Circadian Sleep-Activity Rhythm across Ages in Down Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sleep Data
2.3. Behavioral Data
2.4. Cognitive Data
2.5. Statistical Analyses
3. Results
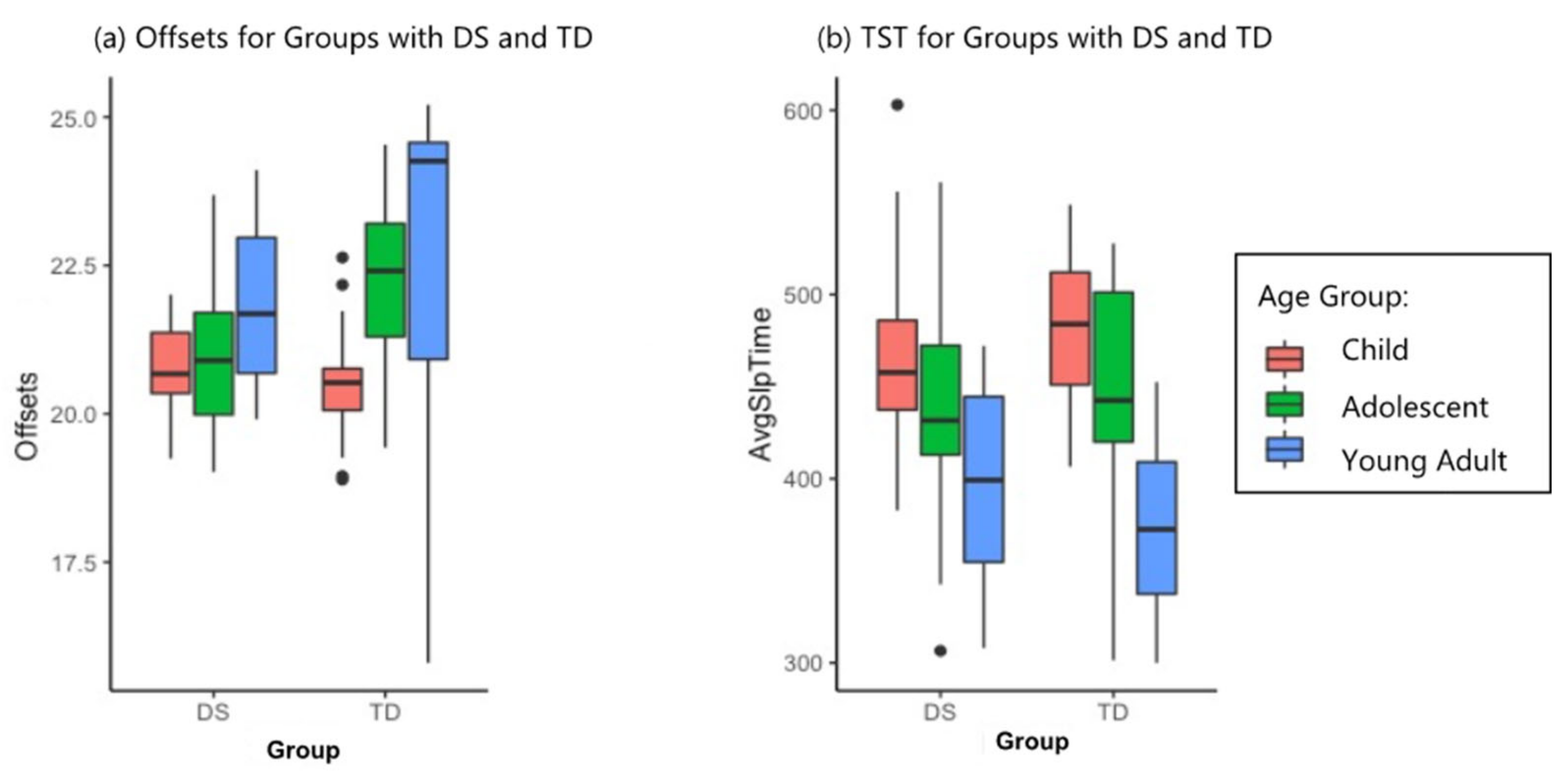
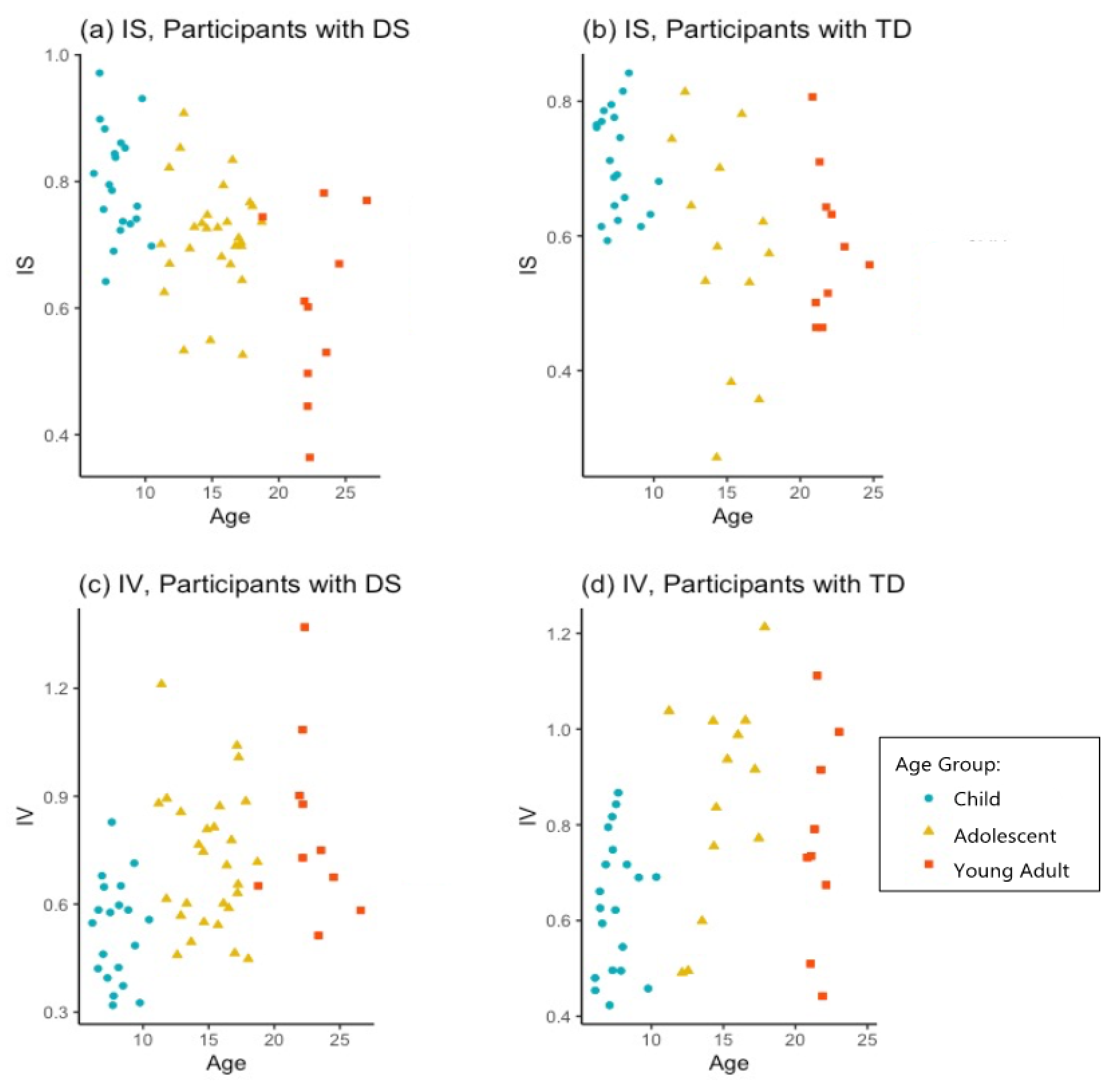
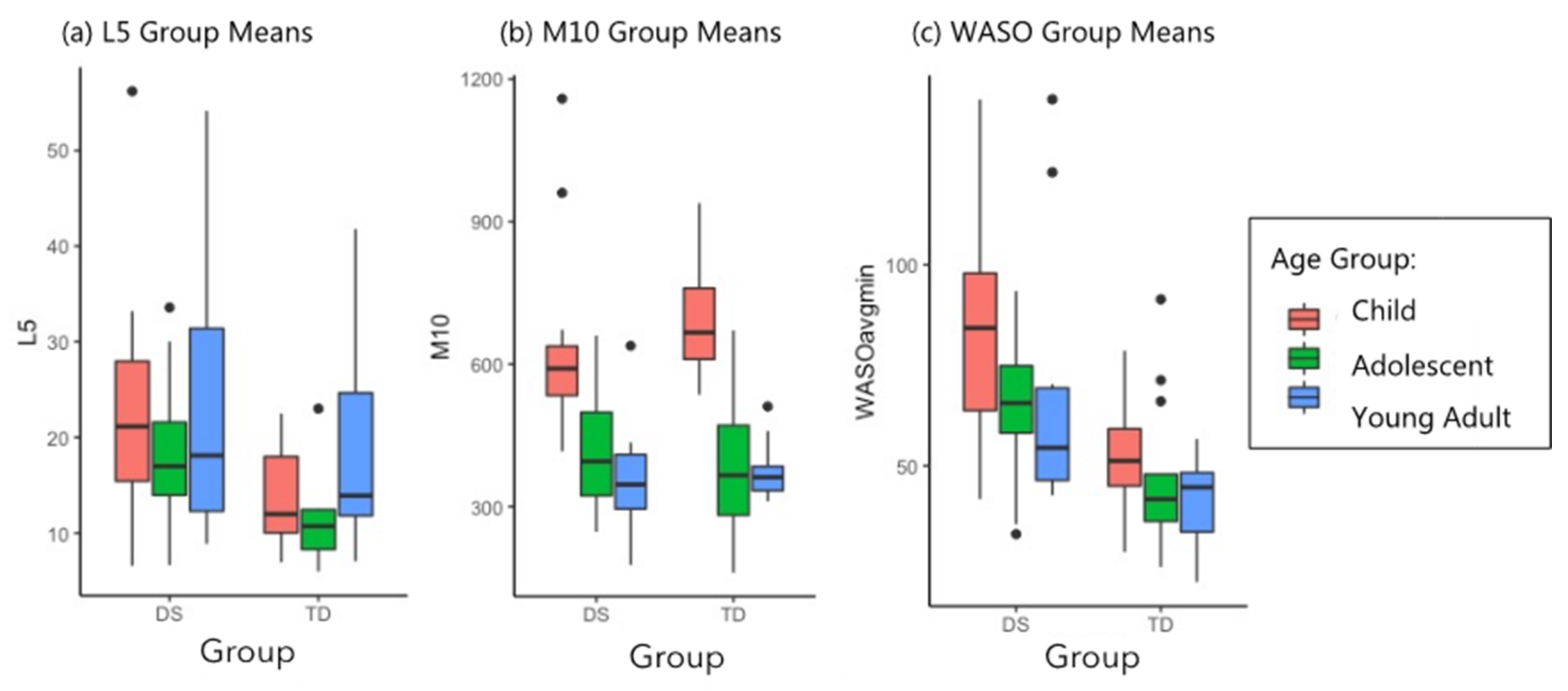
3.1. Sleep and Phase Timing
3.2. Stability and Robustness of Circadian Markers
3.3. Parent-Reported Sleep Data
3.4. Cognitive Outcomes in Participants with DS
4. Discussion
4.1. Sleep and Phase Timing
4.2. Stability and Robustness of Circadian Markers
4.3. Parent-Reported Sleep Health
4.4. Executive Function Measures
4.5. Memory Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edgin, J.O.; Tooley, U.; Demara, B.; Nyhuis, C.; Anand, P.; Spanò, G. Sleep Disturbance and Expressive Language Development in Preschool-Age Children with Down Syndrome. Child Dev. 2015, 86, 1984–1998. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Hill, C.M.; Karmiloff-Smith, A.; Dimitriou, D. Cross Syndrome Comparison of Sleep Problems in Children with Down Syndrome and Williams Syndrome. Res. Dev. Disabil. 2013, 34, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.; Spanò, G.; Bootzin, R.; Anand, P.; Nadel, L.; Edgin, J. Obstructive Sleep Apnea Syndrome and Cognition in Down Syndrome. Dev. Med. Child Neurol. 2014, 56, 657–664. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Schwichtenberg, A.J. Sleep in Neurodevelopmental Disorders. Int. Rev. Res. Dev. Disabil. 2016, 51, 153–191. [Google Scholar] [CrossRef] [Green Version]
- Ng, D.; Hui, H.; Chan, C.; Kwok, K.; Chow, P.; Cheung, J.; Leung, S. Obstructive Sleep Apnoea in Children with Down Syndrome. Singap. Med. J. 2006, 47, 774. [Google Scholar]
- Esbensen, A.J.; Hoffman, E.K. Reliability of Parent Report Measures of Sleep in Children with Down Syndrome. J. Intellect. Disabil. Res. 2017, 61, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Fidler, D.J.; Most, D.E.; Booth-LaForce, C.; Kelly, J.F. Emerging Social Strengths in Young Children With Down Syndrome. Infants Young Child. 2008, 21, 207–220. [Google Scholar] [CrossRef]
- Shott, S.R. Down Syndrome: Common Otolaryngologic Manifestations. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 142C, 131–140. [Google Scholar] [CrossRef]
