression. J. Affect. Disord. 2003, 77, 213–225. [Google Scholar] [CrossRef]
- White, C.; Ratcliff, R.; Vasey, M.; McKoon, G. Dysphoria and memory for emotional material: A diffusion model analysis. Cogn. Emot. 2009, 23, 181–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preglej, L.; Marinković, K.; Hećimović, H. Differences in emotional stimuli processing in subjects with MTLE with and without depression. Epilepsy Behav. 2017, 74, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 2008, 165, 969–977. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Gotlib, I.H. Neural Substrates of Increased Memory Sensitivity for Negative Stimuli in Major Depression. Biol. Psychiatry 2008, 63, 1155–1162. [Google Scholar] [CrossRef] [Green Version]
- Urban, E.J.; Charles, S.T.; Levine, L.J.; Almedia, D.M. Depression history and memory bias for specific daily emotions. PLoS ONE 2018, 13, e0203574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, C.; Rutherford, E.; Campbell, L.; Ebsworthy, G.; Holker, L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J. Abnorm. Psychol. 2002, 111, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, J.D.; Dent, J. Cognitive vulnerability to depression: An investigation of two hypotheses. Br. J. Clin. Psychol. 1987, 26, 113–126. [Google Scholar] [CrossRef]
- Pinabiaux, C.; Bulteau, C.; Fohlen, M.; Dorfmuller, G.; Chiron, C.; Hertz-Pannier, L.; Delalande, O.; Jambaque, I. Impaired emotional memory recognition after early temporal lobe epilepsy surgery: The fearful face exception? Cortex 2013, 49, 1386–1393. [Google Scholar] [CrossRef]
- Műller, N.G.; Wohlarth, B.; Kopp, U.A.; Lengler, U. Emotional content does not interfere with verbal memory in patients with temporal lobe epilepsy. Epilepsy Behav. 2009, 15, 367–371. [Google Scholar] [CrossRef]
- Kircanski, K.; Gotlib, I.H. Processing of emotional information in major depressive disorder: Toward a dimensional understanding. Emotion 2015, 7, 256–264. [Google Scholar] [CrossRef]
