Neurophysiological Correlates of Cognition as Revealed by Virtual Reality: Delving the Brain with a Synergistic Approach
Abstract
1. Introduction
2. The Synergy of Virtual Reality and Neuroimaging
3. Neurophysiological Correlates of Cognition as Revealed by Virtual Reality
3.1. Neurorehabilitation, Neuroprosthetics, and Cognitive Behavioral Therapy
3.2. Neuromodulation and Cognitive State
3.3. Perception
3.4. Sense of Presence
3.5. Spatial Navigation and Memory
3.6. BCI and Feedback Control
3.7. Neuromarketing
3.8. Other Fields
4. Limitations and Future Perspective
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geoffrey, W.G. Using virtual reality to augment perception, enhance sensorimotor adaptation, and change our minds. Front. Syst. Neurosci. 2014, 8, 1–6. [Google Scholar] [CrossRef]
- Pandarinathan, G.; Mishra, S.; Nedumaran, A.M.; Padmanabhan, P.; Gulyás, B. The potential of cognitive neuroimaging: A way forward to the mind-machine interface. J. Imaging 2018, 4, 70. [Google Scholar] [CrossRef]
- Lécuyer, A.; Lotte, F.; Reilly, R.B.; College, T. Brain-Computer Interfaces, Virtual Reality, and Videogames. Computer 2008, 41, 66–72. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Nedumaran, A.M.; Mishra, S.; Pandarinathan, G.; Archunan, G.; Gulyás, B. The Advents of Hybrid Imaging Modalities: A New Era in Neuroimaging Applications. Adv. Biosyst. 2017, 1, 1700019. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.P.; Nakagome, S.; He, Y.; Contreras-Vidal, J.L. Real-time EEG-based brain-computer interface to a virtual avatar enhances cortical involvement in human treadmill walking. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.-L. Effects of a brain-computer interface with virtual reality (VR) neurofeedback: A pilot study in chronic stroke patients. Front. Hum. Neurosci. 2019, 13, 210. [Google Scholar] [CrossRef]
- Tremmel, C.; Herff, C.; Sato, T.; Rechowicz, K.; Yamani, Y.; Krusienski, D.J. Estimating cognitive workload in an interactive virtual reality environment using EEG. Front. Hum. Neurosci. 2019, 13, 13. [Google Scholar] [CrossRef]
- Piccione, J.; Collett, J.; De Foe, A. Virtual skills training: The role of presence and agency. Heliyon 2019, 5, e02583. [Google Scholar] [CrossRef]
- David, N.; Skoruppa, S.; Gulberti, A.; Schultz, J.; Engel, A.K. The sense of agency is more sensitive to manipulations of outcome than movement-related feedback irrespective of sensory modality. PLoS ONE 2016, 11, e0161156. [Google Scholar] [CrossRef]
- Kilteni, K.; Grau-Sánchez, J.; Veciana De Las Heras, M.; Rodríguez-Fornells, A.; Slater, M. Decreased corticospinal excitability after the illusion of missing part of the arm. Front. Hum. Neurosci. 2016, 10, 145. [Google Scholar] [CrossRef]