- Subramanyam, R.; Fleck, R.; McAuliffe, J.; Radhakrishnan, R.; Jung, D.; Patino, M.; Mahmoud, M. Upper Airway Morphology in Down Syndrome Patients under Dexmedetomidine Sedation. Rev. Bras. Anestesiol. 2016, 66, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Horne, R.S.; Roy, B.; Walter, L.M.; Biggs, S.N.; Tamanyan, K.; Weichard, A.; Nixon, G.M.; Davey, M.J.; Ditchfield, M.; Harper, R.M. Regional Brain Tissue Changes and Associations with Disease Severity in Children with Sleep-Disordered Breathing. Sleep 2018, 41, zsx203. [Google Scholar] [CrossRef] [Green Version]
- Diekelmann, S.; Born, J. The Memory Function of Sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.S.C.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and Sleep Disordered Breathing in Children with Down Syndrome: Effects on Behaviour, Neurocognition and the Cardiovascular System. Sleep Med. Rev. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Fernandez, F.; Nyhuis, C.C.; Anand, P.; Demara, B.I.; Ruby, N.F.; Spanò, G.; Clark, C.; Edgin, J.O. Young Children with Down Syndrome Show Normal Development of Circadian Rhythms, but Poor Sleep Efficiency: A Cross-Sectional Study across the First 60 Months of Life. Sleep Med. 2017, 33, 134–144. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Hoffman, E.K. Impact of Sleep on Executive Functioning in School-Age Children with Down Syndrome. J. Intellect. Disabil. Res. 2018, 62, 569–580. [Google Scholar] [CrossRef]
- Nixon, G.M.; Biggs, S.N.; Jitpiriyaroj, S.; Horne, R.S.C. The Relationship Between Sleep-Disordered Breathing Severity and Daytime Adaptive Functioning in Children with Down Syndrome. CNS Neurosci. Ther. 2016, 22, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Perez, M.; Hamner, T.; Adeyemi, E.; Clasen, L.S. A Preliminary Examination of Brain Morphometry in Youth with Down Syndrome with and without Parent-Reported Sleep Difficulties. Res. Dev. Disabil. 2020, 99, 103575. [Google Scholar] [CrossRef] [PubMed]
- Kleitman, N. Sleep and Wakefulness as Alternating Phases in the Cycle of Existence; University of Chicago Press: Chicago, IL, USA, 1939. [Google Scholar]
- Joseph, D.; Chong, N.W.; Shanks, M.E.; Rosato, E.; Taub, N.A.; Petersen, S.A.; Symonds, M.E.; Whitehouse, W.P.; Wailoo, M. Getting Rhythm: How Do Babies Do It? Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F50–F54. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.; Hull, J.; Hughes, R.; Ronda, J.; Czeisler, C. Sleep and Wakefulness Out of Phase with Internal Biological Time Impairs Learning in Humans. J. Cogn. Neurosci. 2006, 18, 508–521. [Google Scholar] [CrossRef]