- Wolitzky-Taylor, K.; Dour, H.; Zinbarg, R.; Mineka, S.; Vrshek-Schallhorn, S.; Epstein, A.; Craske, M.G. Experiencing core symptoms of anxiety and unipolar mood disorders in late adolescence predicts disorder onset in early adulthood. Depress. Anxiety 2014, 31, 207–213. [Google Scholar] [CrossRef]
- Baert, S.; De Raedt, R.; Koster, E.H.W. Depression-related attentional bias: The influence of symptom severity and symptom specificity. Cogn. Emot. 2009, 24, 1044–1052. [Google Scholar] [CrossRef]
- Beevers, C.G.; Carver, C.S. Attentional bias and mood persistence as prospective predictors of dysphoria. Cogn. Ther. Res. 2003, 27, 619–637. [Google Scholar] [CrossRef]
- Hendriks, M.P.H.; Aldenkamp, A.P.; Van der Vlugt, H.; Alperts, W.C.J.; Vermeulen, J. Memory complaints in medically refractory epilepsy: Relationship to epilepsy-related factors. Epilepsy Behav. 2002, 3, 165–172. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beckov Inventar Depresije-II-BDI-II; Naklada Slap: Jastrebarsko, Croatia, 2011. [Google Scholar]
- Cramer, J.A.; Perrine, K.R.; Devinsky, O.; Brayant-Comstok, L.; Meador, K.J.; Hermann, B.P. Development and cross-cultural transitions of a 31-item quality of life in epilepsy inventory. Epilepsia 1998, 39, 81–88. [Google Scholar] [CrossRef]
- Grant, D.M.; Beck, J.G. Attentional biases in social anxiety and dysphoria: Does comorbidity make a difference? J. Anxiety Disord. 2006, 20, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Šarić, L.J. Dictionary of Croatian Synonyms; Jesenski i Turk: Zagreb, Croatia, 2008. [Google Scholar]