- Cebolla, A.M.; Petieau, M.; Cevallos, C.; Leroy, A.; Dan, B.; Cheron, G. Long-lasting cortical reorganization as the result of motor imagery of throwing a ball in a virtual tennis court. Front. Psychol. 2015, 6, 1869. [Google Scholar] [CrossRef] [PubMed]
- Sangani, S.; Lamontagne, A.; Fung, J. Cortical mechanisms underlying sensorimotor enhancement promoted by walking with haptic inputs in a virtual environment. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 218, pp. 313–330. ISBN 0079-6123. [Google Scholar]
- Kober, S.E.; Neuper, C. Sex differences in human EEG theta oscillations during spatial navigation in virtual reality. Int. J. Psychophysiol. 2011, 79, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wamain, Y.; Gabrielli, F.; Coello, Y. EEG μ rhythm in virtual reality reveals that motor coding of visual objects in peripersonal space is task dependent. Cortex 2016, 74, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bulbul, T. Towards an EEG Based Mental Workload Evaluation Method for Construction Workers’ HMD AR Use. In Proceedings of the Construction Research Congress 2020: Computer Applications; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 659–667. [Google Scholar]
- Rho, G.; Callara, A.L.; Condino, S.; Ghiasi, S.; Nardelli, M.; Carbone, M.; Ferrari, V.; Greco, A.; Scilingo, E.P. A preliminary quantitative EEG study on Augmented Reality Guidance of Manual Tasks. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020; pp. 1–5. [Google Scholar]
- Ke, Y.; Liu, P.; An, X.; Song, X.; Ming, D. An online SSVEP-BCI system in an optical see-through augmented reality environment. J. Neural Eng. 2020, 17, 16066. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997, 20, 44–49. [Google Scholar] [CrossRef]
- Leroy, A.; Cevallos, C.; Cebolla, A.-M.; Caharel, S.; Dan, B.; Cheron, G. Short-term EEG dynamics and neural generators evoked by navigational images. PLoS ONE 2017, 12, e0178817. [Google Scholar] [CrossRef]
- Cebolla, A.-M.; Palmero-Soler, E.; Leroy, A.; Cheron, G. EEG spectral generators involved in motor imagery: A swLORETA Study. Front. Psychol. 2017, 8, 2133. [Google Scholar] [CrossRef]
- Roberts, G.; Holmes, N.; Alexander, N.; Boto, E.; Leggett, J.; Hill, R.M.; Shah, V.; Rea, M.; Vaughan, R.; Maguire, E.A.; et al. Towards OPM-MEG in a virtual reality environment. Neuroimage 2019, 199, 408–417. [Google Scholar] [CrossRef]
- Adamovich, S.V.; August, K.; Merians, A.; Tunik, E. A virtual reality-based system integrated with fmri to study neural mechanisms of action observation-execution: A proof of concept study. Restor. Neurol. Neurosci. 2009, 27, 209–223. [Google Scholar] [CrossRef]
- Macaluso, E.; Ogawa, A. Visuo-spatial orienting during active exploratory behavior: Processing of task-related and stimulus-related signals. Cortex 2018, 102, 26–44. [Google Scholar] [CrossRef]