- Ruby, N.F.; Hwang, C.E.; Wessells, C.; Fernandez, F.; Zhang, P.; Sapolsky, R.; Heller, H.C. Hippocampal-Dependent Learning Requires a Functional Circadian System. Proc. Natl. Acad. Sci. USA 2008, 105, 15593–15598. [Google Scholar] [CrossRef] [Green Version]
- Carskadon, M.A. Adolescent sleepiness: Increased risk in a high-risk population. Alcohol Drugs Driv. 1990, 5/6, 317–328. [Google Scholar] [CrossRef]
- Tynjälä, J.; Kannas, L.; Levälahti, E. Perceived Tiredness among Adolescents and Its Association with Sleep Habits and Use of Psychoactive Substances. J. Sleep Res. 1997, 6, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Acebo, C.; Jenni, O.G. Regulation of Adolescent Sleep: Implications for Behavior. Ann. N. Y. Acad. Sci. 2004, 1021, 276–291. [Google Scholar] [CrossRef]
- Wu, J.Q.; Li, P.; Gilbert, K.S.; Hu, K.; Cronin-Golomb, A. Circadian Rest-Activity Rhythms Predict Cognitive Function in Early Parkinson’s Disease Independently of Sleep. Mov. Disord. Clin. Pract. 2018, 5, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.J.; Wang, W.; Ronda, J.M.; Wyatt, J.K.; Duffy, J.F. Circadian and Wake-Dependent Influences on Subjective Sleepiness, Cognitive Throughput, and Reaction Time Performance in Older and Young Adults. Sleep 2010, 33, 481–490. [Google Scholar] [CrossRef]
- Smith, S.; Kilby, S.; Jorgensen, G.; Douglas, J. Napping and Nightshift Work: Effects of a Short Nap on Psychomotor Vigilance and Subjective Sleepiness in Health Workers. Sleep Biol. Rhythms 2007, 5, 117–125. [Google Scholar] [CrossRef]
- Killgore, W.D.S. Effects of Sleep Deprivation on Cognition. In Progress in Brain Research; Kerkhof, G.A., van Dongen, H.P.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 185, pp. 105–129. [Google Scholar] [CrossRef]
- Molzof, H.E.; Prapanjaroensin, A.; Patel, V.H.; Mokashi, M.V.; Gamble, K.L.; Patrician, P.A. Misaligned Core Body Temperature Rhythms Impact Cognitive Performance of Hospital Shift Work Nurses. Neurobiol. Learn. Mem. 2019, 160, 151–159. [Google Scholar] [CrossRef]
- Luber, B.; Stanford, A.D.; Bulow, P.; Nguyen, T.; Rakitin, B.C.; Habeck, C.; Basner, R.; Stern, Y.; Lisanby, S.H. Remediation of Sleep-Deprivation–Induced Working Memory Impairment with FMRI-Guided Transcranial Magnetic Stimulation. Cereb. Cortex 2008, 18, 2077–2085. [Google Scholar] [CrossRef] [Green Version]
- Mann, D.M.A. The Pathological Association between Down Syndrome and Alzheimer Disease. Mech. Ageing Dev. 1988, 43, 99–136. [Google Scholar] [CrossRef]