- Benjamini, Y.; Hochberg, L.R. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Orjuela-Rojas, J.M.; Martínez-Juárez, I.E.; Ruiz-Chow, A.; Crail-Melendez, D. Treatment of depression in patients with temporal lobe epilepsy: A pilot study of cognitive behavioral therapy vs. selective serotonin reuptake inhibitors. Epilepsy Behav. 2015, 51, 176–181. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Jonides, J.; Buschkuehl, M.; Joorman, J. Memory for affectively valenced and neutral stimuli in depression: Evidence from a novel matching task. Cogn. Emot. 2011, 25, 1246–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wingen, G.A.; Van Eijndhoven, P.; Cremers, H.R.; Tendolkar, I.; Verkes, R.J.; Buitelaar, J.K.; Fernandez, G. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. J. Psychiatr. Res. 2010, 44, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Everaert, J.; Duyck, W.; Koster, E.H.W. Emotionally Biased Cognitive Processes: The Weakest Link Predicts Prospective Changes in Depressive Symptom Severity. PLoS ONE 2015, 10, e0124457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, T.W. Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer’s Res. Ther. 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlatar, Z.Z.; Muniz, M.; Galasko, D.; Salmon, D.P. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2018, 3, 1198–1202. [Google Scholar] [CrossRef]
- Lanteaume, L.; Guedj, E.; Bastien-Toniazzo, M.; Magalahaes, A.; Mundler, O.; Bartolomei, F. Cog-nitive and metabolic correlates of emotional vulnerability in patients with temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 2012, 83, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bourgeat, F.; Borg, C.; Bedoin, N.; Convers, P.; Billard, S.; Royer, A.; Grosselin, A.; Bellot, C.; Thomas-Antérion, C. Explicit and implicit emotional processing modifications in pharmacoresistant left temporal lobe epilepsy and anxiodepressive disorders. Epilepsy Behav. 2011, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gois, J.; Valente, K.; Vicentiis, S.; Moschetta, S.; Kuczynski, E.; Fiore, L.; Fuentes, D. Assessment of psychosocial adjustement in patients with temporal lobe epilepsy using a standard measure. Epilepsy Behav. 