- Ku, J.; Mraz, R.; Baker, N.; Zakzanis, K.K.; Lee, J.H.; Kim, I.Y.; Kim, S.I.; Graham, S.J. A data glove with tactile feedback for FMRI of virtual reality experiments. Cyberpsychol. Behav. 2003, 6, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, D.H.; Oh, S.; Lyoo, I.K.; Lee, Y.S.; Renshaw, P.F.; Lukas, S.E. Quantitative electroencephalographic (qEEG) correlates of craving during virtual reality therapy in alcohol-dependent patients. Pharmacol. Biochem. Behav. 2009, 91, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, J.H. Cue exposure treatment in a virtual environment to reduce nicotine craving: A functional MRI study. Cyberpsychol. Behav. 2009, 12, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Ward, P.B.; Naismith, S.L.; Pearson, M.; Lewis, S.J.G. Utilising functional MRI (fMRI) to explore the freezing phenomenon in Parkinson’s disease. J. Clin. Neurosci. 2011, 18, 807–810. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 1–16. [Google Scholar] [CrossRef]
- August, K.; Lewis, J.A.; Chandar, G.; Merians, A.; Biswal, B.; Adamovich, S. fMRI analysis of neural mechanisms underlying rehabilitation in virtual reality: Activating secondary motor areas. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 3692–3695. [Google Scholar] [CrossRef]
- Steinisch, M.; Tana, M.G.; Comani, S. A Post-Stroke Rehabilitation System Integrating Robotics, VR and High-Resolution EEG Imaging. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 849–859. [Google Scholar] [CrossRef]
- Gonçalves, M.G.; Piva, M.F.L.; Marques, C.L.S.; da Costa, R.D.M.; Bazan, R.; Luvizutto, G.J.; Betting, L.E.G.G. Effects of virtual reality therapy on upper limb function after stroke and the role of neuroimaging as a predictor of a better response. Arq. Neuropsiquiatr. 2018, 76, 654–662. [Google Scholar] [CrossRef]
- Merians, A.S.; Tunik, E.; Adamovich, S.V. Virtual reality to maximize function for hand and arm rehabilitation: Exploration of neural mechanisms. Stud. Health Technol. Inform. 2009, 145, 109. [Google Scholar]
- Prochnow, D.; Bermudez, I.; Badia, S.; Schmidt, J.; Duff, A.; Brunheim, S.; Kleiser, R. A functional magnetic resonance imaging study of visuomotor processing in a virtual reality-based paradigm: Rehabilitation gaming system. Eur. J. Neurosci. 2013, 37, 1441–1447. [Google Scholar] [CrossRef]
- Xiao, X.; Lin, Q.; Lo, W.-L.; Mao, Y.-R.; Shi, X.; Cates, R.S.; Zhou, S.-F.; Huang, D.-F.; Li, L. Cerebral reorganization in subacute stroke survivors after virtual reality-based training: A preliminary study. Behav. Neurol. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Çakmak, H.; Maaß, H.; Bekrater-Bodmann, R.; Foell, J.; Diers, M.; Trojan, J.; Fuchs, X.; Flor, H. Illusory hand ownership induced by an MRI compatible immersive virtual reality device. Biomed. Tech. 2012, 57, 718–720. [Google Scholar] [CrossRef][Green Version]