- Horvath, S.; Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Salvioli, S.; Gentilini, D.; Di Blasio, A.M.; Giuliani, C.; Tung, S.; Vinters, H.V. Accelerated Epigenetic Aging in Down Syndrome. Aging Cell 2015, 14, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.; Cabelof, D.C. Down Syndrome as a Model of DNA Polymerase Beta Haploinsufficiency and Accelerated Aging. Mech. Ageing Dev. 2012, 133, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.L.; Handen, B.L.; Devenny, D.; Mihaila, I.; Hardison, R.; Lao, P.J.; Klunk, W.E.; Bulova, P.; Johnson, S.C.; Christian, B.T. Cognitive Decline and Brain Amyloid-β Accumulation across 3 Years in Adults with Down Syndrome. Neurobiol. Aging 2017, 58, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Blackwell, T.; Cawthon, P.M.; Ancoli-Israel, S.; Stone, K.L.; Yaffe, K. Association of Circadian Abnormalities in Older Adults With an Increased Risk of Developing Parkinson Disease. JAMA Neurol. 2020, 77, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. ‘The Clocks That Time Us’—Circadian Rhythms in Neurodegenerative Disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between Puberty and Delayed Phase Preference. Sleep 1993, 16, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, L.S.; Persinger, M.A.; Cortez, M.A.; Snead, O.C. Chronobiometry of Behavioral Activity in the Ts65Dn Model of Down Syndrome. Behav. Genet. 2007, 37, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ruby, N.F.; Fernandez, F.; Zhang, P.; Klima, J.; Heller, H.C.; Garner, C.C. Circadian Locomotor Rhythms Are Normal in Ts65Dn “Down Syndrome” Mice and Unaffected by Pentylenetetrazole. J. Biol. Rhythms 2010, 25, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A. Kaufmann Brief Intelligence Scale, (KBIT-II); WPS: Los Angeles, CA, USA, 2004. [Google Scholar]
- De Souza, L.; Benedito-Silva, A.; Pires, M.; Poyares, D.; Tufik, S.; Calil, H. Further Validation of Actigraphy for Sleep Studies. Sleep 2003, 26, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofstra, W.A.; de Weerd, A.W. How to Assess Circadian Rhythm in Humans: A Review of Literature. Epilepsy Behav. 2008, 13, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, T.; Alessi, C.; Friedman, L.; Owens, J.; Kapur, V.; Boehlecke, B.; Swick, T.J. Practice Parameters for the Use of Actigraphy in the Assessment of Sleep and Sleep Disorders: An Update for 2007. Sleep 2007, 30, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Someren, E.J.W.; Swaab, D.F.; Colenda, C.C.; Cohen, W.; McCall, W.V.; Rosenquist, P.B. Bright Light Therapy: Improved Sensitivity to Its Effects