2011, 20, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lanteaume, L.; Bartolomei, F.; Bastien-Toniazzo, M. How do cognition, emotion, and epileptogenesis meet? A study of emotional cognitive bias in temporal lobe epilepsy. Epilepsy Behav. 2009, 15, 218–224. [Google Scholar] [CrossRef]
| MTLE Group | MTLE -d | MTLE +d | Control Group | |
|---|---|---|---|---|
| N = 39 | N = 39 | |||
| Sex | ||||
| Male | 11 | 8 | 3 | 11 |
| Female | 28 | 20 | 8 | 28 |
| Age (y) | 35 ± 11 | 33.39 ± 11.77 | 38.18 ± 8.86 | 34.54 ± 11.60 |
| Education (y) | 12.7 ± 2.2 | 12.7 ± 2.2 | ||
| ≤12 | 75.21% | 21 (75.00%) | 8 (72.72%) | 75.21% |
| >12 | 24.79% | 7 (25.00%) | 3 (27.27) | 24.79% |
| Total BDI score: | 10.32 ± 8.18 | 5.85 ± 3.77 | 21.27 ± 5.00 | 3.82 ± 2.18 |
| Seizure focus lateralization: | ||||
| Left | 21 (54.85%) | 12 (42.86%) | 6 (54.5%) | |
| Right | 18 (46.15%) | 16 (57.14%) | 5 (45.5%) | |
| Hippocampal sclerosis | 1 (2.56%) | 0 | 1 (9.1%) | |
| Number of seizures/month over past 6 months | 1.54 ± 1.69 | 1.29 ± 1.36 | 2.18 ± 2.32 | |
| Epilepsy duration (y) | 15.46 ± 10.81 | 11.89 ± 9.02 | 24.55 ± 9.89 | |
| Age at seizure onset (y) | 19.10 ± 12.92 | 21.36 ± 12.57 | 13.36 ± 12.54 | |
| AED therapy | ||||
| Monotherapy | 56.40% | 16 (57.14%) | 6 (54.5%) | |
| Polytherapy | 43.60% | 12 (42.86%) | 5 (45.5%) |
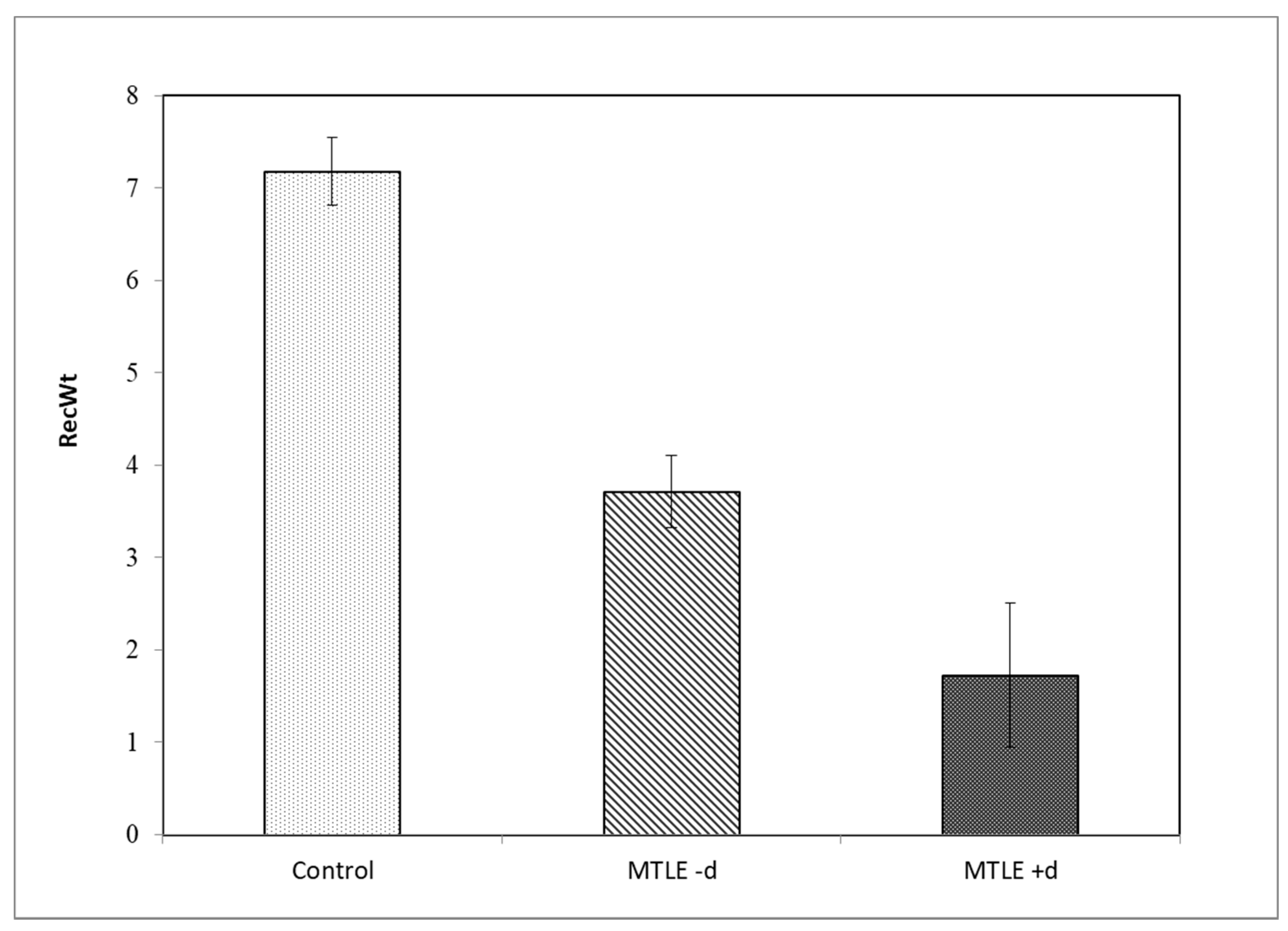
| RecWt | Min | Max | M | SEM | SD |
|---|---|---|---|---|---|
| Control | 1 | 23 | 7.18 | 0.719 | 4.489 |
| MTLE -d | 0 | 9 | 3.71 | 0.496 | 2.623 |
| MTLE +d | 0 | 5 | 1.73 | 0.506 | 1.679 |
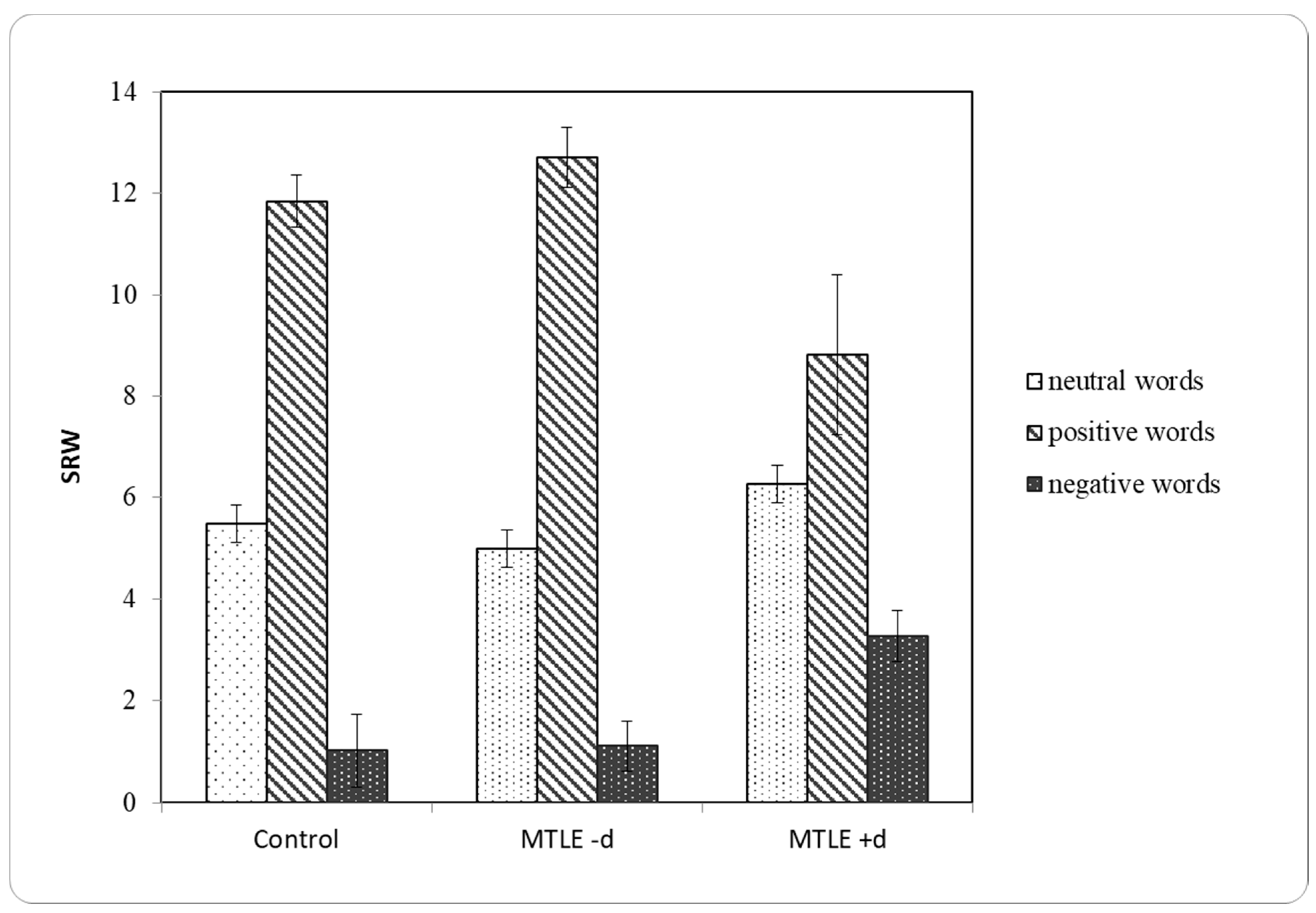
| Control | M | SEM | SD |
|---|---|---|---|
| SRWneg | 1.03 | 0.332 | 2.071 |
| SRWp | 11.85 | 0.516 | 3.224 |
| SRWneut | 5.49 | 0.367 | 2.293 |
| MTLE -d | M | SEM | SD |
| SRWneg | 1.11 | 0.229 | 1.188 |
| SRWp | 12.70 | 0.592 | 3.074 |
| SRWneut | 5.00 | 0.389 | 2.019 |
| MTLE +d | M | SEM | SD |