- Menon, S.; Brantner, G.; Aholt, C.; Kay, K.; Khatib, O. Haptic fMRI: Combining functional neuroimaging with haptics for studying the brain’s motor control representation. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan , 3–7 July 2013; pp. 4137–4142. [Google Scholar] [CrossRef]
- Cho, B.H.; Lee, J.M.; Ku, J.H.; Jang, D.P.; Kim, J.S.; Kim, I.Y.; Lee, J.H.; Kim, S.I. Attention Enhancement System Using Virtual Reality and EEG Biofeedback. In Proceedings of the Virtual Reality Annual International Symposium, Orlando, FL, USA, 24–28 March 2002; pp. 156–163. [Google Scholar] [CrossRef]
- Choo, A.; May, A. Virtual Mindfulness Meditation: Virtual Reality and Electroencephalography for Health Gamification. In Proceedings of the 2014 IEEE Games Media Entertainement Conference, Toronto, ON, Canada, 22–24 October 2014; pp. 11–13. [Google Scholar] [CrossRef]
- Lin, C.T.; Chung, I.F.; Ko, L.W.; Chen, Y.C.; Liang, S.F.; Duann, J.R. EEG-based assessment of driver cognitive responses in a dynamic virtual-reality driving environment. IEEE Trans. Biomed. Eng. 2007, 54, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Martins e Silva, D.C.; Marinho, V.; Teixeira, S.; Teles, G.; Marques, J.; Escórcio, A.; Fernandes, T.; Freitas, A.C.; Nunes, M.; Ayres, M. Non-immersive 3D virtual stimulus alter the time production task performance and increase the EEG theta power in dorsolateral prefrontal cortex. Int. J. Neurosci. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mathiak, K.; Hertrich, I.; Kincses, W.E.; Riecker, A.; Lutzenberger, W.; Ackermann, H. The right supratemporal plane hears the distance of objects: Neuromagnetic correlates of virtual reality. Neuroreport 2003, 14, 307–311. [Google Scholar] [CrossRef]
- Cheetham, M.; Pedroni, A.F.; Antley, A.; Slater, M.; Jäncke, L. Virtual milgram: Empathic concern or personal distress? Evidence from functional MRI and dispositional measures. Front. Hum. Neurosci. 2009, 3, 1–13. [Google Scholar] [CrossRef]
- Baumgartner, T.; Valko, L.; Esslen, M.; Jäncke, L. Neural correlate of spatial presence in an arousing and noninteractive virtual reality: An EEG and psychophysiology study. Cyberpsychol. Behav. 2006, 9, 30–45. [Google Scholar] [CrossRef]
- Slobounov, S.M.; Ray, W.; Johnson, B.; Slobounov, E.; Newell, K.M. Modulation of cortical activity in 2D versus 3D virtual reality environments: An EEG study. Int. J. Psychophysiol. 2015, 95, 254–260. [Google Scholar] [CrossRef]
- Kober, S.E.; Neuper, C. Using auditory event-related EEG potentials to assess presence in virtual reality. Int. J. Hum. Comput. Stud. 2012, 70, 577–587. [Google Scholar] [CrossRef]