on Rest-Activity Rhythms in Alzheimer Patients by Application of Nonparametric Methods. Chronobiol. Int. 1999, 16, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Chapter 4—Actigraphic Monitoring of Sleep and Circadian Rhythms. In Handbook of Clinical Neurology; Montagna, P., Chokroverty, S., Eds.; Sleep Disorders Part I; Elsevier: Amsterdam, The Netherlands, 2011; Volume 98, pp. 55–63. [Google Scholar] [CrossRef]
- Van Someren, E.; Hagebeuk, E.; Lijzenga, C.; Scheltens, P.; de Rooij, S.; Jonker, C.; Swaab, D. Circadian Rest—Activity Rhythm Disturbances in Alzheimer’s Disease. Biol. Psychiatry 1996, 40, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Mumford, R.A.; Mahon, L.V.; Jones, S.; Bigger, B.; Canal, M.; Hare, D.J. Actigraphic Investigation of Circadian Rhythm Functioning and Activity Levels in Children with Mucopolysaccharidosis Type III (Sanfilippo Syndrome). J. Neurodev. Disord. 2015, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.H.; Hare, D.J.; Evershed, K. Actigraphic Assessment of Circadian Activity and Sleep Patterns in Bipolar Disorder. Bipolar Disord. 2005, 7, 176–186. [Google Scholar] [CrossRef]
- Gioia, G.A.; Isquith, P.K.; Guy, S.C.; Kenworthy, L. Test Review Behavior Rating Inventory of Executive Function. Child Neuropsychol. 2000, 6, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric Properties of A Survey Instrument for School-Aged Children. Sleep 2000, 23, 1–9. [Google Scholar] [CrossRef]
- Rosser, T.C.; Edgin, J.O.; Capone, G.T.; Hamilton, D.R.; Allen, E.G.; Dooley, K.J.; Anand, P.; Strang, J.F.; Armour, A.C.; Frank-Crawford, M.A.; et al. Associations between Medical History, Cognition, and Behavior in Youth with down Syndrome: A Report from the down Syndrome Cognition Project. Am. J. Intellect. Dev. Disabil. 2018, 123, 514–528. [Google Scholar] [CrossRef]
- Johnson, C.R.; Smith, T.; DeMand, A.; Lecavalier, L.; Evans, V.; Gurka, M.; Swiezy, N.; Bearss, K.; Scahill, L. Exploring Sleep Quality of Young Children with Autism Spectrum Disorder and Disruptive Behaviors. Sleep Med. 2018, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.C.; Fernandez, F.; Sakhon, S.; Spanò, G.; Edgin, J.O. The Medial Temporal Memory System in Down Syndrome: Translating Animal Models of Hippocampal Compromise. Hippocampus 2017, 27, 683–691. [Google Scholar] [CrossRef]
- CDC—How Much Sleep Do I Need?—Sleep and Sleep Disorders. Available online: https://www.cdc.gov/sleep/about_sleep/how_much_sleep.html (accessed on 19 August 2021).