| SRWneg | 3.27 | 0.854 | 2.832 |
| SRWp | 8.82 | 1.583 | 5.250 |
| SRWneut | 6.27 | 0.776 | 2.573 |
| F | Ss | p | η2 | |
|---|---|---|---|---|
| Within-subjects effects | ||||
| Emotional word valence | 48.09 | 2/146 | <0.001 | 0.397 |
| Emotional word valence × age | 7.16 | 2/146 | <0.001 | 0.089 |
| Emotional word valence × group | 5.12 | 4/146 | <0.001 | 0.123 |
| Between-subjects effects | ||||
| Group | 0.06 | 2/73 | 0.938 | |
| Age | 1.42 | 1/73 | 0.238 |
| QOLIE CS | Confidence Interval 95% | BDI | Confidence Interval 95% | |
|---|---|---|---|---|
| RecWt | 0.43 ** | 0.047–0.591 | −0.33 * | −0.455–0.130 |
| RecWp | 0.38 * | 0.052–0.577 | −0.27 ′ | −0.311–0.204 |
| SRWp | 0.58 ** | 0.125–0.674 | −0.46 ** | −0.574–0.173 |
| SRWneg | −0.46 ** | −0.507–0.114 | −0.54 ** | −0.088–0.564 |
| SF | −0.33 | −0.514–0.109 | 0.21 | −0.172–0.563 |
| ED | −0.62 ** | −0.645–(−0.172) | 0.49 ** | −0.216–0.359 |
| ASO | 0.17 | −0.307–0.271 | −0.25 | −0.330–0.270 |
| Age | −0.42 * | −0.642–(−0.136) | 0.19 | −0.265–0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preglej, L.; Marinkovic, K.; Hećimović, H. Recall and Self-Relevance of Emotional Words Predict Subjective Self-Evaluation of Cognition in Patients with MTLE with or without Depressive Symptoms. Brain Sci. 2021, 11, 1402. https://doi.org/10.3390/brainsci11111402
Preglej L, Marinkovic K, Hećimović H. Recall and Self-Relevance of Emotional Words Predict Subjective Self-Evaluation of Cognition in Patients with MTLE with or without Depressive Symptoms. Brain Sciences. 2021; 11(11):1402. https://doi.org/10.3390/brainsci11111402
Chicago/Turabian StylePreglej, Lidija, Ksenija Marinkovic, and Hrvoje Hećimović. 2021. "Recall and Self-Relevance of Emotional Words Predict Subjective Self-Evaluation of Cognition in Patients with MTLE with or without Depressive Symptoms" Brain Sciences 11, no. 11: 1402. https://doi.org/10.3390/brainsci11111402