- Clemente, M.; Rodríguez, A.; Rey, B.; Alcañiz, M. Assessment of the influence of navigation control and screen size on the sense of presence in virtual reality using EEG. Expert Syst. Appl. 2014, 41, 1584–1592. [Google Scholar] [CrossRef]
- Slobounov, S.M.; Zhang, K.; Pennell, D.; Ray, W.; Johnson, B.; Sebastianelli, W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: A functional MRI study. Exp. Brain Res. 2010, 202, 341–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hung, Y.; Vetivelu, A.; Hird, M.A.; Yan, M.; Tam, F.; Graham, S.J.; Cusimano, M.; Schweizer, T.A. Using fMRI virtual-reality technology to predict driving ability after brain damage: A preliminary report. Neurosci. Lett. 2014, 558, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ehinger, B.V.; Fischer, P.; Gert, A.L.; Kaufhold, L.; Weber, F.; Pipa, G.; König, P. Kinesthetic and vestibular information modulate alpha activity during spatial navigation: A mobile EEG study. Front. Hum. Neurosci. 2014, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, D.B.; Baffa, O.; Wakai, R.T. Theta oscillations and human navigation: A magnetoencephalography study. J. Cogn. Neurosci. 2002, 14, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Ray, W.; Slobounov, S. Encoding of visual-spatial information in working memory requires more cerebral efforts than retrieval: Evidence from an EEG and virtual reality study. Brain Res. 2010, 1347, 80–89. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Scherer, R.; Leeb, R.; Keinrath, C.; Neuper, C.; Lee, F.; Bischof, H. Viewing moving objects in virtual reality can change the dynamics of sensorimotor EEG rhythms. Presence Teleoper. Virtual Environ. 2007, 16, 111–118. [Google Scholar] [CrossRef]
- Ron-Angevin, R.; Díaz-Estrella, A. Brain-computer interface: Changes in performance using virtual reality techniques. Neurosci. Lett. 2009, 449, 123–127. [Google Scholar] [CrossRef]
- Othmer, S.; Kaiser, D. Implementation of virtual reality in EEG biofeedback. Cyberpsychol. Behav. 2000, 3, 415–420. [Google Scholar] [CrossRef]
- Zarka, D.; Cevallos, C.; Petieau, M.; Hoellinger, T.; Dan, B.; Cheron, G. Neural rhythmic symphony of human walking observation: Upside-down and Uncoordinated condition on cortical theta, alpha, beta and gamma oscillations. Front. Syst. Neurosci. 2014, 8, 169. [Google Scholar] [CrossRef]
- Ariely, D.; Berns, G.S. Neuromarketing: The hope and hype of neuroimaging in business. Nat. Rev. Neurosci. 2010, 11, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S. Neuromarketing: Neural Explanations for Consumer Behaviours. Biomed. J. Sci. Tech. Res. 2019, 16. [Google Scholar] [CrossRef]