- Heise, I.; Fisher, S.P.; Banks, G.T.; Wells, S.; Peirson, S.N.; Foster, R.G.; Nolan, P.M. Sleep-like Behavior and 24-h Rhythm Disruption in the Tc1 Mouse Model of Down Syndrome. Genes Brain Behav. 2015, 14, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Markovich, A.; Gendron, M.; Corkum, P. Validating the Children’s Sleep Habits Questionnaire against Polysomnography and Actigraphy in School-Aged Children. Front. Psychiatry 2014, 5, 188. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Marttunen, V.; Hagen, T.; Espeseth, T. Proactive and Reactive Modes of Cognitive Control Can Operate Independently and Simultaneously. Acta Psychol. 2019, 199, 102891. [Google Scholar] [CrossRef]
- Carter, C.S.; Braver, T.S.; Barch, D.M.; Botvinick, M.M.; Cohen, J.D. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science 1998, 280, 747–749. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.X.; Cavanagh, J.F. Single-Trial Regression Elucidates the Role of Prefrontal Theta Oscillations in Response Conflict. Front. Psychol. 2011, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, J.F.; Zambrano-Vazquez, L.; Allen, J.J.B. Theta Lingua Franca: A Common Mid-Frontal Substrate for Action Monitoring Processes. Psychophysiology 2012, 49, 220–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astill, R.G.; Van der Heijden, K.B.; Van IJzendoorn, M.H.; Van Someren, E.J. Sleep, Cognition, and Behavioral Problems in School-Age Children: A Century of Research Meta-Analyzed. Psychol. Bull. 2012, 138, 1109. [Google Scholar] [CrossRef] [PubMed]
- Spanò, G.; Gómez, R.L.; Demara, B.I.; Alt, M.; Cowen, S.L.; Edgin, J.O. REM Sleep in Naps Differentially Relates to Memory Consolidation in Typical Preschoolers and Children with Down Syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, 11844–11849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Trejo, N.; Angulo-Chavira, A.Q.; Demara, B.; Figueroa, C.; Edgin, J. The influence of sleep on language production modalities in preschool children with Down syndrome. J. Sleep Res. 2021, 30, e13120. [Google Scholar] [CrossRef]


| Age, Group | Total (Females) | Mean Ages (Mean Female Ages) |
|---|---|---|
| Children with DS | 20 (8) | 7.95 ± 1.16 (7.75 ± 1.32) |
| Children, TD | 20 (10) | 7.53 ± 1.15 (7.27 ± 0.84) |
| Teenagers with DS | 28 (16) | 15.11 ± 2.21 (15.59 ± 2.02) |
| Teenagers, TD | 13 (5) | 14.84 ± 2.12 (16.51 ± 1.74) |
| Adults with DS | 10 (3) | 22.76 ± 2.02 (24.42 ± 2.22) |
| Adults, TD | 10 (6) | 21.94 ± 1.17 (22.07 ± 1.38) |
| Measures | Children, DS n = 20 | Children, TD n = 20 | Adolescents, DS n = 28 | Adolescents, TD n = 13 | Adults, DS n = 10 | Adults, TD n = 10 | F, Age F, Group F, Interaction | p, Age p, Group p, Interaction | p, Corrected, Age p, Corrected, Group p, Corrected, Interaction |
|---|---|---|---|---|---|---|---|---|---|