- Burris, H.R.; Sheikh, S.A. Virtual reality and neuroimaging technologies: Synergistic approaches in neuromarketing. In Virtual Technologies for Business and Industrial Applications: Innovative and Synergistic Approaches; IGI Global: Hershey, PA, USA, 2010; pp. 118–128. [Google Scholar] [CrossRef][Green Version]
- Castellanos, M.C.; Ausin, J.M.; Guixeres, J.; Bigné, E. Emotion in a 360-Degree vs. Traditional Format Through EDA, EEG and Facial Expressions. Adv. Advert. Res. 2018, 9, 3–15. [Google Scholar] [CrossRef]
- Lin, C.T.; Chuang, S.W.; Chen, Y.C.; Ko, L.W.; Liang, S.F.; Jung, T.P. EEG effects of motion sickness induced in a dynamic virtual reality environment. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 3872–3875. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Salvadore, G.; Colon-Rosario, V.; Latov, D.R.; Holroyd, T.; Carver, F.W.; Coppola, R.; Manji, H.K.; Zarate, C.A.; Grillon, C. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: Evidence from whole-head magnetoencephalography. Am. J. Psychiatry 2010, 167, 836–844. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Ciorciari, J.; Carbis, C.; Liley, D. EEG correlates of virtual reality hypnosis. Int. J. Clin. Exp. Hypn. 2009, 57, 94–116. [Google Scholar] [CrossRef]
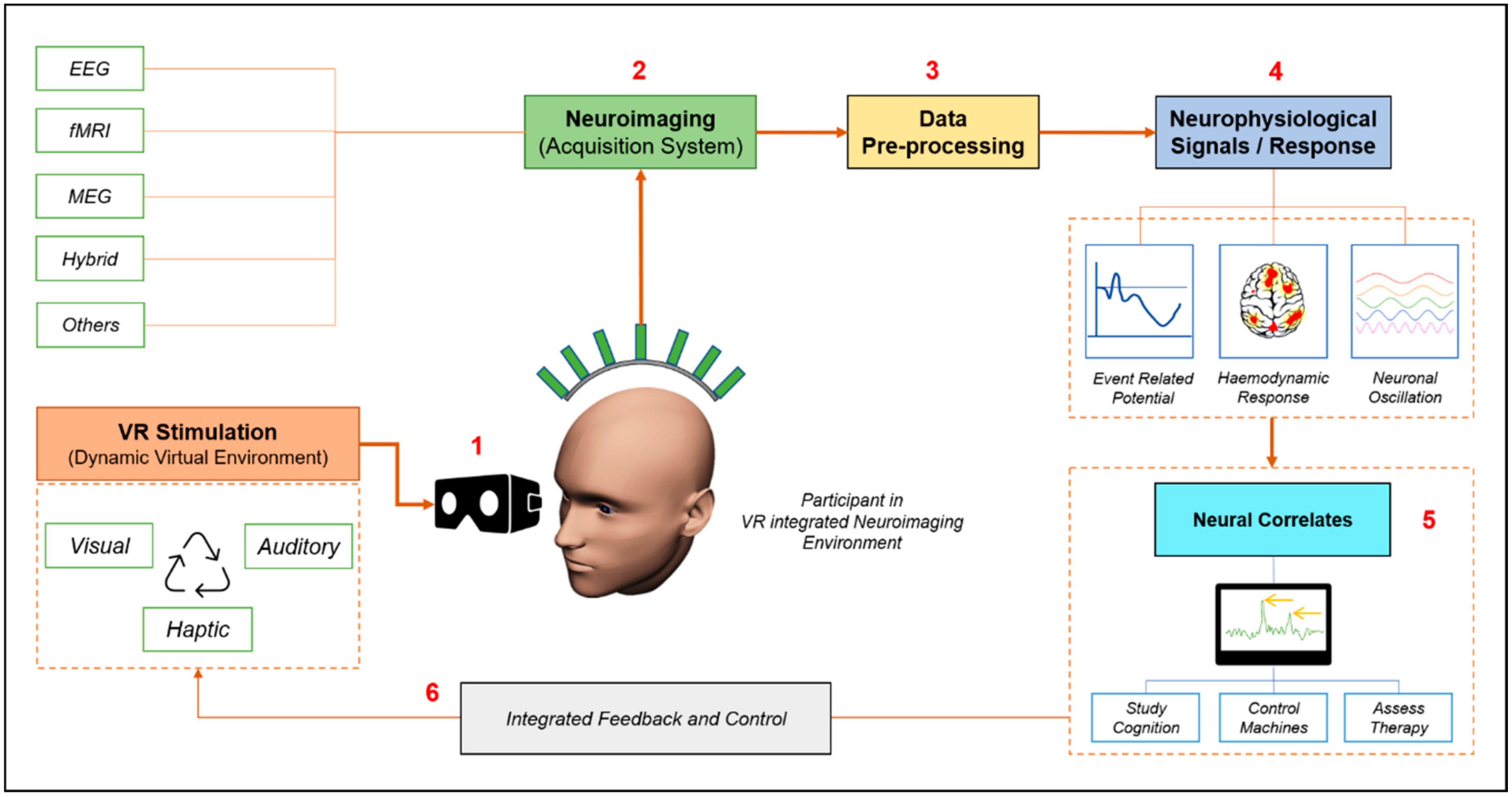




| Serial. Number | Neuroimaging Technique | VR Stimulation System | Neural Correlates/Area of Interest | References |
|---|---|---|---|---|
| 1. (a) | EEG | VR system with a series of scenes associated with virtual alcohol cues, relaxation, and aversive stimulation. | Study of Alpha activity in the frontal region. | [25] |
| (b) | Robotic-assisted gait training device together with VR scenes for neurorehabilitation. | Entrainment of several brain areas and stronger cortical activations due to VR feedback. | [28] | |
| (c) | VR-based prosthetics training for stroke patients. | Activation of primary and secondary motor areas through VR-based exercise, working as neuromotor rehabilitation. | [29,30] | |
| (d) | VR-based attention enhancement system, integrated with EEG biofeedback. | Treatment for Attention disorder, using Beta wave as feedback control and showed significant results in attention enhancement. | [37] | |
| (e) | VR device, which can perform traffic-light motion simulation to study driver cognitive response, by kinetic audiovisual stimuli delivering. | ERP signals processed with a self-constructing neural fuzzy inference network, which recognized different brain potentials corresponding to red/green/yellow traffic events. | [39] | |
| (f) | Varying motion speed of a non-immersive 3D virtual stimulus. | Theta band power in the dorsolateral prefrontal cortex. | [40] | |
| (g) | VR system for inducing the visual perception of manipulable objects for motor coding of visual objects in peripersonal space. | Centro-parietal region was studied while perceptually judging the intrinsic or extrinsic properties of visual objects. | [14] | |