| TST | 469.37 (51.59) | 482.40 (40.43) | 438.32 (53.40) | 439.61 (66.73) | 398.61 (55.83) | 376.12 (51.49) | F = 19.02 F = 0.81 F = 0.47 | 1.13 × 10−7 *** ns ns | 2.71 × 10−6 *** ns ns |
| SE | 83.85 (5.05) | 89.32 (2.68) | 85.98 (3.37) | 89.63 (2.62) | 83.73 (6.67) | 87.36 (3.52) | F = 2.19 F = 24.72 F=0.60 | ns 2.94 × 10−6 *** ns | ns 5.58 × 10−5 *** ns |
| WASO | 83.18 (24.93) | 53.23 (13.01) | 64.74 (16.80) | 46.86 (18.91) | 69.11 (34.71) | 41.27 (10.96) | F = 5.81 F = 38.13 F = 0.25 | 4.17 × 10−3 ** 1.63 × 10−8 *** ns | 0.03 * 4.07 × 10−7 *** ns |
| Onsets | 6.69 (0.60) | 7.014 (1.140) | 6.59 (1.08) | 7.57 (1.21) | 7.19 (1.24) | 7.80 (2.97) | F = 5.92 F = 14.92 F = 2.11 | 3.8 × 10−3 ** 2.1 × 10−4 *** ns | 0.03 * 3.32 × 10−3 ** ns |
| Offsets | 20.76 (0.77) | 20.48 (0.96) | 20.92 (1.21) | 22.23 (1.59) | 21.83 (1.47) | 22.40 (3.53) | χ2 = 16.03 χ2 = 2.00 | 3.3 × 10−4 *** ns | 4.95 × 10−3 ** ns |
| Acrophase | 13.62 (0.87) | 13.59 (0.83) | 14.16 (1.04) | 14.88 (1.54) | 14.30 (1.30) | 15.82 (1.80) | F = 16.74 F = 14.04 F = 5.23 | 5.57 × 10−7 *** 3.3 × 10−4 *** 5.82 × 10−3 ** | 1.23 × 10−5 *** 4.95 × 10−3 ** 0.03 * |
| IS | 0.80 (0.09) | 0.71 (0.08) | 0.71 (0.09) | 0.58 (0.17) | 0.60 (0.14) | 0.59 (0.11) | F = 15.71 F = 9.53 F = 2.30 | 1.32 × 10−6 *** 2.66 × 10−3 ** ns | 2.78 × 10−5 *** 2.66 × 10−2 * ns |
| IV | 0.53 (0.141) | 0.64 (0.14) | 0.72 (0.19) | 0.85 (0.22) | 0.81 (0.26) | 0.77 (0.22) | F = 14.90 F = 3.41 F = 0.96 | 2.44 × 10−6 *** ns ns | 4.89 × 10−5 *** ns ns |
| FFT | 0.31 (0.07) | 0.27 (0.06) | 0.22 (0.06) | 0.14 (0.06) | 0.23 (0.17) | 0.15 (0.040) | χ2 = 29.10 χ2 = 6.52 | 4.79 × 10−7 *** 0.01069 | 1.10 × 10−5 *** 0.034 * ns |
| L5 | 22.53 (10.93) | 13.76 (5.02) | 17.64 (6.08) | 14.94 (14.89) | 23.00 (15.30) | 18.53 (10.72) | F = 1.90 F = 8.26 F = 0.75 | ns 5.01 × 10−3 ** ns | ns 0.034 * ns |
| M10 | 614.73 (171.56) | 687.0 (107.28) | 403.75 (110.36) | 376.74 (138.78) | 363.89 (122.82) | 376.69 (62.81) | F = 49.83 F = 0.42 F = 1.88 | 1.77 × 10−15 *** ns ns | 4.59 × 10−14 *** ns ns |
| CSHQ Measures | Children, DS n = 20 | Children, TD n = 20 | Teens, DS n = 28 | Teens, TD n = 13 | Adults, DS n = 10 | χ2 or F, Age χ2 or F, Group | p, Age p, Group | p, Corrected, Age p, Corrected, Group |
|---|---|---|---|---|---|---|---|---|
| Bedtime Resistance | 8.05 (3.52) | 7.35 (2.41) | 8.96 (3.72) | 6.25 (0.62) | 13 (1.32) | χ2 = 15.75 χ2 = 9.36 | 3.80 × 10−4 2.21 × 10−3 | 4.95 × 10−3 ** 0.02 * |
| Sleep Onset Delay | 1.25 (0.64) | 1.2 (0.41) | 1.32 (0.7) | 1.42 (0.52) | 1.11 (0.33) | χ2 = 1.76 χ2 = 0.89 | ns ns | ns ns |
| Sleep Duration | 4.2 (1.51) | 3.7 (1.42) | 4.79 (1.83) | 4.08 (1.8) | 7 (0.5) | χ2 = 19.64 χ2 = 7.09 | 5.45 × 10−5 7.75 × 10−3 | 9.81 × 10−4 *** 0.03 * |
| Sleep Anxiety | 5.75 (1.89) | 5.15 (2.01) | 5.61 (1.99) | 4 (0.43) | 4.67 (0.71) | χ2 = 1.1 χ2 = 7.18 | ns 7.39 × 10−3 | ns 0.03 * |
| Night Wakings | 5.2 (2.12) | 4.05 (1.15) | 4.07 (1.63) | 3.54 (0.66) | 3.22 (0.67) | χ2 = 10.78 χ2 = 0.004 | 4.57 × 10−3 ns | 0.03 * ns |
| Parasomnias | 9.2 (2.19) | 8.75 (1.94) | 9.11 (2.27) | 8.08 (1.38) | 7.67 (1.00) | F = 2.24 F = 2.01 | ns ns | ns ns |
| Sleep Disordered Breathing | 4.7 (2.23) | 3.25 (0.55) | 4.64 (2.57) | 3.08 (0.28) | 3.78 (1.09) | χ2 = 0.16 χ2 = 14.40 | ns 1.48 × 10−4 | ns 2.52 × 10−3 ** |
| Daytime Sleepiness | 12.8 (7.76) | 11.3 (2.87) | 13.36 (2.83) | 11.58 (2.81) | 13.11 (1.76) | χ2 = 6.83 χ2 = 4.88 | 3.29 × 10−2 2.72 × 10−2 | ns ns |
| CSHQ Total | 46.15 (6.54) | 42.15 (7.37) | 13.36 (2.83) | 40.54 (4.68) | 48.22 (2.99) | F = 0.14 F = 10.91 | ns 1.41 × 10−3 | ns 0.02 * |
| Executive, Cognitive Outcomes | Model F Statistic | Degrees of Freedom | Interdaily Stability (IS) p Value | Sleep Efficiency (SE) p Value | Age p Value | Gender p Value | IS × Age Interaction p Value | Model Multiple R2 | Significant p Values, Corrected |
|---|---|---|---|---|---|---|---|---|---|
| Reaction Time | 5.363 | 6.32 | 0.00278 ** | 0.693734 | 0.03137 * | 0.808016 | 0.02159 * | 0.5014 | IS: 0.011 * Age: 0.043 * Is × Age: ns |
| BRIEF | 0.198 | 6.45 | 0.3658 | 0.7704 | 0.5104 | 0.7697 | 0.487 | 0.02152 | ns |
| Verbal Recall | 4.557 | 4.28 | 0.00274 ** | 0.22320 | 0.95120 | 0.59583 | na | 0.3943 | IS: 0.014 * |
| Object-Context Binding | 6.939 | 5.48 | 0.22554 | 0.48540 | 0.00112 ** | 0.03389 * | na | 0.4195 | Age 6.72 × 10−3 ** |
| Spatial Recall | 5.228 | 5.32 | 0.1266 | 0.6160 | 0.0272 * | 0.5816 | na | 0.4496 | ns |
| Scene Recall | 3.142 | 5.33 | 0.0078 ** | 0.2005 | 0.9070 | 0.3846 | na | 0.3225 | IS: 0.03 * |
| Visual Recall | 3.868 | 5.51 | 0.1183 | 0.6864 | 0.0616 | 0.6172 | 0.101 | 0.275 | ns |
| KBIT-II Verbal | 0.138 | 4.52 | 0.542 | 0.713 | 0.992 | 0.783 | na | 0.01697 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovos, A.; Bottrill, K.; Sakhon, S.; Nyhuis, C.; Egleson, E.; Luongo, A.; Murphy, M.; Thurman, A.J.; Abbeduto, L.; Lee, N.R.; et al. Circadian Sleep-Activity Rhythm across Ages in Down Syndrome. Brain Sci. 2021, 11, 1403. https://doi.org/10.3390/brainsci11111403
Lovos A, Bottrill K, Sakhon S, Nyhuis C, Egleson E, Luongo A, Murphy M, Thurman AJ, Abbeduto L, Lee NR, et al. Circadian Sleep-Activity Rhythm across Ages in Down Syndrome. Brain Sciences. 2021; 11(11):1403. https://doi.org/10.3390/brainsci11111403
Chicago/Turabian StyleLovos, Annalysa, Kenneth Bottrill, Stella Sakhon, Casandra Nyhuis, Elizabeth Egleson, Alison Luongo, Melanie Murphy, Angela John Thurman, Leonard Abbeduto, Nancy Raitano Lee, and et al. 2021. "Circadian Sleep-Activity Rhythm across Ages in Down Syndrome" Brain Sciences 11, no. 11: 1403. https://doi.org/10.3390/brainsci11111403
APA StyleLovos, A., Bottrill, K., Sakhon, S., Nyhuis, C., Egleson, E., Luongo, A., Murphy, M., Thurman, A. J., Abbeduto, L., Lee, N. R., Hughes, K., & Edgin, J. (2021). Circadian Sleep-Activity Rhythm across Ages in Down Syndrome. Brain Sciences, 11(11), 1403. https://doi.org/10.3390/brainsci11111403