| (h) | Virtual roller coaster scenarios to study the neural correlates of sense of presence. | Activation increased in prefrontal areas that are involved in the control of executive functions. | [43] | |
| (i) | Spatial navigation and memory-based experiments with a virtual maze. | Theta oscillations study during spatial navigation. Observing absolute band power and event-related synchronization. | [13] | |
| (j) | Free navigation experiment in a virtual environment, which was compared with video and photographs. | EEG activity of the right Insula for the theta band oscillation. | [46] | |
| (k) | VR system in which the subject was traversing on a triangular path. | Modulated alpha activity in spatial navigation due to kinesthetic and vestibular information. Alpha suppression during turning movement, in parietal, occipital, and temporal clusters | [49] | |
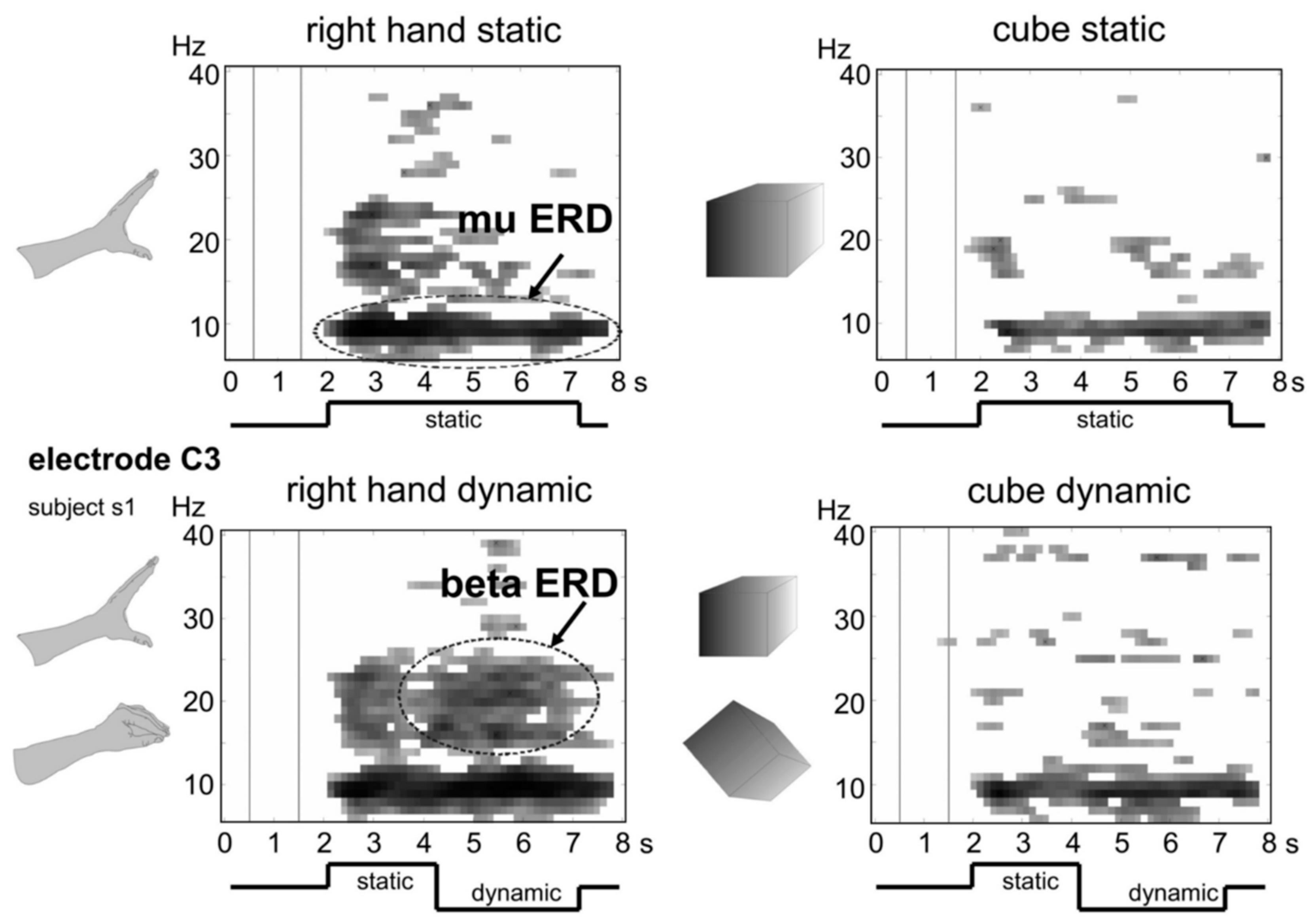
| (l) | Brain computer interface (BCI) system in which moving objects were presented through VR. | Dynamics of sensorimotor rhythms. | [52] | |
| (m) | VR-based BCI neurofeedback system for stroke patient rehabilitation, with the function of the movement of a virtual avatar arm. | EEG based neurofeedback beneficial for severe motor impairment patients. | [6] | |
| (n) | Digital video advertising with EEG measurements 360-degree screen for neuromarketing study. | Study the effect of interactivity in emotion during the viewing of the advertisements. The power spectral density (PSD) values of all frequency bands were computed with an index of frontal asymmetry. | [59] | |
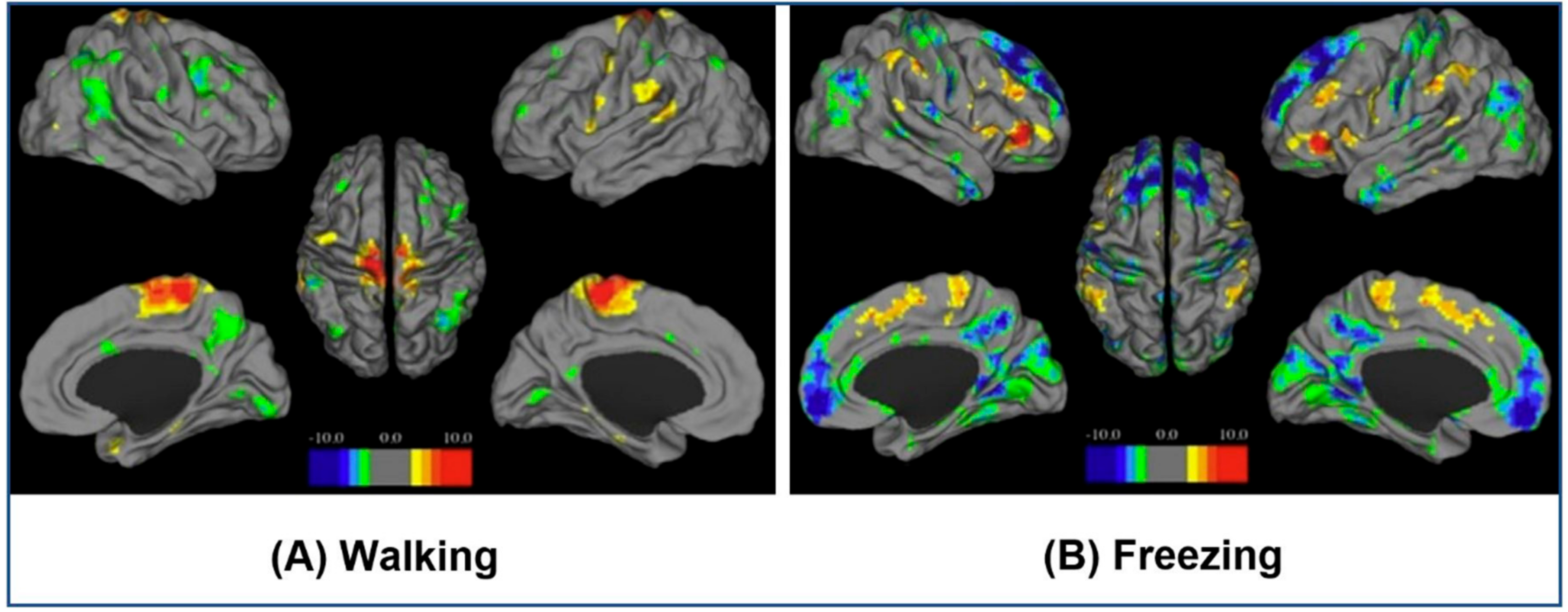
| 2. (a) | fMRI | Motor functions in neurodegenerative diseases, such as freezing behavior, provoked through VR-based stimulation method. | BOLD patterns observed during walking and episodes of freezing. | [27] |
| (b) | VR-based prosthetics training for stroke patients. | Activation of primary and secondary motor areas through VR-based exercise, working as neuromotor rehabilitation. | [32] | |
| (c) | VR-based rehabilitation gaming system for stroke patients. | Object processing, attention, and mirror neuron system involvement. Virtual target catching can activate frontal, parietal, temporal, cingulate, and cerebellar regions. | [33] | |
| (d) | Virtual reality enhanced treadmill. | BOLD response for ankle dorsiflexion activity showed increased activation in the primary sensorimotor cortex of the lesioned hemisphere and in supplementary motor areas. | [34] | |
| (e) | MRI compatible VR-based setup that can trigger illusory sensations with visual and tactile stimulations. | BOLD response with illusory ownership experiences for a virtual limb. The bilateral activity of ventral premotor cortex, secondary somatosensory cortex, extrastriate visual cortex, as well as right-hemispheric activation of the cerebellum. | [35] | |
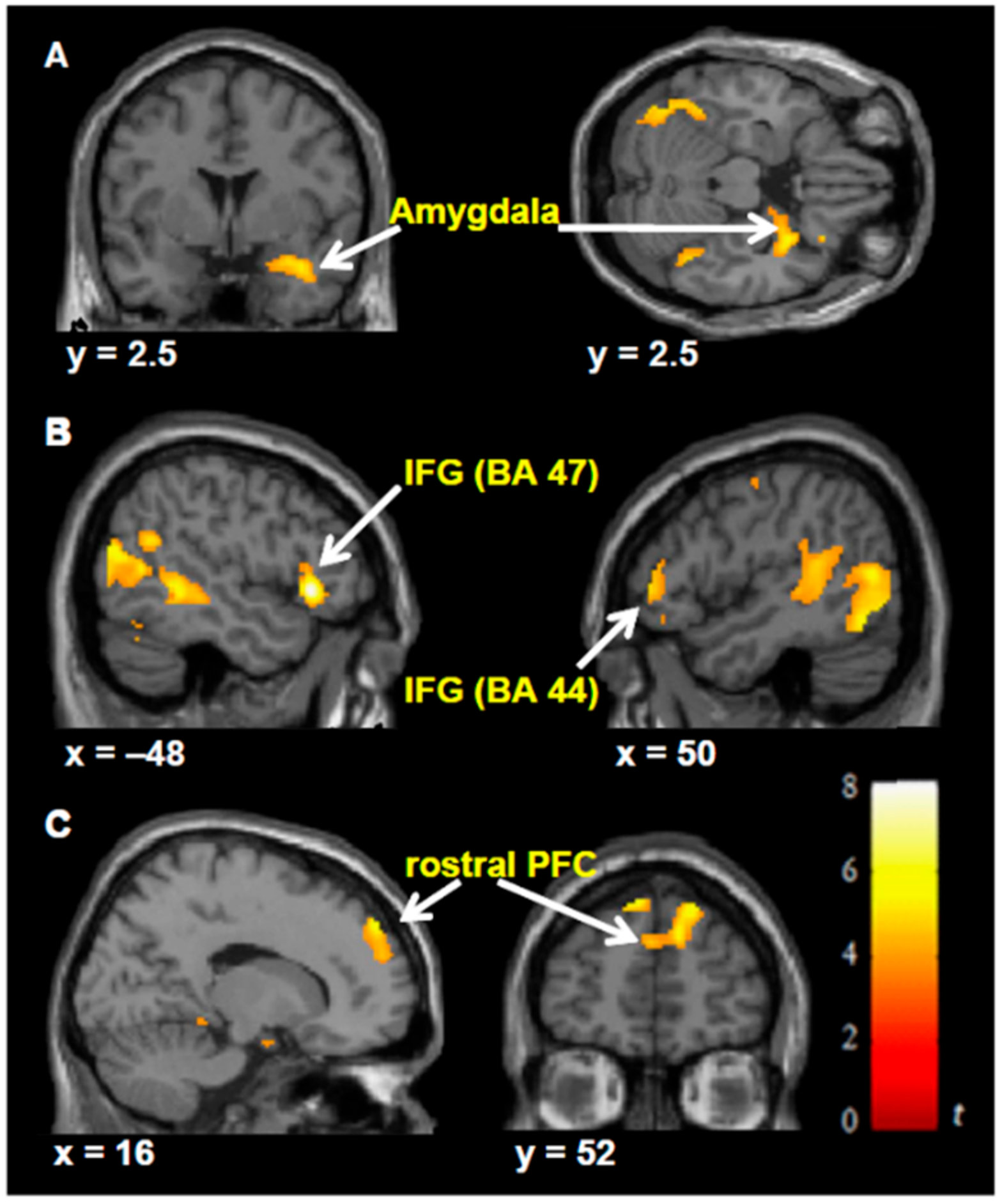
| (f) | Milgram’s obedience paradigm in VR. | Event-related BOLD response during the observation of pain. | [42] | |
| (g) | Navigable virtual corridor for mild traumatic brain injury patients | BOLD response showing that concussed subjects have larger cluster sizes during encoding at the parietal cortex, right hippocampus and, right dorsolateral prefrontal cortex. | [47] | |
| (h) | VR-based driving simulator to study motor-speed coordination in basic driving maneuvers. | Mean cerebellar activation and the average time to complete right turns, which shows compromised motor coordination in patients. | [48] | |
| 3. (a) | MEG | Virtual system to study neural correlates of auditory distance perception. | MEG signals as variation in amplitude over both supratemporal planes. Hemispheric lateralization was indicated by enhanced pre-attentive responses over the right temporal lobe. | [41] |
| (b) | VR-based navigation in a virtual town. | Alpha and theta band activity. Showed a link between navigation and theta activity. | [50] | |
| (c) | Virtual Morris water maze to find a hidden platform as a virtual reality navigation task. | Theta oscillations mapping across the brain. Dysfunction of the right anterior hippocampus and para- hippocampal cortices were indicated as the reason for depression due to observation of the comparative difference in theta activity in right medial temporal cortices. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.; Kumar, A.; Padmanabhan, P.; Gulyás, B. Neurophysiological Correlates of Cognition as Revealed by Virtual Reality: Delving the Brain with a Synergistic Approach. Brain Sci. 2021, 11, 51. https://doi.org/10.3390/brainsci11010051
Mishra S, Kumar A, Padmanabhan P, Gulyás B. Neurophysiological Correlates of Cognition as Revealed by Virtual Reality: Delving the Brain with a Synergistic Approach. Brain Sciences. 2021; 11(1):51. https://doi.org/10.3390/brainsci11010051
Chicago/Turabian StyleMishra, Sachin, Ajay Kumar, Parasuraman Padmanabhan, and Balázs Gulyás. 2021. "Neurophysiological Correlates of Cognition as Revealed by Virtual Reality: Delving the Brain with a Synergistic Approach" Brain Sciences 11, no. 1: 51. https://doi.org/10.3390/brainsci11010051
APA StyleMishra, S., Kumar, A., Padmanabhan, P., & Gulyás, B. (2021). Neurophysiological Correlates of Cognition as Revealed by Virtual Reality: Delving the Brain with a Synergistic Approach. Brain Sciences, 11(1), 51. https://doi.org/10.3390/brainsci11010051





